The Prostaglandin EP3 Receptor Is an Independent Negative Prognostic Factor for Cervical Cancer Patients
Abstract
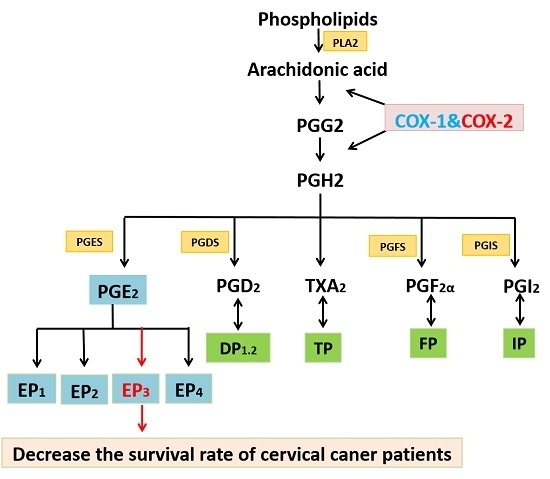
:1. Introduction
2. Results
2.1. Positive Control of EP3 Staining
2.2. EP3 Staining in Cervical Carcinoma
2.3. Correlation Analysis between Prostaglandin E Receptor Type 3 (EP3) and Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) Classification
2.4. Role of EP3 for Overall Survival
2.5. Survival Function of Squamous Cell Carcinoma Versus Adenocarcinoma
2.6. EP3 Staining of Squamous Cell Carcinoma Versus Adenocarcinoma
2.7. Cox Regression of EP3 Immunoreactive Score (IRS) with Clinic Pathological Variables
3. Discussion
4. Materials and Methods
4.1. Patients and Specimens
4.2. Ethics Approval
4.3. Immunohistochemistry
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in globocan 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Diaz, M.; Castellsague, X.; Ferrer, E.; Bosch, F.X.; de Sanjose, S. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010, 202, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Munoz, N.; Bosch, F.X.; Castellsague, X.; Diaz, M.; de Sanjose, S.; Hammouda, D.; Shah, K.V.; Meijer, C.J. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int. J. Cancer 2004, 111, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Knabl, J.; Huttenbrenner, R.; Hutter, S.; Gunthner-Biller, M.; Vrekoussis, T.; Karl, K.; Friese, K.; Kainer, F.; Jeschke, U. Peroxisome proliferator-activated receptor-γ (PPARγ) is down regulated in trophoblast cells of gestational diabetes mellitus (GDM) and in trophoblast tumour cells bewo in vitro after stimulation with ppargamma agonists. J. Perinat. Med. 2014, 42, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Karnezis, T.; Shayan, R.; Fox, S.; Achen, M.G.; Stacker, S.A. The connection between lymphangiogenic signalling and prostaglandin biology: A missing link in the metastatic pathway. Oncotarget 2012, 3, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid receptors: Structures, properties, and functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef] [PubMed]
- Sugita, R.; Kuwabara, H.; Kubota, K.; Sugimoto, K.; Kiho, T.; Tengeiji, A.; Kawakami, K.; Shimada, K. Simultaneous inhibition of PGE2 and PGI2 signals is necessary to suppress hyperalgesia in rat inflammatory pain models. Mediat. Inflamm. 2016, 2016, 9847840. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Hayashi, I.; Endo, H.; Kitasato, H.; Yamashina, S.; Maruyama, T.; Kobayashi, M.; Satoh, K.; Narita, M.; Sugimoto, Y.; et al. Host prostaglandin E2-EP3 signaling regulates tumor-associated angiogenesis and tumor growth. J. Exp. Med. 2003, 197, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Sales, K.J.; Katz, A.A.; Davis, M.; Hinz, S.; Soeters, R.P.; Hofmeyr, M.D.; Millar, R.P.; Jabbour, H.N. Cyclooxygenase-2 expression and prostaglandin E2 synthesis are up-regulated in carcinomas of the cervix: A possible autocrine/paracrine regulation of neoplastic cell function via EP2/EP4 receptors. J. Clin. Endocrinol. Metab. 2001, 86, 2243–2249. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, M.; Kawano, S.; DuBois, R.N. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc. Natl. Acad. Sci. USA 1997, 94, 3336–3340. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.W.; Bailey, T.J.; Donello, J.E.; Pierce, K.L.; Pepperl, D.J.; Zhang, D.; Kedzie, K.M.; Fairbairn, C.E.; Bogardus, A.M.; Woodward, D.F.; et al. Molecular cloning and expression of human EP3 receptors: Evidence of three variants with differing carboxyl termini. Br. J. Pharmacol. 1994, 112, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Israel, D.D.; Regan, J.W. EP3 prostanoid receptor isoforms utilize distinct mechanisms to regulate ERK 1/2 activation. Biochim. Biophys. Acta 2009, 1791, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Kotani, M.; Tanaka, I.; Ogawa, Y.; Usui, T.; Mori, K.; Ichikawa, A.; Narumiya, S.; Yoshimi, T.; Nakao, K. Molecular cloning and expression of multiple isoforms of human prostaglandin e receptor EP3 subtype generated by alternative messenger RNA splicing: Multiple second messenger systems and tissue-specific distributions. Mol. Pharmacol. 1995, 48, 869–879. [Google Scholar] [PubMed]
- Herfs, M.; Herman, L.; Hubert, P.; Minner, F.; Arafa, M.; Roncarati, P.; Henrotin, Y.; Boniver, J.; Delvenne, P. High expression of PGE2 enzymatic pathways in cervical (pre)neoplastic lesions and functional consequences for antigen-presenting cells. Cancer Immunol. Immunother. 2009, 58, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Rader, J.S.; Zhang, F.; Liapis, H.; Koki, A.T.; Masferrer, J.L.; Subbaramaiah, K.; Dannenberg, A.J. Cyclooxygenase-2 is overexpressed in human cervical cancer. Clin. Cancer Res. 2001, 7, 429–434. [Google Scholar] [PubMed]
- Beyer, S.; Zhu, J.; Mayr, D.; Kuhn, C.; Schulze, S.; Hofmann, S.; Dannecker, C.; Jeschke, U.; Kost, B.P. Histone H3 acetyl K9 and histone H3 tri methyl K4 as prognostic markers for patients with cervical cancer. Int. J. Mol. Sci. 2017, 18, 477. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Hsueh, S.; Hong, J.H.; Chang, T.C.; Tseng, C.J.; Chou, H.H.; Huang, K.G.; Lin, J.D. Are adenocarcinomas and adenosquamous carcinomas different from squamous carcinomas in stage IB and II cervical cancer patients undergoing primary radical surgery? Int. J. Gynecol. Cancer 1999, 9, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Jawanjal, P.; Salhan, S.; Dhawan, I.; Das, N.; Aggarwal, R.; Tripathi, R.; Rath, G. Augmented activity of cyclooxygenase-2 in tissue and serum of patients with cervical cancer. J. Clin. Lab. Anal. 2016, 30, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology 2009, 17, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.S.; Chang, K.H.; Yang, H.W.; Kim, M.S.; Kwon, H.C.; Oh, K.S. High cyclooxygenase-2 expression in stage IB cervical cancer with lymph node metastasis or parametrial invasion. Gynecol. Oncol. 2000, 76, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Galic, V.; Herzog, T.J.; Lewin, S.N.; Neugut, A.I.; Burke, W.M.; Lu, Y.S.; Hershman, D.L.; Wright, J.D. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol. Oncol. 2012, 125, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Bean, L.M.; Ward, K.K.; Plaxe, S.C.; McHale, M.T. Survival of women with microinvasive adenocarcinoma of the cervix is not improved by radical surgery. Am. J. Obstet. Gynecol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Young, R.H.; Clement, P.B. Endocervical adenocarcinoma and its variants: Their morphology and differential diagnosis. Histopathology 2002, 41, 185–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, C.X.; Sun, X.X.; Wang, C.; Liu, T.F.; Wang, D.J. Long non-coding RNA CCHE1 overexpression predicts a poor prognosis for cervical cancer. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 479–483. [Google Scholar] [PubMed]
- Miyata, Y.; Ohba, K.; Matsuo, T.; Watanabe, S.; Hayashi, T.; Sakai, H.; Kanetake, H. Tumor-associated stromal cells expressing E-prostanoid 2 or 3 receptors in prostate cancer: Correlation with tumor aggressiveness and outcome by angiogenesis and lymphangiogenesis. Urology 2013, 81, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Zissel, G.; Muller-Qernheim, J.; Jae Shin, S.; Satoh, H.; Ichikawa, T. Prostaglandin E2 reinforces the activation of ras signal pathway in lung adenocarcinoma cells via EP3. FEBS Lett. 2002, 518, 154–158. [Google Scholar] [CrossRef]
- Shoji, Y.; Takahashi, M.; Kitamura, T.; Watanabe, K.; Kawamori, T.; Maruyama, T.; Sugimoto, Y.; Negishi, M.; Narumiya, S.; Sugimura, T.; et al. Downregulation of prostaglandin E receptor subtype EP3 during colon cancer development. Gut 2004, 53, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, E.; Shiota, M.; Yokomizo, A.; Itsumi, M.; Inokuchi, J.; Uchiumi, T.; Naito, S. Prostaglandin receptor EP3 mediates growth inhibitory effect of aspirin through androgen receptor and contributes to castration resistance in prostate cancer cells. Endocr. Relat. Cancer 2013, 20, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.F.; Shu, P.; Murphy, T.F.; Aisner, S.; Fitzhugh, V.A.; Jordan, M.L. Significance of divergent expression of prostaglandin EP4 and EP3 receptors in human prostate cancer. Mol. Cancer Res. 2013, 11, 427–439. [Google Scholar] [CrossRef] [PubMed]


| Variables | p (NPAR) | Correlation Coefficient |
|---|---|---|
| Histology | 0.700 | (−0.025) |
| pN | 0.229 | 0.078 |
| pT | 0.018 | 0.154 |
| FIGO | 0.040 | 0.133 |
| Variable | Significance | Hazard Ratio of Exp (B) | Lower 95% CI of Exp (B) | Upper 95% CI of Exp (B) | B |
|---|---|---|---|---|---|
| EP3 IRS | 0.007 | 1.264 | 1.066 | 1.498 | 0.234 |
| Histology | 0.002 | 3.118 | 1.538 | 6.322 | 1.137 |
| pT | 0.001 | 1.32 | 1.115 | 1.562 | 0.277 |
| pN | 0.025 | 2.208 | 1.103 | 4.42 | 0.792 |
| FIGO | 0.398 | 0.971 | 0.905 | 1.04 | −0.030 |
| Grading | 0.242 | 1.381 | 0.804 | 2.372 | 0.323 |
| Age | 0.136 | 1.021 | 0.993 | 1.05 | 0.021 |
| pM | 0.261 | 2.214 | 0.554 | 8.857 | 2.214 |
| Item | Numbers/Total Numbers | Percentage |
|---|---|---|
| Age | ||
| <49 | 139/250 | 55.6% |
| >49 | 111/250 | 44.4% |
| Number of positive lymph nodes | ||
| 0 | 151/250 | 60.4% |
| >1 | 97/250 | 38.8% |
| Not available | 2/250 | 0.8% |
| pT, Tumour size | ||
| pT1 | 110/250 | 44,0% |
| pT2/3/4 | 137/250 | 54.8% |
| Not available | 3/250 | 1.2% |
| FIGO | ||
| I | 64/250 | 25.6% |
| II/III/IV | 92/250 | 36.8% |
| Not available | 94/250 | 37.6% |
| Tumour grade | ||
| I | 21/250 | 8.4% |
| II | 143/250 | 57.2% |
| III | 78/250 | 31.2% |
| Not available | 8/250 | 3.2% |
| Tumour subtype | ||
| Squamous | 202/250 | 80.8% |
| Adenocarcinoma | 48/250 | 19.2% |
| Progression (over 235 months) | ||
| None | 210/250 | 84,0% |
| At least one event | 21/250 | 11.6% |
| Not available | 11/250 | 4.4% |
| Survival (over 235 months) | ||
| Right censured | 190/250 | 76,0% |
| Died | 49/250 | 19.6% |
| Not available | 11/250 | 4.4% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidegger, H.; Dietlmeier, S.; Ye, Y.; Kuhn, C.; Vattai, A.; Aberl, C.; Jeschke, U.; Mahner, S.; Kost, B. The Prostaglandin EP3 Receptor Is an Independent Negative Prognostic Factor for Cervical Cancer Patients. Int. J. Mol. Sci. 2017, 18, 1571. https://doi.org/10.3390/ijms18071571
Heidegger H, Dietlmeier S, Ye Y, Kuhn C, Vattai A, Aberl C, Jeschke U, Mahner S, Kost B. The Prostaglandin EP3 Receptor Is an Independent Negative Prognostic Factor for Cervical Cancer Patients. International Journal of Molecular Sciences. 2017; 18(7):1571. https://doi.org/10.3390/ijms18071571
Chicago/Turabian StyleHeidegger, Helene, Sebastian Dietlmeier, Yao Ye, Christina Kuhn, Aurelia Vattai, Caroline Aberl, Udo Jeschke, Sven Mahner, and Bernd Kost. 2017. "The Prostaglandin EP3 Receptor Is an Independent Negative Prognostic Factor for Cervical Cancer Patients" International Journal of Molecular Sciences 18, no. 7: 1571. https://doi.org/10.3390/ijms18071571
APA StyleHeidegger, H., Dietlmeier, S., Ye, Y., Kuhn, C., Vattai, A., Aberl, C., Jeschke, U., Mahner, S., & Kost, B. (2017). The Prostaglandin EP3 Receptor Is an Independent Negative Prognostic Factor for Cervical Cancer Patients. International Journal of Molecular Sciences, 18(7), 1571. https://doi.org/10.3390/ijms18071571