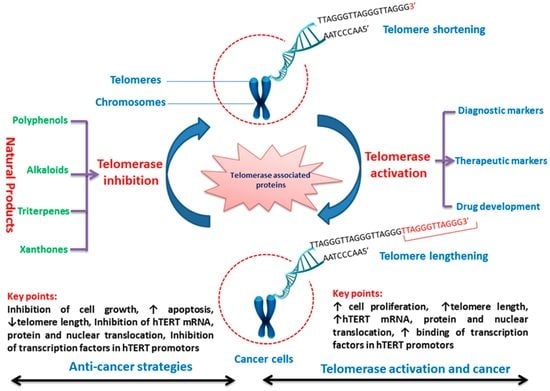
Telomerase Inhibitors from Natural Products and Their Anticancer Potential
Abstract
:1. Introduction
2. Expression of Telomerase in Cancer Cells
3. Telomerase Inhibitors from Natural Products
3.1. Polyphenols
3.1.1. Curcumin
3.1.2. Quercetin
3.1.3. Resveratrol
3.1.4. Tannic Acid
3.2. Alkaloids
3.2.1. Boldine
3.2.2. Berberine
3.3. Triterpenoid
3.3.1. Pristimerin
3.3.2. Oleanane
3.4. Xanthones
Gambogic Acid and Gambogenic Acid
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Popli, D.B.; Sircar, K.; Chowdhry, A. Telomerase: An exploration toward the end of cancer. Indian J. Dent. Res. 2017, 28, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Wang, J.P. A multi-target protein of hTERTR-FAM96A presents significant anticancer potent in the treatment of hepatocellular carcinoma. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Ivancich, M.; Schrank, Z.; Wojdyla, L.; Leviskas, B.; Kuckovic, A.; Sanjali, A.; Puri, N. Treating cancer by targeting telomeres and telomerase. Antioxidants 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Adekoya, D.; Enenmoh, I.; Clarke, O.; Wang, P.; Sarkyssian, M.; Wu, Y.; Vadgama, J.V. Salinomycin abolished STAT3 and STAT1 interactions and reduced telomerase activity in colorectal cancer cells. Anticancer Res. 2017, 37, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Odago, F.O.; Gerson, S.L. Telomerase inhibition and telomere erosion: A two-pronged strategy in cancer therapy. Trends Pharmacol. Sci. 2003, 24, 328–331. [Google Scholar] [CrossRef]
- Parkinson, E.K. Telomerase as a novel and potentially selective target for cancer chemotherapy. Ann. Med. 2003, 35, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Cian, A.D.; Lacroix, L.; Douarre, C.; Temime-Smaali, N.; Trentesaux, C.; Riou, J.F.; Mergny, J.L. Targeting telomeres and telomerase. Biochimie 2008, 90, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Autexier, C.; Lue, N.F. The structure and function of telomerase reverse transcriptase. Ann. Rev. Biochem. 2006, 75, 493–517. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, E.; Hiyama, K. Telomere and telomerase in stem cells. Br. J. Cancer 2007, 96, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Masutomi, K.; Possemato, R.; Wong, J.M.Y.; Currier, J.L.; Tothova, Z.; Manola, J.B.; Ganesan, S.; Lansdorp, P.M.; Collins, K.; Hahn, W.C. The telomerase reverse transcriptase regulates chromatin state and DNA damage responses. Proc. Natl. Acad. Sci. USA 2005, 102, 8222–8227. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.P.; Hoare, S.F.; Glasspool, R.M.; Keith, W.N. Lack of telomerase gene expression in alternative lengthening of telomere cells is associated with chromatin remodeling of the hTR and hTERT gene promoters. Cancer Res. 2005, 65, 7585–7590. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Bisoffi, M.; Heaphy, C.M.; Griffith, J.K. Telomeres: Prognostic markers for solid tumors. Int. J. Cancer 2006, 119, 2255–2260. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Lomedasht, F.; Rami, A.; Zarghami, N. Comparison of inhibitory effect of curcumin nanoparticles and free curcumin in human telomerase reverse transcriptase gene expression in breast cancer. Adv. Pharm. Bull. 2013, 3, 127–130. [Google Scholar] [PubMed]
- Badrzadeh, F.; Akbarzadeh, A.; Zarghami, N.; Yamchi, M.R.; Zeighamian, V.; Tabatabae, F.S.; Taheri, M.; Kafil, H.S. Comparison between effects of free curcumin and curcumin loaded NIPAAm-MAA nanoparticles on telomerase and PinX1 gene expression in lung cancer cells. Asian Pac. J. Cancer Prev. 2014, 15, 8931–8936. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, M.; Zarghami, N.; Koshki, K.N.; Mollazadeh, M.; Moghaddam, M.P.; Yamchi, M.R.; Esfahlan, R.J.; Barkhordari, A.; Alibakhshi, A. Curcumin and silibinin inhibit telomerase expression in T47D human breast cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 3449–3453. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.W.-L.; Blackburn, E.H. New ways not to make ends meet: Telomerase, DNA damage proteins and heterochromatin. Oncogene 2002, 21, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Celli, G.B.; de Lange, T. DNA processing is not required for ATM mediated telomere damage response after TRF2 deletion. Nat. Cell Biol. 2005, 7, 712–718. [Google Scholar] [CrossRef] [PubMed]
- De Lange, T. How telomeres solve the end-protection problem. Science 2009, 326, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Sfeir, A.; Kabir, S.; van Overbeek, M.; Celli, G.B.; de Lange, T. Loss of Rap1 induces telomere recombination in the absence of NHEJ or a DNA damage signal. Science 2010, 327, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.H.; Liu, Y.C.; Su, J.H.; El-Shazly, M.; Wu, C.F.; Du, Y.C.; Hsu, Y.M.; Yang, J.C.; Weng, M.K.; Chou, C.H.; et al. Antileukemic scalarane sesterterpenoids and meroditerpenoid from Carteriospongia (Phyllospongia) sp., induce apoptosis via dual inhibitory effects on topoisomerase II and Hsp90. Sci. Rep. 2016, 6, 36170. [Google Scholar] [CrossRef] [PubMed]
- Chini, M.G.; Malafronte, N.; Vaccaro, M.C.; Gualtieri, M.J.; Vassallo, A.; Vasaturo, M.; Castellano, S.; Milite, C.; Leone, A.; Bifulco, G.; et al. Identification of limonol derivatives as heat shock protein 90 (Hsp90) inhibitors through a multidisciplinary approach. Chemistry 2016, 22, 13236–13250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xue, N.; Bian, C.; Yan, R.; Jin, L.; Chen, X.; Yu, X. C15-methoxyphenylated 18-deoxy-herbimycin A analogues, their in vitro anticancer activity and heat shock protein 90 binding affinity. Bioorg. Med. Chem. Lett. 2016, 26, 4287–4291. [Google Scholar] [CrossRef] [PubMed]
- Noureini, S.K.; Tanavar, F. Boldine, a natural aporphine alkaloid, inhibits telomerase at non-toxic concentrations. Chem. Biol. Interact. 2015, 231, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Platella, C.; Guida, S.; Bonmassar, L.; Aquino, A.; Bonmassar, E.; Ravagnan, G.; Montesarchio, D.; Roviello, G.N.; Musumeci, D.; Fuggetta, M.P. Antitumour activity of resveratrol on human melanoma cells: A possible mechanism related to its interaction with malignant cell telomerase. Biochim. Biophys. Acta 2017, 1861, 2843–2851. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Lee, D.S.; Jeong, J.W.; Hong, S.H.; Choi, I.W.; Cha, H.J.; Kim, S.; Kim, H.S.; Park, C.; Kim, G.Y.; et al. Fucoidan induces ROS-dependent apoptosis in 5637 human bladder cancer cells by downregulating telomerase activity via inactivation of the PI3K/Akt signaling pathway. Drug Dev. Res. 2017, 78, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kwon, H.C.; Ko, H.; Park, J.H.; Kim, H.Y.; Yoo, J.H.; Yang, H.O. Anti-tumor activity of the ginsenoside Rk1 in human hepatocellular carcinoma cells through inhibition of telomerase activity and induction of apoptosis. Biol. Pharm. Bull. 2008, 31, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Moirangthem, D.S.; Laishram, S.; Borah, J.C.; Kalita, M.C.; Talukdar, N.C. Cephalotaxus griffithii Hook.f. needle extract induces cell cycle arrest, apoptosis and suppression of hTERT and hTR expression on human breast cancer cells. BMC Complement. Altern. Med. 2014, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Wang, C.Y.; Yang, R.C.; Chu, C.J.; Wu, H.T.; Pang, J.H. Phyllanthus urinaria increases apoptosis and reduces telomerase activity in human nasopharyngeal carcinoma cells. Forsch. Komplementmed. 2009, 16, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Wang, C.Y.; Yang, R.C.; Chu, C.J.; Wu, H.T.; Pang, J.H. Wogonin, an active compound in Scutellaria baicalensis, induces apoptosis and reduces telomerase activity in the HL-60 leukemia cells. Phytomedicine 2010, 17, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.J.; Choi, Y.H. Growth inhibition of A549 human lung carcinoma cells by beta-lapachone through induction of apoptosis and inhibition of telomerase activity. Int. J. Oncol. 2005, 26, 1017–1023. [Google Scholar] [PubMed]
- Oyama, J.I.; Shiraki, A.; Nishikido, T.; Maeda, T.; Komoda, H.; Shimizu, T.; Makino, N.; Node, K. EGCG, a green tea catechin, attenuates the progression of heart failure induced by the heart/muscle-specific deletion of MnSOD in mice. J. Cardiol. 2017, 69, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.Y.; Lee, C.L.; Wang, H.C.; Liang, S.S.; Kung, P.H.; Wu, Y.C.; Chang, F.R.; Wu, C.C. CLL2-1, a chemical derivative of orchid 1,4-phenanthrenequinones, inhibits human platelet aggregation through thiol modification of calcium-diacylglycerol guanine nucleotide exchange factor-I (CalDAG-GEFI). Free Radic. Biol. Med. 2015, 78, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Sheremet, M.; Kapoor, S.; Schröder, P.; Kumar, K.; Ziegler, S.; Waldmann, H. Small molecules inspired by the natural product withanolides as potent inhibitors of Wnt signaling. Chembiochem 2017, 18, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Bian, H.; Ouyang, J.; Bi, Y.; Yang, L.; Ye, S. Wenyang Huazhuo Tongluo formula, a Chinese herbal decoction, improves skin fibrosis by promoting apoptosis and inhibiting proliferation through down-regulation of survivin and cyclin D1 in systemic sclerosis. BMC Complement. Altern. Med. 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Franceschin, M.; Rossetti, L.; D’Ambrosio, A.; Schirripa, S.; Bianco, A.; Ortaggi, G.; Savino, M.; Schultes, C.; Neidle, S. Natural and synthetic G-quadruplex interactive berberine derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 1707–1711. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.O.; Moon, D.O.; Choi, Y.H.; Shin, D.Y.; Kang, H.S.; Choi, B.T.; Lee, J.D.; Li, W.; Kim, G.Y. Platycodin D induces apoptosis and decreases telomerase activity in human leukemia cells. Cancer Lett. 2008, 261, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Park, C.; Kim, S.H.; Hossain, M.A.; Kim, M.Y.; Chung, H.Y.; Son, W.S.; Kim, G.Y.; Choi, Y.H.; Kim, N.D. Korean red ginseng extract induces apoptosis and decreases telomerase activity in human leukemia cells. J. Ethnopharmacol. 2009, 121, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Chiruvella, K.K.; Raghavan, S.C. A natural compound, methyl angolensate, induces mitochondrial pathway of apoptosis in Daudi cells. Invest. New Drugs 2011, 29, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Guo, B.; Kong, X.; Wu, B. Evodiamine enhances the radiosensitivity of esophageal squamous cell cancer Eca-109 cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2016, 32, 940–944. [Google Scholar] [PubMed]
- Shirode, A.B.; Kovvuru, P.; Chittur, S.V.; Henning, S.M.; Heber, D.; Reliene, R. Antiproliferative effects of pomegranate extract in MCF-7 breast cancer cells are associated with reduced DNA repair gene expression and induction of double strand breaks. Mol. Carcinog. 2014, 53, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.H.C.; Zeng, F.; Zhang, S.; Xu, J. Isolation and identification of alkaloids from Menispermum dauricum growing in Xianning. Zhong Yao Cai 1998, 21, 456–458. [Google Scholar] [PubMed]
- Ji, X.; Sun, H.; Zhou, H.; Xiang, J.; Tang, Y.; Zhao, C. The interaction of telomeric DNA and C-myc22 G-quadruplex with 11 natural alkaloids. Nucleic Acid Ther. 2012, 22, 127–136. [Google Scholar] [PubMed]
- Kumari, S.; Nayak, G.; Lukose, S.T.; Kalthur, S.G.; Bhat, N.; Hegde, A.R.; Mutalik, S.; Kalthur, G.; Adiga, S.K. Indian propolis ameliorates the mitomycin C-induced testicular toxicity by reducing DNA damage and elevating the antioxidant activity. Biomed. Pharmacother. 2017, 95, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, D.; Kremb, S.; Sioud, S.; Emwas, A.H.; Voolstra, C.R.; Ravasi, T. Anti-cancer agents in Saudi Arabian herbals revealed by automated high-content imaging. PLoS ONE 2017, 12, e0177316. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.F.; Chang, T.T.; Chang, K.W.; Huang, H.J.; Chen, H.Y.; Tsai, F.J.; Lin, J.G.; Chen, C.Y. Blocking the DNA repair system by traditional Chinese medicine? J. Biomol. Struct. Dyn. 2011, 28, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Us Saqib, Q.N.; Waheed, A. Cytotoxic activity of extracts and crude saponins from Zanthoxylum armatum DC. against human breast (MCF-7, MDA-MB-468) and colorectal (Caco-2) cancer cell lines. BMC Complement. Altern. Med. 2017, 17, 368. [Google Scholar] [CrossRef] [PubMed]
- Sarvesvaran, J.; Going, J.J.; Milroy, R.; Kaye, S.B.; Keith, W.N. Is small cell lung cancer the perfect target for anti-telomerase treatment? Carcinogenesis 1999, 20, 1649–1651. [Google Scholar] [CrossRef] [PubMed]
- Kumaki, F.; Kawai, T.; Hiroi, S.; Shinomiya, N.; Ozeki, Y.; Ferrans, V.J.; Torikata, C. Telomerase activity and expression of human telomerase RNA component and human telomerase reverse transcriptase in lung carcinomas. Hum. Pathol. 2001, 32, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Oztas, E.; Kara, H.; Kara, Z.P.; Aydogan, M.U.; Uras, C.; Ozhan, G. Association between human telomerase reverse transcriptase gene variations and risk of developing breast cancer. Genet. Test. Mol. Biomarkers 2016, 20, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zheng, J.; Liu, C.; Tan, G.; Qing, Z.; Yang, S.; Yang, J.; Tan, Y.; Yang, R. SERS assay of telomerase activity at single-cell level and colon cancer tissues via quadratic signal amplification. Biosens. Bioelectron. 2016, 77, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.A.; Peng, C.H.; Wu, R.J.; Zheng, Z.D.; Chen, K.; Fu, Z.N. Detection of telomerase activity of exfoliated cells in bile and its clinical impact. Ai Zheng 2002, 21, 177–180. [Google Scholar] [PubMed]
- Barbosa, L.C.; da Silva, I.D.; Corrêa, J.C.; Ribalta, J.C. Survivin and telomerase expression in the uterine cervix of women with human papillomavirus-induced lesions. Int. J. Gynecol. Cancer 2011, 21, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, X.; Tang, M. Quantitative assessment of the diagnostic role of human telomerase activity from pancreatic juice in pancreatic cancer. Tumour Biol. 2014, 35, 7897–7904. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.; Yoshida, K.; Osaki, A.; Toge, T.; Tahara, H.; Ide, T.; Yasui, W. Expression and distribution of human telomerase catalytic component, hTERT, in human breast tissues. Anticancer Res. 2002, 22, 4101–4107. [Google Scholar] [PubMed]
- Boldrini, L.; Faviana, P.; Gisfredi, S.; Zucconi, Y.; Di Quirico, D.; Donati, V.; Berti, P.; Spisni, R.; Galleri, D.; Materazzi, G.; et al. Evaluation of telomerase mRNA (hTERT) in colon cancer. Int. J. Oncol. 2002, 21, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Bonatz, G.; Frahm, S.O.; Klapper, W.; Helfenstein, A.; Heidorn, K.; Jonat, W.; Krupp, G.; Parwaresch, R.; Rudolph, P. High telomerase activity is associated with cell cycle deregulation and rapid progression in endometrioid adenocarcinoma of the uterus. Hum. Pathol. 2001, 32, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chen, R.J. Prevalence of telomerase activity in human cancer. J. Formos. Med. Assoc. 2011, 110, 275–289. [Google Scholar] [CrossRef]
- Sidorova, N.; Zavalishina, L.; Kurchashova, S.; Korsakova, N.; Nazhimov, V.; Frank, G.; Kuimov, A. Immunohistochemical detection of tankyrase 2 in human breast tumors and normal renal tissue. Cell Tissue Res. 2006, 323, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Itoi, T.; Shinohara, Y.; Takeda, K.; Nakamura, K.; Shimizu, M.; Ohyashiki, K.; Hisatomi, H.; Nakano, H.; Moriyasu, F. Detection of telomerase reverse transcriptase mRNA in biopsy specimens and bile for diagnosis of biliary tract cancers. Int. J. Mol. Med. 2001, 7, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.; Cao, J.; Jin, S.; Xu, G.; Pan, B.; Shang, L.; Che, D.; Yu, Q.; Yu, Y. Newly identified biomarkers for detecting circulating tumor cells in lung adenocarcinoma. Tohoku J. Exp. Med. 2014, 234, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Hilal, G.; Reitzel, R.; Al Hamal, Z.; Chaftari, A.M.; Al Wohoush, I.; Jiang, Y.; Hachem, R.; Raad, I.I. Novel plasma telomerase detection method to improve cancer diagnostic assessment. PLoS ONE 2017, 12, e0174266. [Google Scholar] [CrossRef] [PubMed]
- Glybochko, P.V.; Zezerov, E.G.; Glukhov, A.I.; Alyaev, Y.G.; Severin, S.E.; Polyakovsky, K.A.; Varshavsky, V.A.; Severin, E.S.; Vinarov, A.Z. Telomerase as a tumor marker in diagnosis of prostatic intraepithelial neoplasia and prostate cancer. Prostate 2014, 74, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.; Lerma, E. Thyroid Neoplasia Study Group. Telomerase activity in thyroid fine needle aspirates. Acta Cytol. 2004, 48, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Capezzone, M.; Marchisotta, S.; Cantara, S.; Pacini, F. Telomeres and thyroid cancer. Curr. Genom. 2009, 10, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Koonrungsesomboon, N.; Wadagni, A.C.; Mbanefo, E.C. Molecular markers and Schistosoma-associated bladder carcinoma: A systematic review and meta-analysis. Cancer Epidemiol. 2015, 39, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Zhuang, Y.; Zuo, X.; Jia, Y.; Hong, Y.; Min, X.; Zhang, Z.; Xu, X.; Liu, N.; Xia, F.; et al. Real-time, quantitative lighting-up detection of telomerase in urines of bladder cancer patients by AIEgens. Anal. Chem. 2015, 87, 6822–6827. [Google Scholar] [CrossRef] [PubMed]
- Hapangama, D.K.; Kamal, A.; Saretzki, G. Implications of telomeres and telomerase in endometrial pathology. Hum. Reprod. Update 2017, 23, 166–187. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, R.; Seidi, K.; Monfaredan, A.; Shafie-Irannejad, V.; Abbasi, M.M.; Karimian, A.; Yousefi, B. The herbal medicine Melissa officinalis extract effects on gene expression of p53, Bcl-2, Her2, VEGF-A and hTERT in human lung, breast and prostate cancer cell lines. Gene 2017, 613, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Yokoyama, M.; Watanabe, S.; Uchiyama, M.; Nakao, Y.; Hara, K.; Iwasaka, T. Inhibitory effect of the tea polyphenol, (-)-epigallocatechin gallate, on growth of cervical adenocarcinoma cell lines. Cancer Lett. 2006, 234, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Turan, I.; Aliyazicioglu, Y.; Kilinc, K.; Yaman, S.O.; Ayazoglu Demir, E.; Arslan, A.; Mentese, A.; Deger, O. Morus rubra extract induces cell cycle arrest and apoptosis in human colon cancer cells through endoplasmic reticulum stress and telomerase. Nutr. Cancer 2017, 69, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.Q.; Li, L.Z.; He, Z.Y.; Zhang, Q.; Liu, J.; Hu, C.Y.; Qin, F.J.; Wang, T.Y. Anti-proliferative effects of Atractylis lancea (Thunb.) DC. via down-regulation of the c-myc/hTERT/telomerase pathway in Hep-G2 cells. Asian Pac. J. Cancer Prev. 2013, 14, 6363–6367. [Google Scholar] [CrossRef] [PubMed]
- Abliz, G.; Mijit, F.; Hua, L.; Abdixkur, G.; Ablimit, T.; Amat, N.; Upur, H. Anti-carcinogenic effects of the phenolic-rich extract from abnormal Savda Munziq in association with its cytotoxicity, apoptosis-inducing properties and telomerase activity in human cervical cancer cells (SiHa). BMC Complement. Altern. Med. 2015, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Sung, C.K. Screening of Telomerase Inhibitor from Natural Products and Their Anticancer Activities. Ph.D. Thesis, Chungnam National University, Taejon, Korea, 5 February 2005. [Google Scholar]
- Chen, J.L.-Y.; Sperry, J.; Ip, N.Y.; Brimble, M.A. Natural products targeting telomere maintenance. MedChemComm 2011, 2, 229. [Google Scholar] [CrossRef]
- Xu, B.; Wang, Q.; Sung, C.K. Telomerase inhibitory effects of red pigment rubropunctatin and Statin monacolin L isolated from red yeast rice. Genes 2017, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, R.Y.; Somayyeh, G.; Gholamreza, H.; Majid, M.; Yousef, R. Diosgenin inhibits hTERT gene expression in the A549 lung cancer cell line. Asian Pac. J. Cancer Prev. 2013, 14, 6945–6948. [Google Scholar] [CrossRef] [PubMed]
- Rahmati-Yamchi, M.; Ghareghomi, S.; Haddadchi, G.; Milani, M.; Aghazadeh, M.; Daroushnejad, H. Fenugreek extract diosgenin and pure diosgenin inhibit the hTERT gene expression in A549 lung cancer cell line. Mol. Biol. Rep. 2014, 41, 6247–6252. [Google Scholar] [CrossRef] [PubMed]
- Noureini, S.K.; Wink, M. Antiproliferative effect of the isoquinoline alkaloid papaverine in hepatocarcinoma HepG-2 cells—Inhibition of telomerase and induction of senescence. Molecules 2014, 19, 11846–11859. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y. Functional and mechanistic analysis of telomerase: An antitumor drug target. Pharmacol. Ther. 2016, 163, 24–47. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, C.; Sung, C.K. Telomerase inhibitory effects of medicinal mushrooms and lichens, and their anticancer activity. Int. J. Med. Mushrooms 2014, 16, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Sung, C.K. Telomerase inhibitory effects and anti-proliferative properties of onion and other natural spices against cancer cells. Food Biosci. 2015, 10, 80–85. [Google Scholar] [CrossRef]
- Marconett, C.N.; Sundar, S.N.; Tseng, M.; Tin, A.S.; Tran, K.Q.; Mahuron, K.M.; Bjeldanes, L.F.; Firestone, G.L. Indole-3-carbinol downregulation of telomerase gene expression requires the inhibition of estrogen receptor-alpha and Sp1 transcription factor interactions within the hTERT promoter and mediates the G1 cell cycle arrest of human breast cancer cells. Carcinogenesis 2011, 32, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Li, W.C.; Shih, J.W.; Hong, K.F.; Pan, Y.R.; Lin, J.J. The tea polyphenols EGCG and EGC repress mRNA expression of human telomerase reverse transcriptase (hTERT) in carcinoma cells. Cancer Lett. 2006, 236, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Tuntiwechapikul, W.; Taka, T.; Songsomboon, C.; Kaewtunjai, N.; Imsumran, A.; Makonkawkeyoon, L.; Pompimon, W.; Lee, T.R. Ginger extract inhibits human telomerase reverse transcriptase and c-Myc expression in A549 lung cancer cells. J. Med. Food 2010, 13, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Lin, S.Z.; Chen, Y.L.; Chang, J.S.; Ho, L.I.; Liu, P.Y.; Chang, L.F.; Harn, Y.C.; Chen, S.P.; Sun, L.Y.; et al. Butylidenephthalide suppresses human telomerase reverse transcriptase (TERT) in human glioblastomas. Ann. Surg. Oncol. 2011, 18, 3514–3527. [Google Scholar] [CrossRef] [PubMed]
- Lanzilli, G.; Fuggetta, M.P.; Tricarico, M.; Cottarelli, A.; Serafino, A.; Falchetti, R.; Ravagnan, G.; Turriziani, M.; Adamo, R.; Franzese, O.; et al. Resveratrol down-regulates the growth and telomerase activity of breast cancer cells in vitro. Int. J. Oncol. 2006, 28, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Noureini, S.K.; Wink, M. Antiproliferative effects of crocin in HepG2 cells by telomerase inhibition and hTERT down-regulation. Asian Pac. J. Cancer Prev. 2012, 13, 2305–2309. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Pratap, R. The biological potential of flavones. Nat. Prod. Rep. 2010, 27, 1571–1593. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Jung, J.H.; Kim, N.D.; Choi, Y.H. Inhibition of cyclooxygenase-2 and telomerase activities in human leukemia cells by dideoxypetrosynol A, a polyacetylene from the marine sponge Petrosia sp. Int. J. Oncol. 2007, 30, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Kim, G.Y.; Kim, W.I.; Hong, S.H.; Park, D.I.; Kim, N.D.; Bae, S.J.; Jung, J.H.; Choi, Y.H. Induction of apoptosis by (Z)-stellettic acid C, an acetylenic acid from the sponge Stelletta sp., is associated with inhibition of telomerase activity in human leukemic U937 cells. Chemotherapy 2007, 53, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Park, D.I.; Lee, J.H.; Moon, S.K.; Kim, C.H.; Lee, Y.T.; Cheong, J.; Choi, B.T.; Choi, Y.H. Induction of apoptosis and inhibition of telomerase activity by aqueous extract from Platycodon grandiflorum in human lung carcinoma cells. Pharmacol. Res. 2005, 51, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Chen, G.Y.; Wang, M.; Yang, Z.Y.; Hong, X. Effects of vinorelbine on apoptosis and expression of telomerase activity in human lung adenocarcinoma cells in vitro. Zhonghua Zhong Liu Za Zhi 2010, 32, 743–747. [Google Scholar] [PubMed]
- Jagadeesh, S.; Kyo, S.; Banerjee, P.P. Genistein represses telomerase activity via both transcriptional and posttranslational mechanisms in human prostate cancer cells. Cancer Res. 2006, 66, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Andrews, L.G.; Tollefsbol, T.O. Genistein depletes telomerase activity through cross-talk between genetic and epigenetic mechanisms. Int. J. Cancer 2009, 125, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.J.; Lee, S.J.; Choi, B.T.; Park, Y.M.; Choi, Y.H. Induction of apoptosis and inhibition of telomerase activity by trichostatin A, a histone deacetylase inhibitor, in human leukemic U937 cells. Exp. Mol. Pathol. 2007, 82, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Pate, M.S.; Wylie, R.C.; Tollefsbol, T.O.; Katiyar, S.K. EGCG down-regulates telomerase in human breast carcinoma MCF-7 cells, leading to suppression of cell viability and induction of apoptosis. Int. J. Oncol. 2004, 24, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Patel, S.N.; Chan, T.H.; Tollefsbol, T.O. A novel prodrug of epigallocatechin-3-gallate: Differential epigenetic hTERT repression in human breast cancer cells. Cancer Prev. Res. 2011, 4, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.; Rashid, G.; Klein, A. Indole-3-carbinol inhibits telomerase activity and gene expression in prostate cancer cell lines. Anticancer Res. 2011, 31, 3733–3737. [Google Scholar] [PubMed]
- Ramachandran, C.; Fonseca, H.B.; Jhabvala, P.; Escalon, E.A.; Melnick, S.J. Curcumin inhibits telomerase activity through human telomerase reverse transcritpase in MCF-7 breast cancer cell line. Cancer Lett. 2002, 184, 1–6. [Google Scholar] [CrossRef]
- Mukherjee Nee Chakraborty, S.; Ghosh, U.; Bhattacharyya, N.P.; Bhattacharya, R.K.; Dey, S.; Roy, M. Curcumin-induced apoptosis in human leukemia cell HL-60 is associated with inhibition of telomerase activity. Mol. Cell. Biochem. 2007, 297, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, N. Molecular mechanism of curcumin induced cytotoxicity in human cervical carcinoma cells. Mol. Cell. Biochem. 2009, 325, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lyu, S.Y.; Park, W.B. Mistletoe lectin induces apoptosis and telomerase inhibition in human A253 cancer cells through dephosphorylation of Akt. Arch. Pharm. Res. 2004, 27, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Hsiao, Y.M.; Hsu, C.P.; Lin, M.Y.; Wang, J.C.; Huang, Y.L.; Ko, J.L. Transcriptionally mediated inhibition of telomerase of fungal immunomodulatory protein from Ganoderma tsugae in A549 human lung adenocarcinoma cell line. Mol. Carcinog. 2006, 45, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Kim, G.Y.; Moon, S.K.; Kim, W.J.; Nam, T.J.; Choi, Y.H. Apoptosis induction by glycoprotein isolated from Laminaria japonica is associated with down-regulation of telomerase activity and prostaglandin E2 synthesis in AGS human gastric cancer cells. Int. J. Oncol. 2011, 38, 577–584. [Google Scholar] [PubMed]
- Chakrabarti, M.; Banik, N.L.; Ray, S.K. Sequential hTERT knockdown and apigenin treatment inhibited invasion and proliferation and induced apoptosis in human malignant neuroblastoma SK-N-DZ and SK-N-BE2 cells. J. Mol. Neurosci. 2013, 51, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Jayasooriya, R.G.; Kang, S.H.; Kang, C.H.; Choi, Y.H.; Moon, D.O.; Hyun, J.W.; Chang, W.Y.; Kim, G.Y. Apigenin decreases cell viability and telomerase activity in human leukemia cell lines. Food Chem. Toxicol. 2012, 50, 2605–2611. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-S.; Lim, S.-E. Growth and telomerase inhibition of SK-MEL 28 melanoma cell line by a plant flavonoid, apigenin. BMB Rep. 1998, 31, 339–344. [Google Scholar]
- Park, S.E.; Yoo, H.S.; Jin, C.Y.; Hong, S.H.; Lee, Y.W.; Kim, B.W.; Lee, S.H.; Kim, W.J.; Cho, CK.; Choi, Y.H. Induction of apoptosis and inhibition of telomerase activity in human lung carcinoma cells by the water extract of Cordyceps militaris. Food Chem. Toxicol. 2009, 47, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Vale, P.; de M Sampayo, M.A. Pectenotoxin-2 seco acid, 7-epi-pectenotoxin-2 seco acid and pectenotoxin-2 in shellfish and plankton from Portugal. Toxicon 2002, 40, 979–987. [Google Scholar] [CrossRef]
- Han, Q.B.; Xu, H.X. Caged Garcinia xanthones: Development since 1937. Curr. Med. Chem. 2009, 16, 3775–3796. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Guo, Q.L.; You, Q.D.; Lin, S.S.; Li, Z.; Gu, H.Y.; Zhang, H.W.; Tan, Z.; Wang, X. Repression of telomerase reverse transcriptase mRNA and hTERT promoter by gambogic acid in human gastric carcinoma cells. Cancer Chemother. Pharmacol. 2006, 58, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, X. Effects of allicin on both telomerase activity and apoptosis in gastric cancer SGC-7901 cells. World J. Gastroenterol. 2003, 9, 1930–1934. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Yang, H.Y.; Wu, J.; Li, M.; Min, J.M.; Cui, J.R. Z-ajoene causes cell cycle arrest at G2/M and decrease of telomerase activity in HL-60 cells. Zhonghua Zhong Liu Za Zhi 2005, 27, 516–520. [Google Scholar] [PubMed]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar] [PubMed]
- Kala, R.; Shah, H.N.; Martin, S.L.; Tollefsbol, T.O. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent γ-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer 2015, 15, 672. [Google Scholar] [CrossRef] [PubMed]
- Kanno, S.; Kitajima, Y.; Kakuta, M.; Osanai, Y.; Kurauchi, K.; Ujibe, M.; Ishikawa, M. Costunolide-induced apoptosis is caused by receptor-mediated pathway and inhibition of telomerase activity in NALM-6 cells. Biol. Pharm. Bull. 2008, 31, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Im, E.; Kang, H.K.; Lee, J.H.; Kwak, H.S.; Bae, Y.T.; Park, H.J.; Kim, N.D. Inhibitory effects of costunolide on the telomerase activity in human breast carcinoma cells. Cancer Lett. 2005, 227, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Thelen, P.; Wuttke, W.; Jarry, H.; Grzmil, M.; Ringert, R.H. Inhibition of telomerase activity and secretion of prostate specific antigen by silibinin in prostate cancer cells. J. Urol. 2004, 171, 1934–1938. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Wang, J.; Guo, W.; Wang, H.; Wang, C.; Liu, Y.; Sun, X. Triptolide inhibits transcription of hTERT through down-regulation of transcription factor specificity protein 1 in primary effusion lymphoma cells. Biochem. Biophys. Res. Commun. 2016, 469, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Kim, M.O.; Choi, Y.H.; Lee, H.G.; Kim, N.D.; Kim, G.Y. Gossypol suppresses telomerase activity in human leukemia cells via regulating hTERT. FEBS Lett. 2008, 582, 3367–3373. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.O.; Moon, D.O.; Kang, S.H.; Heo, M.S.; Choi, Y.H.; Jung, J.H.; Lee, J.D.; Kim, G.Y. Pectenotoxin-2 represses telomerase activity in human leukemia cells through suppression of hTERT gene expression and Akt-dependent hTERT phosphorylation. FEBS Lett. 2008, 582, 3263–3269. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Hsiao, Y.M.; Sheu, G.T.; Chang, J.T.; Wang, P.H.; Wu, M.F.; Shieh, G.J.; Hsu, C.P.; Ko, J.L. Nuclear translocation of telomerase reverse transcriptase and calcium signaling in repression of telomerase activity in human lung cancer cells by fungal immunomodulatory protein from Ganoderma tsugae. Biochem. Pharmacol. 2007, 74, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Kang, S.H.; Kim, K.C.; Kim, M.O.; Choi, Y.H.; Kim, G.Y. Sulforaphane decreases viability and telomerase activity in hepatocellular carcinoma Hep3B cells through the reactive oxygen species-dependent pathway. Cancer Lett. 2010, 295, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.; Sengupta, S.B. Role of diallyl disulfide-mediated cleavage of c-Myc and Sp-1 in the regulation of telomerase activity in human lymphoma cell line U937. Nutrition 2015, 31, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.N.; Ye, W.C.; Liu, G.Q.; Huang, Y. Inhibition of telomerase activity and bcl-2 expression in berbamine-induced apoptosis in HL-60 cells. Planta Med. 2002, 68, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Tippani, R.; Prakhya, L.J.; Porika, M.; Sirisha, K.; Abbagani, S.; Thammidala, C. Pterostilbene as a potential novel telomerase inhibitor: Molecular docking studies and its in vitro evaluation. Curr. Pharm. Biotechnol. 2014, 14, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Herz, C.; Tran, H.T.T.; Landerer, S.; Gaus, J.; Schlotz, N.; Lehr, L.; Schäfer, W.R.; Treeck, O.; Odongo, G.A.; Skatchkov, I.; et al. Normal human immune cells are sensitive to telomerase inhibition by Brassica-derived 3, 3-diindolylmethane, partly mediated via ERα/β-AP1 signaling. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Cai, Y.-J.; Li, Z.-X.; Chen, Q.; Liu, Z.-L.; Wang, R. Structure determination, apoptosis induction, and telomerase inhibition of CFP-2, a novel lichenin from Cladonia furcata. Biochim. Biophys. Acta 2003, 1622, 99–108. [Google Scholar] [CrossRef]
- Lyu, S.Y.; Choi, S.H.; Park, W.B. Korean mistletoe lectin-induced apoptosis in hepatocarcinoma cells is associated with inhibition of telomerase via mitochondrial controlled pathway independent of p53. Arch. Pharm. Res. 2002, 25, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Xin, N.; Hasan, M.; Li, W.; Li, Y. Juglans mandshurica Maxim extracts exhibit antitumor activity on HeLa cells in vitro. Mol. Med. Rep. 2014, 9, 1313–1318. [Google Scholar] [PubMed]
- Warabi, K.; Matsunaga, S.; van Soest, R.W.; Fusetani, N. Dictyodendrins A-E, the first telomerase-inhibitory marine natural products from the sponge Dictyodendrilla verongiformis. J. Org. Chem. 2003, 68, 2765–2770. [Google Scholar] [CrossRef] [PubMed]
- Warabi, K.; Hamada, T.; Nakao, Y.; Matsunaga, S.; Hirota, H.; van Soest, R.W.; Fusetani, N. Axinelloside A, an unprecedented highly sulfated lipopolysaccharide inhibiting telomerase, from the marine sponge, Axinella infundibula. J. Am. Chem. Soc. 2005, 127, 13262–13270. [Google Scholar] [CrossRef] [PubMed]
- Herz, C.; Hertrampf, A.; Zimmermann, S.; Stetter, N.; Wagner, M.; Kleinhans, C.; Erlacher, M.; Schuler, J.; Platz, S.; Rohn, S.; et al. The isothiocyanate erucin abrogates telomerase in hepatocellular carcinoma cells in vitro and in an orthotopic xenograft tumour model of HCC. J. Cell Mol. Med. 2014, 18, 2393–2403. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, P.; Somasundaram, S.T.; Perumal, K.; Vishwakarma, R.A.; Karthikeyan, N.P.; Velmurugan, R.; Balakrishnan, A. Novel substituted methylenedioxy lignin suppresses proliferation of cancer cells by inhibiting telomerase and activation of c-myc and caspases leading to apoptosis. Br. J. Cancer 2002, 87, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.S.L.; Yang, Y.M.; Wang, X.J.; Huang, G.Q. Alteration of activities of telomerase in tanshinone IIA inducing apoptosis of the leukemia cells. Zhongguo Zhong Yao Za Zhi 2005, 30, 207–211. [Google Scholar] [PubMed]
- Faezizadeh, Z.; Mesbah-Namin, S.A.; Allameh, A. The effect of silymarin on telomerase activity in the human leukemia cell line K562. Planta Med. 2012, 78, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Yurtcu, E.; Darcansoy Iseri, O.; Iffet Sahin, F. Effects of silymarin and silymarin-doxorubicin applications on telomerase activity of human hepatocellularcarcinoma cell line HepG2. J. BUON 2015, 20, 555–561. [Google Scholar] [PubMed]
- Kim, M.Y.; Vankayalapati, H.; Shin-Ya, K.; Wierzba, K.; Hurley, L.H. Telomestatin, a potent telomerase inhibitor that interacts quite specifically with the human telomeric intramolecular G-quadruplex. J. Am. Chem. Soc. 2002, 124, 2098–2099. [Google Scholar] [CrossRef] [PubMed]
- Duangmano, S.; Dakeng, S.; Jiratchariyakul, W.; Suksamrarn, A.; Smith, D.R.; Patmasiriwat, P. Antiproliferative effects of cucurbitacin B in breast cancer cells: Down-regulation of the c-Myc/hTERT/telomerase pathway and obstruction of the cell cycle. Int. J. Mol. Sci. 2010, 11, 5323–5338. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Jiang, J.F.; Xiao, D.; Ding, J. Down-regulation of telomerase activity via protein phosphatase 2A activation in salvicine-induced human leukemia HL-60 cell apoptosis. Biochem. Pharmacol. 2002, 64, 1677–1687. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, H.; Zhou, Y.; Ji, S.; Li, M. Effects of three diterpenoids on tumour cell proliferation and telomerase activity. Nat. Prod. Res. 2009, 23, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.M.; Kang, G.Z.; Xiao, B.X.; Li, D.H. Zhang. S. Effect of daidzein on cell growth, cell cycle, and telomerase activity of human cervical cancer in vitro. Int. J. Gynecol. Cancer 2004, 14, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jia, Z.; Deng, Z.; Wie, Y.; Zheng, R.; Yu, L. In vitro modulation of telomerase activity, telomere length and cell cycle in MKN45 cells by verbascoside. Planta Med. 2002, 68, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.J.; DeGuzman, F.S.; Hossain, M.B.; van der Helm, D. Cytotoxic aromatic alkaloids from the ascidian Amphicarpa meridiana and Leptoclinides sp.: Meridine and 11-hydroxyascididemin. J. Org. Chem. 1991, 56, 804–808. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, W.; Ding, J.; Cai, J.; Duan, W. Shikonin derivatives: Synthesis and inhibition of human telomerase. Bioorg. Med. Chem. Lett. 2002, 12, 1375–1378. [Google Scholar] [CrossRef]
- Guittat, L.; Alberti, P.; Rosu, F.; Van Miert, S.; Thetiot, E.; Pieters, L.; Gabelica, V.; De Pauw, E.; Ottaviani, A.; Riou, J.-F.; et al. Interactions of cryptolepine and neocryptolepine with unusual DNA structures. Biochimie 2003, 85, 535–547. [Google Scholar] [CrossRef]
- Li, W.; Zhang, M.; Zhang, J.L.; Li, H.Q.; Zhang, X.C.; Sun, Q.; Qiu, C.M. Interactions of daidzin with intramolecular G-quadruplex. FEBS Lett. 2006, 580, 4905–4910. [Google Scholar] [CrossRef] [PubMed]
- Rafii, F. The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites 2015, 5, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Tomar, J.S. In-silico modeling studies of G-quadruplex with soy isoflavones having anticancerous activity. J. Mol. Model. 2015, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Fu, Y.; Zheng, L.; Li, W.; Li, H.; Sun, Q.; Xiao, Y.; Geng, F. Natural isoflavones regulate the quadruplex-duplex competition in human telomeric DNA. Nucleic Acids Res. 2009, 37, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Guittat, L.; De Cian, A.; Rosu, F.; Gabelica, V.; De Pauw, E.; Delfourne, E.; Mergny, J.L. Ascididemin and meridine stabilise G-quadruplexes and inhibit telomerase in vitro. Biochim. Biophys. Acta 2005, 1724, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.P.; Hagihara, M.; Jiang, Z.H.; Nakatani, K. Ligand binding to tandem G quadruplexes from human telomeric DNA. Chembiochem 2008, 9, 2583–2587. [Google Scholar] [CrossRef] [PubMed]
- Banik, U.; Parasuraman, S.; Adhikary, A.K.; Othman, N.H. Curcumin: The spicy modulator of breast carcinogenesis. J. Exp. Clin. Cancer Res. 2017, 36, 98. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Aggarwal, B.B.; Singh, R.B.; Buttar, H.S.; Wilson, D.; De Meester, F. Food antioxidants and their anti-inflammatory properties: A potential role in cardiovascular diseases and cancer prevention. Diseases 2016, 4, 28. [Google Scholar] [CrossRef]
- Siddappa, G.; Kulsum, S.; Ravindra, D.R.; Kumar, V.V.; Raju, N.; Raghavan, N.; Sudheendra, H.V.; Sharma, A.; Sunny, S.P.; Jacob, T.; et al. Curcumin and metformin-mediated chemoprevention of oral cancer is associated with inhibition of cancer stem cells. Mol. Carcinog. 2017, 56, 2446–2460. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Wang, Q.X.; Lin, H.P.; Chang, N. Anti-tumor bioactivities of curcumin on mice loaded with gastric carcinoma. Food Funct. 2017, 8, 3319–3326. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.X.; Qu, X.J.; Xie, Y.Y.; Zhou, L.; Nakata, M.; Makuuchi, M.; Tang, W. Curcumin inhibits telomerase activity in human cancer cell lines. Int. J. Mol. Med. 2006, 18, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Ghosh, U.; Bhattacharyya, N.P.; Bhattacharya, R.K.; Roy, M. Inhibition of telomerase activity and induction of apoptosis by curcumin in K-562 cells. Mutat. Res. 2006, 596, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chung, I.K. Curcumin inhibits nuclear localization of telomerase by dissociating the Hsp90 co-chaperone p23 from hTERT. Cancer Lett. 2010, 290, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Hsin, I.L.; Sheu, G.T.; Chen, H.H.; Chiu, L.Y.; Wang, H.D.; Chan, H.W.; Hsu, C.P.; Ko, J.L. N-acetyl cysteine mitigates curcumin-mediated telomerase inhibition through rescuing of Sp1 reduction in A549 cells. Mutat. Res. 2010, 688, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, N. Curcumin counteracts the proliferative effect of estradiol and induces apoptosis in cervical cancer cells. Mol. Cell. Biochem. 2011, 347, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lou, M.; Zhang, L.N.; Ji, P.G.; Feng, F.Q.; Liu, J.H.; Yang, C.; Li, B.F.; Wang, L. Quercetin nanoparticles induced autophagy and apoptosis through AKT/ERK/Caspase-3 signaling pathway in human neuroglioma cells: In vitro and in vivo. Biomed. Pharmacother. 2016, 84, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.W.; Li, Y.H.; Wu, G.; Ren, J.Z.; Lu, H.B.; Li, Z.M.; Han, X.W. Quercetin nanoparticles display antitumor activity via proliferation inhibition and apoptosis induction in liver cancer cells. Int. J. Oncol. 2017, 50, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Naasani, I.; Oh-Hashi, F.; Oh-Hara, T.; Feng, W.Y.; Johnston, J.; Chan, K.; Tsuruo, T. Blocking telomerase by dietary polyphenols is a major mechanism for limiting the growth of human cancer cells in vitro and in vivo. Cancer Res. 2003, 63, 824–830. [Google Scholar] [PubMed]
- Avci, C.B.; Yilmaz, S.; Dogan, Z.O.; Saydam, G.; Dodurga, Y.; Ekiz, H.A.; Kartal, M.; Sahin, F.; Baran, Y.; Gunduz, C. Quercetin-induced apoptosis involves increased hTERT enzyme activity of leukemic cells. Hematology 2011, 16, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Cosan, D.T.; Soyocak, A.; Basaran, A.; Degirmenci, I.; Gunes, H.V.; Sahin, F.M. Effects of various agents on DNA fragmentation and telomerase enzyme activities in adenocarcinoma cell lines. Mol. Biol. Rep. 2011, 38, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Sakamoto, H.; Satoh, K.; Yamamoto, T. Tamoxifen and gonadal steroids inhibit colon cancer growth in association with inhibition of thymidylate synthase, survivin and telomerase expression through estrogen receptor beta mediated system. Cancer Lett. 2000, 161, 63–71. [Google Scholar] [CrossRef]
- Choi, J.A.; Kim, J.Y.; Lee, J.Y.; Kang, C.M.; Kwon, H.J.; Yoo, Y.D.; Kim, T.W.; Lee, Y.S.; Lee, S.J. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int. J. Oncol. 2001, 19, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.C.; Liu, H.F.; Chao, J.I. Survivin and p53 Modulate quercetin- induced cell growth inhibition and apoptosis in human lung carcinoma cells. J. Biol. Chem. 2004, 279, 55875–55885. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.J.; Kim, O.H.; Kim, Y.H.; Lim, J.H.; Kim, S.; Park, J.W.; Kwon, T.K. Quercetin arrests G2/M phase and induces caspase-dependent cell death in U937 cells. Cancer Lett. 2006, 240, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.M. Antiproliferative potency of structurally distinct dietary flavonoids on human colon cancer cells. Cancer Lett. 1996, 110, 41–48. [Google Scholar]
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; Biasi, S.D.; Roat, E.; Bertoncelli, L.; Cooper, E.L.; Cossarizza, A. Quercetin and cancer chemoprevention. Evid. Based Complement. Altern. Med. 2011, 2011, 591356. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Han, Y.; Yang, X.; Sun, Y.; Zhao, Y. Protective effects of quercetin and quercetin-5′, 8-disulfonate against carbon tetrachloride-caused oxidative liver injury in mice. Molecules 2013, 19, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gundala, S.R.; Mukkavilli, R.; Vangala, S.; Reid, M.D.; Aneja, R. Synergistic interactions among flavonoids and acetogenins in Graviola (Annona muricata) leaves confer protection against prostate cancer. Carcinogenesis 2015, 36, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Doğan, Z.; Kocahan, S.; Erdemli, E.; Köse, E.; Yılmaz, I.; Ekincioğlu, Z.; Ekinci, N.; Turkoz, Y. Effect of chemotherapy exposure prior to pregnancy on fetal brain tissue and the potential protective role of quercetin. Cytotechnology 2015, 67, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Karač, I.; Sirovina, D.; Kukolj, M.; Kunštić, M.; Gajski, G.; Garaj-Vrhovac, V.; Štajcar, D. Chemotherapeutic potential of quercetin on human bladder cancer cells. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2016, 51, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Parvaresh, A.; Razavi, R.; Rafie, N.; Ghiasvand, R.; Pourmasoumi, M.; Miraghajani, M. Quercetin and ovarian cancer: An evaluation based on a systematic review. J. Res. Med. Sci. 2016, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.H.; Gaascht, F.; Dicato, M.; Diederich, M. Targeting the wingless signaling pathway with natural compounds as chemopreventive or chemotherapeutic agents. Curr. Pharm. Biotechnol. 2012, 13, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Nien, S.; Wu, C.H.; Liu, C.L.; Chang, Y.C.; Lin, Y.S. Reappraisal of the anticancer efficacy of quercetin in oral cancer cells. J. Chin. Med. Assoc. 2013, 76, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.N.; Singh, C.; Nall, D.; Meeker, D.; Shankar, S.; Srivastava, R.K. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelialmesenchymal transition. J. Mol. Signal. 2010, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, P.H.; Tu, Z.G. Effects of quercetin on proliferation of lung cancer cell line A549 by down-regulating hTERT gene expression. J. Third Mil. Med. Univ. 2007, 29, 1852–1854. [Google Scholar]
- Wei, J.W.; Fan, Y.; Zhang, Y.L.; Wu, Y. Effects of quercetin on telomerase activity and apoptosis in gastric cancer cells. Shandong Med. J. 2007, 35, 15. [Google Scholar]
- Behjati, M.; Hashemi, M.; Kazemi, M.; Salehi, M.; Javanmard, S.H. Evaluation of energy balance on human telomerase reverse transcriptase (hTERT) alternative splicing by semi-quantitative RT-PCR in human umbilical vein endothelial cells. Adv. Biomed. Res. 2017, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.S.; Chen, L.S. Triterpenoids from Ganoderma lucidum inhibit the activation of EBV antigens as telomerase inhibitors. Exp. Ther. Med. 2017, 14, 3273–3278. [Google Scholar] [PubMed]
- Kuhar, M.; Imran, S.; Singh, N. Curcumin and quercetin combined with cisplatin to induce apoptosis in human laryngeal carcinoma Hep-2 cells through the mitochondrial pathway. J. Cancer Mol. 2007, 3, 121–128. [Google Scholar]
- Zamin, L.L.; Filippi-Chiela, E.C.; Dillenburg-Pilla, P.; Horn, F.; Salbego, C.; Lenz, G. Resveratrol and quercetin cooperate to induce senescence-like growth arrest in C6 rat glioma cells. Cancer Sci. 2009, 100, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Ak, A.; Basaran, A.; Dikmen, M.; Turgut Cosan, D.; Degirmenci, I.; Gunes, H.V. Evaluation of effects of quercetin (3, 3′, 4′, 5, 7-pentohidroxyfl avon) on apoptosis and telomerase enzyme activity in MCF-7 and NIH-3T3 cell lines compared with tamoxifen. Balkan Med. J. 2011, 28, 293–299. [Google Scholar]
- Shen, X.; Wang, M.; Bi, X.; Zhang, J.; Wen, S.; Fu, G.; Xia, L. Resveratrol prevents endothelial progenitor cells from senescence and reduces the oxidative reaction via PPARγ/HO1 pathways. Mol. Med. Rep. 2016, 14, 5528–5534. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Tollefsbol, T.O. Pterostilbene down-regulates hTERT at physiological concentrations in breast cancer cells: Potentially through the inhibition of cMyc. J. Cell. Biochem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Wu, P.H.; Ho, C.T.; Way, T.D.; Pan, M.H.; Chen, H.M.; Ho, Y.S.; Wang, Y.J. P53-dependent downregulation of hTERT protein expression and telomerase activity induces senescence in lung cancer cells as a result of pterostilbene treatment. Cell Death Dis. 2017, 8, e2985. [Google Scholar] [CrossRef] [PubMed]
- Fuggetta, M.P.; Lanzilli, G.; Tricarico, M.; Cottarelli, A.; Falchetti, R.; Ravagnan, G.; Bonmassar, E. Effect of resveratrol on proliferation and telomerase activity of human colon cancer cells in vitro. J. Exp. Clin. Cancer Res. 2006, 25, 189–193. [Google Scholar] [PubMed]
- Perrone, D.; Fuggetta, M.P.; Ardito, F.; Cottarelli, A.; De Filippis, A.; Ravagnan, G.; De Maria, S.; Lo Muzio, L. Resveratrol (3, 5, 4′-trihydroxystilbene) and its properties in oral diseases. Exp. Ther. Med. 2017, 14, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.W.; Aziz, M.H. Protective molecular mechanisms of resveratrol in UVR-induced skin carcinogenesis. Photodermatol. Photoimmunol. Photomed. 2017. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, A.R.; Chow, H.S.; Martinez, J.A. Effects of resveratrol on drug- and carcinogen-metabolizing enzymes, implications for cancer prevention. Pharmacol. Res. Perspect. 2017, 5, e00294. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Fan, Y.; Zhang, Y.L.; Zhong, X.M. Effect of resveratrol on promoter and human telomerase reverse transcriptase (hTERT) expression of human colorectal carcinoma cell. J. Jiangsu Univ. 2010, 23, 3–23. [Google Scholar]
- Zhai, X.X.; Ding, J.C.; Tang, Z.M.; Li, J.G.; Li, Y.C.; Yan, Y.H.; Sun, J.C.; Zhang, C.X. Effects of resveratrol on the proliferation, apoptosis and telomerase ability of human A431 epidermoid carcinoma cells. Oncol. Lett. 2016, 11, 3015–3018. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Przyjemska, M.; Kaczmarek, M.; Krajka-Kuźniak, V.; Łuczak, M.; Baer-Dubowska, W. The effect of resveratrol, its naturally occurring derivatives and tannic acid on the induction of cell cycle arrest and apoptosis in rat C6 and human T98G glioma cell lines. Toxicol. In Vitro 2017, 43, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Jordan, L.G.; Booth, B.W. HER2+ breast cancer cells undergo apoptosis upon exposure to tannic acid released from remodeled cross-linked collagen type I. J. Biomed. Mater. Res. A 2017. [CrossRef] [PubMed]
- Zhang, J.; Cui, L.; Han, X.; Zhang, Y.; Zhang, X.; Chu, X.; Zhang, F.; Zhang, Y.; Chu, L. Protective effects of tannic acid on acute doxorubicin-induced cardiotoxicity: Involvement of suppression in oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 2017, 93, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Shimozu, Y.; Kimura, Y.; Esumi, A.; Aoyama, H.; Kuroda, T.; Sakagami, H.; Hatano, T. Ellagitannins of Davidia involucrata L. structure of davicratinic acid a and effects of davidia tannins on drug-resistant bacteria and human oral squamous cell carcinomas. Molecules 2017, 22, 470. [Google Scholar] [CrossRef]
- Fu, S.; Yang, Y.; Liu, D.; Luo, Y.; Ye, X.; Liu, Y.; Chen, X.; Wang, S.; Wu, H.; Wang, Y.; et al. Flavonoids and tannins from Smilax china L. rhizome induce apoptosis via mitochondrial pathway and MDM2-p53 signaling in human lung adenocarcinoma cells. Am. J. Chin. Med. 2017, 45, 369–384. [Google Scholar] [PubMed]
- Darvin, P.; Joung, Y.H.; Kang, D.Y.; Sp, N.; Byun, H.J.; Hwang, T.S.; Sasidharakurup, H.; Lee, C.H.; Cho, K.H.; Park, K.D.; et al. Tannic acid inhibits EGFR/STAT1/3 and enhances p38/STAT1 signalling axis in breast cancer cells. J. Cell Mol. Med. 2017, 21, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Adaramoye, O.; Erguen, B.; Nitzsche, B.; Höpfner, M.; Jung, K.; Rabien, A. Punicalagin, a polyphenol from pomegranate fruit, induces growth inhibition and apoptosis in human PC-3 and LNCaP cells. Chem. Biol. Interact. 2017, 274, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Pumiputavon, K.; Chaowasku, T.; Saenjum, C.; Osathanunkul, M.; Wungsintaweekul, B.; Chawansuntati, K.; Wipasa, J.; Lithanatudom, P. Cell cycle arrest and apoptosis induction by methanolic leaves extracts of four Annonaceae plants. BMC Complement. Altern. Med. 2017, 17, 294. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.U.; Yamout, S.Z.; Sidani, M.M. Tannins protect against skin tumor promotion induced by ultraviolet-B radiation in hairless mice. Nutr. Cancer 2000, 37, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Tietbohl, L.A.C.; Oliveira, A.P.; Esteves, R.S.; Albuquerque, R.D.D.G.; Folly, D.; Machado, F.P.; Corrêa, A.L.; Santos, M.G.; Ruiz, A.L.G.; Rocha, L. Antiproliferative activity in tumor cell lines, antioxidant capacity and total phenolic, flavonoid and tannin contents of Myrciaria floribunda. An. Acad. Bras. Cienc. 2017, 89, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive components in Moringa Oleifera leaves protect against chronic disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dubey, V.; Singh, D.K.; Fatima, K.; Ahmad, A.; Luqman, S. Antiproliferative and antimicrobial efficacy of the compounds isolated from the roots of Oenothera biennis L. J. Pharm. Pharmacol. 2017, 69, 1230–1243. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Biswas, S.; Mathew, A.E.; Varghese, S.; Mathew, J.E.; Nandakumar, K.; Aranjani, J.M.; Lobo, R. Pro-apoptotic and cytotoxic effects of enriched fraction of Elytranthe parasitica (L.) Danser against HepG2 hepatocellular carcinoma. BMC Complement. Altern Med. 2016, 16, 420. [Google Scholar] [CrossRef] [PubMed]
- Colomer, R.; Sarrats, A.; Lupu, R.; Puig, T. Natural polyphenols and their synthetic analogs as emerging anticancer agents. Curr. Drug Target 2017, 18, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.B.; Gao, X.; Deeb, D.; Pindolia, K.; Gautam, S.C. Role of telomerase in anticancer activity of pristimerin in prostate cancer cells. J. Exp. Ther. Oncol. 2017, 11, 41–49. [Google Scholar] [PubMed]
- Moradzadeh, M.; Hosseini, A.; Erfanian, S.; Rezaei, H. Epigallocatechin-3-gallate promotes apoptosis in human breast cancer T47D cells through down-regulation of PI3K/AKT and telomerase. Pharmacol. Rep. 2017, 69, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Gurung, R.L.; Lim, S.N.; Low, G.K.; Hande, M.P. MST-312 alters telomere dynamics, gene expression profiles and growth in human breast cancer cells. J. Nutrigenet. Nutrigenom. 2014, 7, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, A.; Safa, M.; Kazemi, A. MST-312 induces G2/M cell cycle arrest and apoptosis in APL cells through inhibition of telomerase activity and suppression of NF-κB pathway. Tumour Biol. 2015, 36, 8425–8437. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zuo, J.; Wang, G. Epigallocatechin-3-gallate suppresses cell proliferation and promotes apoptosis in Ec9706 and Eca109 esophageal carcinoma cells. Oncol. Lett. 2017, 14, 4391–4395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, P.; Gao, F.; Yang, J.; Yao, K. Effects of epigallocatechin gallate on the proliferation and apoptosis of the nasopharyngeal carcinoma cell line CNE2. Exp. Ther. Med. 2014, 8, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, D.; Bertola, G.; Dietrich, F.; Figueiró, F.; Zanotto-Filho, A.; Moreira Fonseca, J.C.; Morrone, F.B.; Barrios, C.H.; Battastini, A.M.; Salbego, C.G. Boldine induces cell cycle arrest and apoptosis in T24 human bladder cancer cell line via regulation of ERK, AKT, and GSK-3β. Urol. Oncol. 2014, 32, 36.e1–36.e9. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, D.; Horn, A.P.; Gaelzer, M.M.; Frozza, R.L.; Delgado-Cañedo, A.; Pelegrini, A.L.; Henriques, A.T.; Lenz, G.; Salbego, C. Boldine: A potential new antiproliferative drug against glioma cell lines. Invest. New Drugs 2009, 27, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Noureini, S.K.; Wink, M. Dose-dependent cytotoxic effects of boldine in HepG-2 cells-telomerase inhibition and apoptosis induction. Molecules 2015, 20, 3730–3743. [Google Scholar] [CrossRef] [PubMed]
- Paydar, M.; Kamalidehghan, B.; Wong, Y.L.; Wong, W.F.; Looi, C.Y.; Mustafa, M.R. Evaluation of cytotoxic and chemotherapeutic properties of boldine in breast cancer using in vitro and in vivo models. Drug Des. Dev. Ther. 2014, 8, 719–733. [Google Scholar]
- Imanshahidi, M.; Hosseinzadeh, H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother. Res. 2008, 22, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Hsu, C.Y.; Liu, W.H.; Yung, B.Y. Berberine-induced apoptosis of human leukemia HL-60 cells is associated with down-regulation of nucleophosmin/B23 and telomerase activity. Int. J. Cancer 1999, 81, 923–929. [Google Scholar] [CrossRef]
- Naasani, I.; Seimiya, H.; Yamori, T.; Tsuruo, T. FJ5002: A potent telomerase inhibitor identified by exploiting the disease-oriented screening program with COMPARE analysis. Cancer Res. 1999, 59, 4004–4011. [Google Scholar] [PubMed]
- Zhang, W.J.; Ou, T.M.; Lu, Y.J.; Huang, Y.Y.; Wu, W.B.; Huang, Z.S.; Zhou, J.L.; Wong, K.Y.; Gu, L.Q. 9-Substituted berberine derivatives as G-quadruplex stabilizing ligands in telomeric DNA. Bioorg. Med. Chem. 2007, 15, 5493–5501. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ou, T.M.; Hou, J.Q.; Lu, Y.J.; Tan, J.H.; Gu, L.Q.; Huang, Z.S. 9-N-Substituted berberine derivatives: Stabilization of G-quadruplex DNA and down-regulation of oncogene c-myc. Bioorg. Med. Chem. 2008, 16, 7582–7591. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ou, T.M.; Tan, J.H.; Hou, J.Q.; Huang, S.L.; Gu, L.Q.; Huang, Z.S. Synthesis and evaluation of 9-O-substituted berberine derivatives containing aza-aromatic terminal group as highly selective telomeric G-quadruplex stabilizing ligands. Bioorg. Med. Chem. Lett. 2009, 19, 3414–3417. [Google Scholar] [CrossRef] [PubMed]
- Cevatemre, B.; Erkısa, M.; Aztopal, N.; Karakas, D.; Alper, P.; Tsimplouli, C.; Sereti, E.; Dimas, K.; Armutak, E.I.I.; Gurevin, E.G.; et al. A promising natural product, pristimerin, results in cytotoxicity against breast cancer stem cells in vitro and xenografts in vivo through apoptosis and an incomplete autopaghy in breast cancer. Pharmacol. Res. 2017. [Google Scholar] [CrossRef]
- Wu, C.C.; Chan, M.L.; Chen, W.Y.; Tsai, C.Y.; Chang, F.R.; Wu, Y.C. Pristimerin induces caspase-dependent apoptosis in MDA-MB-231 cells via direct effects on mitochondria. Mol. Cancer Ther. 2005, 4, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Landis-Piwowar, K.R.; Lu, D.; Yuan, P.; Li, L.; Reddy, G.P.; Yuan, X.; Dou, Q.P. Pristimerin induces apoptosis by targeting the proteasome in prostate cancer cells. J. Cell. Biochem. 2008, 103, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.Y.; Kim, M.J.; Eum, D.Y.; Yoon, C.H.; Seo, W.D.; Park, K.H.; Hyun, J.W.; Lee, Y.S.; Lee, J.S.; Yoon, M.Y.; et al. Reactive oxygen species-dependent activation of Bax and poly(ADP-ribose) polymerase-1 is required for mitochondrial cell death induced by triterpenoid pristimerin in human cervical cancer cells. Mol. Pharmacol. 2009, 76, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Tiedemann, R.E.; Schmidt, J.; Keats, J.J.; Shi, C.X.; Zhu, Y.X.; Palmer, S.E.; Mao, X.; Schimmer, A.D.; Stewart, A.K. Identification of a potent natural triterpenoid inhibitor of proteosome chymotrypsin-like activity and NF-kappaB with antimyeloma activity in vitro and in vivo. Blood 2009, 113, 4027–4037. [Google Scholar] [CrossRef] [PubMed]
- Deeb, D.; Gao, X.; Liu, Y.; Pindolia, K.; Gautam, S.C. Inhibition of hTERT/telomerase contributes to the antitumor activity of pristimerin in pancreatic ductal adenocarcinoma cells. Oncol. Rep. 2015, 34, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Deeb, D.; Gao, X.; Liu, Y.; Varma, N.R.; Arbab, A.S.; Gautam, S.C. Inhibition of telomerase activity by oleanane triterpenoid CDDO-Me in pancreatic cancer cells is ROS-dependent. Molecules 2013, 18, 3250–3265. [Google Scholar] [CrossRef] [PubMed]
- Yore, M.M.; Liby, K.T.; Honda, T.; Gribble, G.W.; Sporn, M.B. The synthetic triterpenoid 1-[2-cyano-3, 12-dioxooleana-1, 9-dien-28-oyl]imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol. Cancer Ther. 2006, 5, 3232–3239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, X.; Deeb, D.; Arbab, A.S.; Gautam, S.C. Telomerase reverse transcriptase (TERT) is a therapeutic target of oleanane triterpenoid CDDO-Me in prostate cancer. Molecules 2012, 17, 14795–14809. [Google Scholar] [CrossRef] [PubMed]
- Deeb, D.; Gao, X.; Liu, Y.; Kim, S.H.; Pindolia, K.R.; Arbab, A.S.; Gautam, S.C. Inhibition of cell proliferation and induction of apoptosis by oleanane triterpenoid (CDDO-Me) in pancreatic cancer cells is associated with the suppression of hTERT gene expression and its telomerase activity. Biochem. Biophys. Res. Commun. 2012, 422, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Han, Q.B.; Chan, C.Y.; Wang, H.; Liu, Z.; Cheng, C.H.; Yew, D.T.; Lin, M.C.; He, M.L.; et al. Proteomic identification of molecular targets of gambogic acid: Role of stathmin in hepatocellular carcinoma. Proteomics 2009, 9, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wang, H.J.; Zhao, W.W.; Sun, Y.L.; Hu, L.K. Gambogic acid improves non-small cell lung cancer progression by inhibition of mTOR signaling pathway. Kaohsiung J. Med. Sci. 2017, 33, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zou, Z.; Ren, W.; Qian, H.; Cheng, Q.; Ji, L.; Liu, B.; Liu, Q. Gambogic acid-loaded PEG-PCL nanoparticles act as an effective antitumor agent against gastric cancer. Pharm. Dev. Technol. 2017, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Jansson, K.H.; Beshiri, M.L.; Yin, J.; Fang, L.; Agarwal, S.; Nguyen, H.; Corey, E.; Zhang, Y.; Liu, J.; et al. Gambogic acid inhibits thioredoxin activity and induces ROS-mediated cell death in castration-resistant prostate cancer. Oncotarget 2017, 8, 77181–77194. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cheng, H.; Zhu, G.; Yang, L.; Zhou, A.; Wang, X.; Fang, N.; Xia, L.; Su, J.; Wang, M.; et al. Gambogenic acid inhibits proliferation of A549 cells through apoptosis-inducing and cell cycle arresting. Biol. Pharm. Bull. 2010, 33, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.M.; Zhang, J.F.; Wang, H.; Tan, H.S.; Wang, W.M.; Chen, S.C.; Zhu, X.; Chan, T.M.; Tse, C.M.; Leung, K.S.; et al. Apoptosis induced by 1, 3, 6, 7-tetrahydroxyxanthone in hepatocellular carcinoma and proteomic analysis. Apoptosis 2012, 17, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.L.; Lin, S.S.; You, Q.D.; Gu, H.Y.; Yu, J.; Zhao, L.; Qi, Q.; Liang, F.; Tan, Z.; Wang, X. Inhibition of human telomerase reverse transcriptase gene expression by gambogic acid in human hepatoma SMMC-7721 cells. Life Sci. 2006, 78, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Guo, Q.L.; You, Q.D.; Zhao, L.; Gu, H.Y.; Yang, Y.; Zhang, H.W.; Tan, Z.; Wang, X. Gambogic acid-induced G2/M phase cell-cycle arrest via disturbing CDK7-mediated phosphorylation of CDC2/p34 in human gastric carcinoma BGC-823 cells. Carcinogenesis 2007, 28, 632–638. [Google Scholar] [CrossRef] [PubMed]
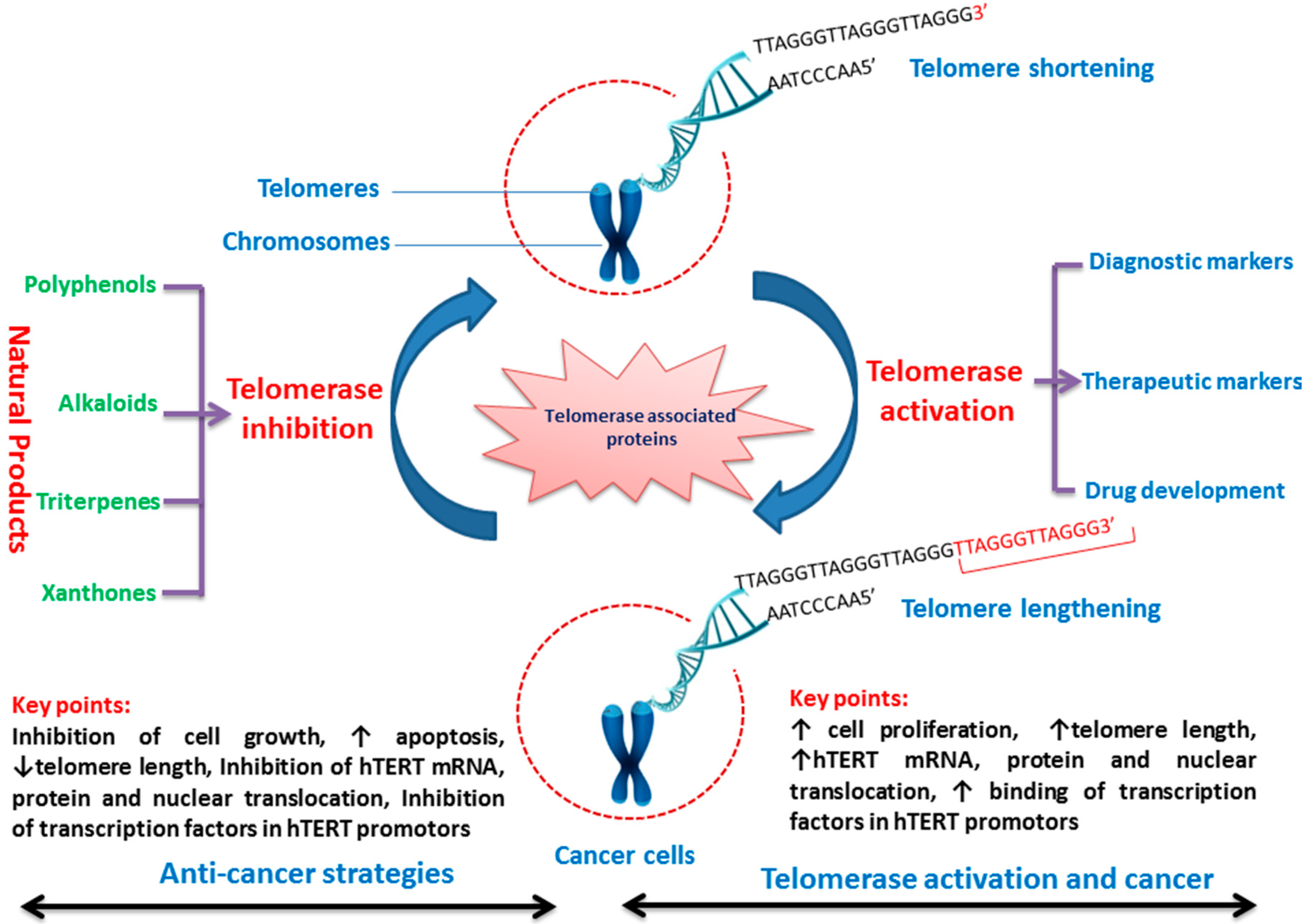
| Protein | Functions | References |
|---|---|---|
| Telomerase Components | ||
| Heat shock 90 kDa protein (Hsp90) | Hsp90 is a molecular chaperone, involved in the activation of disparate client proteins | [21,22,23] |
| Human telomerase reverse transcriptase (hTERT) | Encodes a rate-limiting catalytic subunit of telomerase that maintains genomic integrity | [24,25,26] |
| Human telomerase RNA component (hTERC) | Encodes the RNA component of human telomerase that acts as a template for the addition of the repeat sequence | [27,28] |
| Telomerase-associated protein 1 (TP1) | Associated with a catalytic subunit in a multicomponent telomerase complex | [29,30] |
| Telomere Binding Proteins | ||
| Dyskerin, | Catalyzes pseudouridylation of rRNA required for correct intranuclear trafficking of TERC, the RNA component of the TERT enzyme | [31] |
| PINX1 (PIN2/TERF1-interacting telomerase inhibitor 1) | Potential telomerase inhibitor, negatively regulating telomere length by interacting with TRF1. | [32,33] |
| Rap 1 (Repressor activator protein 1) | Mammalian Rap1, whose function is still unclear, | [34] |
| TANK1 and TANK2; Tankyrase (TANK) telomere-associated poly (ADP-ribose) polymerase (PARP) 1 | Positive regulator of telomere length through inhibition of TRF1 | [35] |
| Tankyrase, TRF1-interacting ankyrin-related poly (ADP-ribose) polymerase (PARP) | Mediates poly-ADP-ribosylation of TERF1, thereby contributing to the regulation of telomere length | [36] |
| Telomere-end-binding protein—Protection of telomeres 1 (POT1) | Essential for the replication of chromosome termini and involved in the regulation of telomere length by cis-inhibition of telomerase | [37] |
| Telomeric-repeat-binding factor 1 (TRF1) | Telomere length regulation | [38] |
| TERF1-interacting nuclear factor 2 (TINF2) | Involves in the regulation of telomere length and protection | [39] |
| Telomere Repairing Proteins | ||
| KU70 (Thyroid autoantigen 70 kDa (Ku antigen) | Acts as a negative regulator of telomerase and required for maintenance of the telomeric C-rich strand | [40,41] |
| MRE11 (Meiotic recombination 11 homologue) | A component of the MRN complex, which plays a central role in double-strand break (DSB) repair, DNA recombination, maintenance of telomere integrity and meiosis | [42] |
| Rad50 (S. cerevisiae) homologue | Single-strand endonuclease activity and double-strand-specific 3′-5′ exonuclease activity, which are provided by MRE11 | [42] |
| Tripeptidyl-peptidase I (TPP1) | Plays a role in telomere capping by interacting with TIN2 and POT1 | [43,44] |
| XRCC5/KU80 (X-ray repair (double-strand-break rejoining; Ku autoantigen, 80 kDa) | Works in the 3′-5′ direction and binds to DNA mediated by XRCC6 | [44] |
| H2AX (Histone 2 AX) | Requires for checkpoint-mediated arrest of cell cycle progression in response to low doses of ionizing radiation and for efficient repair of DNA double-strand breaks | [45,46] |
| Ku86 (Ku autoantigen, 80 kDa) | Negative regulator of telomere length, role in telomere capping, regulation of telomerase recruitment | [47] |
| DNA-PK (DNA-dependent protein kinase) | Plays a role in telomere capping, putative role in post-replicative processing of telomeres | [41,48] |
| Cancer | Findings | Implications | References |
|---|---|---|---|
| Diagnostic Implications of Telomerase Activity | |||
| Breast | The telomerase activity in breast fine-needle aspirates has higher sensitivity (86% vs. 70% for cytology) and is detectable in stage 1 cancer cells. | Telomerase assays might play a potentially useful adjunct role in noninvasive screening and diagnosis of breast cancer. | [51] |
| Cervix | Telomerase activity is expressed in cervical fluid of patients. | Telomerase assay gives a promising diagnostic biomarker for early cervical cancer detection. | [54] |
| Colon | Telomerase is detected in the intestinal fluid of patients (80–90%) with colorectal carcinoma. | Telomerase assay holds great promising as a diagnostic biomarker for early colon cancer detection and monitoring and has considerable potential for developing anticancer therapy. | [52,59] |
| Kidney | Telomerase activity is expressed in kidney abscess of patients (77%) with kidney carcinoma. | Telomerase assay gives a promising diagnostic biomarker for kidney cancer detection. | [60] |
| Liver and biliary | Telomerase activity is expressed in liver and biliary abscess of patients (70%) with liver and biliary carcinoma. | Telomerase assay gives a promising diagnostic biomarker for early liver cancer detection. | [53,61] |
| Lung | The telomerase activity and circulating tumor cells in lung adenocarcinoma fluid has a higher sensitivity (78% vs. 65% for circulating tumor cells). | The combination of the circulating tumor cells and telomerase assays provide high sensitivity in lung adenocarcinoma diagnosis and follow up. | [62] |
| Pancreas | Telomerase activity is expressed in pancreas fluid and abscess of patients (82% and 85%) with prostate carcinoma. | Telomerase assay gives a promising diagnostic biomarker for pancreatic cancer detection. | [55,63] |
| Prostate | Telomerase activity is expressed in prostate abscess of patients (75%) with prostate carcinoma. | Telomerase assay gives a promising diagnostic biomarker for early prostate cancer detection. | [64] |
| Thyroid | Telomerase activity is expressed in thyroid abscess of patients (80%) with thyroid carcinoma. | Telomerase assay gives a promising diagnostic biomarker for early thyroid cancer detection. | [65,66] |
| Urinary bladder | Telomerase activity is expressed in bladder abscess of patients (80%) with bladder carcinoma. | Telomerase assays might play a potentially useful adjunct role in noninvasive screening and diagnosis of bladder cancer. | [67,68] |
| Uterine | Telomerase activity is expressed in uterine abscess of patients (90%) with liver and biliary carcinoma. | Telomerase assay gives a promising diagnostic biomarker for early uterine cancer detection. | [69] |
| Therapeutic Implications of Telomerase Inhibition in Human Cancers by Natural Products | |||
| Breast | Treatment with Melissa officinalis extract can inhibit telomerase activity in human breast cancer cell line. | Telomerase inhibition might be useful in the treatment of various cancers with telomerase-positive cells. | [70] |
| Cervical | Treatment with (−)-epigallocatechin gallate can inhibit telomerase activity in human cervical cancer cell line. | [71] | |
| Colon | Treatment with Morus Rubra extract can inhibit telomerase activity in human colon cancer cell line. | [72] | |
| Liver | Treatment with Atractylis lancea (Thunb.) DC extract can inhibit telomerase activity in human liver cancer cell line. | [73] | |
| Lung | Treatment with Melissa officinalis extract can inhibit telomerase activity in human lung adenocarcinoma cell line. | [70] | |
| Prostate | Treatment with Melissa officinalis extract can inhibit telomerase activity in human prostate cancer cell line. | [70] | |
| Uterine | Treatment with phenolic-rich extracts from Savda Munziq can inhibit telomerase activity in human uterine cancer cell line. | [74] | |
| Plant Source | Compounds | Mechanism of Action | Reference |
|---|---|---|---|
| Targeting hTERT—Inhibition of the Catalytic Function | |||
| Brassica oleracea | Indole-3-carbinol | Inhibition of telomerase and downregulated expression of the catalytic subunit of hTERT | [84] |
| Camellia sinensis | Epigallocatechin gallate | Binding competitively at the active site of hTERT | [32,33,85] |
| Trigonella foenum-graecum | Diosgenin | Prevention of telomerase activity by down regulation of the hTERT gene expression | [78,79] |
| Zingiber officinale Roscoe | Gingerol | Reduction of hTERT expression and decrease of c-Myc (myelocytomatosis viral oncogene) | [86] |
| Suppression of Transcriptional and Post-Transcriptional Regulation | |||
| Angelica sinensis | Butylidenephthalide | Down-regulation of the telomerase activity and hTERT expression | [70,76,80,87,88,89,90,91,92,93,94,95,96,97] |
| Asian coniferous evergreen trees Cephalotaxus sp. | Cephalotaxus alkaloids | ||
| Papaveraceae | Papaverine | ||
| Blueberries | Resveratrol | ||
| Crocus sativus L. | Crocin | ||
| Marine sponge Petrosia sp. | Dideoxypetrosynol A | ||
| Marine sponge Stelletta sp. | (Z)-Stellettic acid C | ||
| Melissa officinalis | Luteolin-7-0-glucoside | ||
| Secondary plant metabolites | Genistein | ||
| Fruits and vegetables | Quercetin | ||
| Platycodon grandiflorum | Saponins | ||
| Streptomyces sp. | Trichostatin A | ||
| Streptomyces sp. | Vinorelbine | ||
| Salvia miltiorrhiza | Tanshinone I | ||
| Arnica montana | Helenalin | Down-regulation of hTERT transcription through inhibition of nuclear factor kappa beta (NF-κB) | [76] |
| Atractylis lancea (Thunb.) DC. | Atractylenolide | Inhibition of hTERT expression and decreased the expression of both mRNA and protein | [73,98,99,100,101,102,103,104,105,106] |
| Ganoderma tsugae | Fungal immuno-modulatory protein-gts | ||
| Camellia sinensis | Epigallocatechin gallate | ||
| Curcuma longa | Curcumin | ||
| Laminaria japonica | Glycoprotein LJPG (Lamanaria japonica glycoprotein) | ||
| European mistletoe, Viscum album | Mistletoe lectin | ||
| Cruciferous vegetables | Indole-3-carbinol | ||
| Common fruits and vegetables | Apigenin | Inhibition of telomerase activity with down-regulation of hTERT expression, attenuating the binding of c-Myc and special protein 1 (Sp1) to the regulatory regions of hTERT | [107,108,109,110,111] |
| Cordyceps militaris | Phenolic acids | ||
| Dinophysis fortii | Pectenotoxin-2 | ||
| Garcinia hurburyi tree | Gambogic acid | Down-regulation of hTERT transcription via inhibition of the transcription activator c-myc, and by the inhibition of the phosphorylation of serine/threonine-protein kinase (Akt); down regulation of the activity of hTERT in a post-translational manner | [112,113] |
| Garlic (Allium sativum) | Allicin and Ajoene | Reduction of hTERT mRNA levels | [114,115] |
| Hellbore (Veratrum grandiflorum O. Loes), peanuts (Arachis hypogea), legumes (Cassia sp.) and grapes (Vitis vinifera) | Resveratrol | Down-regulation of the telomerase activity and the nuclear levels of hTERT | [116,117] |
| Vitis vinifera | Resveratrol and pterostilbene | ||
| Magnolia sieboldii | Costunolide | Inhibition of telomerase activity, reduction of hTERT mRNA and protein levels, decreasing the bindings of transcription factors in hTERT promoters | [118,119] |
| Panax ginseng C. A. MEYER, Sun Ginseng | Ginsenoside Rk1 | Inhibition telomerase activity with down-regulation of levels of hTERT and c-Myc mRNA | [27,30,120] |
| Scutellaria baicalensis | Baicalin and wogonoside | ||
| Silybum marianum L. Gaertn | Silibinin | ||
| Peumus boldus | Boldine | Inhibition of hTERT expression | [24] |
| Tripterygium wilfordii | Triptolide | Inhibition of transcription of hTERT through down-regulation of transcription factor specificity protein 1 | [121] |
| Translocation | |||
| Cottonseed | Gossypol | Inhibition of telomerase activity with reducing the phosphorylation and nuclear translocation of hTERT | [95,96,111,122,123,124] |
| Dinophysis fortii | Pectenotoxin-2 | ||
| Ganoderma tsugae | Recombinant fungal immunomodulatory protein-gts | ||
| Secondary plant metabolites | Genistein | ||
| Post-Translational Modification | |||
| Broccoli and cauliflower | Sulforaphane | Inhibition of telomerase activity and post-translational modification of hTERT | [122,125] |
| Cottonseed | Gossypol | ||
| Inhibition of Telomerase Activity | |||
| Red yeast rice | Rubropunctatin | Inhibition of telomerase activity | [29,77,82,83,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141] |
| Mushrooms, onion, and other spices | Crude extract | ||
| Allium sativum L. | Diallyl disulfide | ||
| Berberis vulgaris | Berbarine | ||
| Blueberries | Pterostilbene | ||
| European mistletoe, Viscum album | Coloratum agglutinin | ||
| Juglans mandshurica | Polyphenols | ||
| Marine sponge Dictyodendrilla verongiformis | Dictyodendrins | ||
| Phyllanthus urinaria | Gallic acid, ellagic acid, quercetin and cisplatin | ||
| Salvia miltiorrhiza | Tanshinone IIA | ||
| Silybum marianum | Silymarin | ||
| Streptomyces anulatus | Telomestatin | ||
| Trichosanthes cucumerina L. | Cucurbitacins | ||
| Marine sponge Axinellan fundibula | Axinelloside A | ||
| Phyllanthus urinaria | 7′-Hydroxy-3′,4′,5,9,9′-pentamethoxy-3,4-methylene dioxylignan | ||
| Metabolites of sulforaphane from broccoli | MTBITC(erucin) | ||
| Brassica oleracea | Indole-3-carbinol and 3,3′-diindolylmethane | ||
| Cladonia furcate | Lichenin CFP-2 | ||
| Diterpenoid quinone | Salvicine | Induce apoptosis and Inhibition of telomerase activity | [114,142,143] |
| Garlic (Allium sativum) | Allicin and Ajoene | ||
| ent-kaurene Diterpenoids | Xerophilusin B, Macrocalin B, and Eeriocalyxin B | ||
| Glycine max | Daidzein | Inhibition of cell growth and cell cycle in G2/M phase. Induce apoptosis and Inhibition of telomerase activity and reduced telomere length | [38,39,144,145] |
| Panax ginseng C.A. Meyer Radix rubra | Korean red ginseng | ||
| Platycodon grandiflorum | Platycodin | ||
| Pedicularis striata Pall | Verbascoside | ||
| Targeting hTR (human telomerase RNA component)—Transcriptional Level | |||
| Tabebuia avellanedae(Lapacho tree) | Beta-Lapachone | Inhibition of telomerase activity, down-regulation of the levels of hTR and c-myc expression | [31] |
| Targeting the Telomerase Substrate and Associated Protein-Competitor for Substrate | |||
| Camellia sinensis | Epigallocatechin gallate | Binding competitively with respect to the RNA substrate primer | [32,33,85] |
| G4 DNA-Interactive Compounds | |||
| Ascidian Amphicarpa meridian | Meridine | Inhibition of telomerase activity and stabilization of G4 | [37,43,44,146,147,148,149,150,151,152,153] |
| Berberis vulgaris chinensis (Coptis or goldenthread) | Berberine | ||
| Cryptolepis triangularis | Cryptolepine | ||
| Glycine max | Daidzin | ||
| Glycine max | Daidzein, daidzin, genistein and genistin | ||
| Menispermum dauricum and Rhizoma Menispermi | Daurisoline, dauricinoline and daurinoline | ||
| Okinawan tunicate Didenum sp. | Ascididemin | ||
| Boraginaceae family (mainly in the genus of Alkanna Lithospermum) | Shikonin and its derivatives | ||
| Coptidis rhizoma | Palmatine | Formation of C-myc22 G4 and Hum24 G4 | [44,154] |
| North American herb bloodroot (Sanguinaria canadensis) | Sanguinarine | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganesan, K.; Xu, B. Telomerase Inhibitors from Natural Products and Their Anticancer Potential. Int. J. Mol. Sci. 2018, 19, 13. https://doi.org/10.3390/ijms19010013
Ganesan K, Xu B. Telomerase Inhibitors from Natural Products and Their Anticancer Potential. International Journal of Molecular Sciences. 2018; 19(1):13. https://doi.org/10.3390/ijms19010013
Chicago/Turabian StyleGanesan, Kumar, and Baojun Xu. 2018. "Telomerase Inhibitors from Natural Products and Their Anticancer Potential" International Journal of Molecular Sciences 19, no. 1: 13. https://doi.org/10.3390/ijms19010013
APA StyleGanesan, K., & Xu, B. (2018). Telomerase Inhibitors from Natural Products and Their Anticancer Potential. International Journal of Molecular Sciences, 19(1), 13. https://doi.org/10.3390/ijms19010013