Radical Scavenging and Anti-Inflammatory Activities of Representative Anthocyanin Groupings from Pigment-Rich Fruits and Vegetables
Abstract
:1. Introduction
2. Results and Discussion
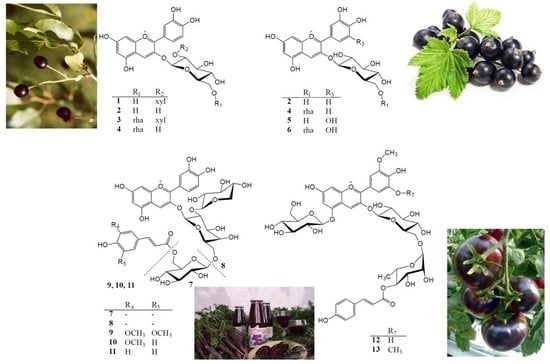
2.1. Anthocyanin Composition of Selected Fruits and Vegetables
2.2. Antioxidative Capacity of Different Anthocyanin Groupings from Selected Fruits and Vegetables
2.3. Vascular Anti-Inflammatory Properties of Selected Anthocyanin Groupings in Endothelial Cells
3. Materials and Methods
3.1. Standards and Chemical Reagents
3.2. Plant Material, Preparation of Purified Anthocyanin Samples (PASs) and Anthocyanin Identification
3.3. Quantification of Anthocyanin Content
3.4. Antioxidant Activity Assays
3.5. Cell Culture and Treatments
3.6. Detection of Endothelial Cell Molecules
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PAS | Purified Anthocyanin Sample |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| ORAC | Oxygen Radical Absorbance Capacity |
| VCAM-1 | Vascular Cell Adhesion Molecule-1 |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| HMEC-1 | Human Microvascular Endothelial Cell-1 |
| TNF-α | Tumour Necrosis Factor-α |
| EIA | Cell-Surface Enzyme Immunoassay |
References
- Andersen, Ø.M.; Jordheim, M. The anthocyanins. In Flavonoids: Chemistry, Biochemistry and Applications; Andersen, Ø.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 471–551. [Google Scholar]
- Andersen, Ø.M.; University of Bergen, Norway. Unpublished data. January 2018.
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Battino, M.; Beekwilder, J.; Denoyes-Rothan, B.; Laimer, M.; McDougall, G.J.; Mezzetti, B. Bioactive compounds in berries relevant to human health. Nutr. Rev. 2009, 67. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Ø.M.; Jordheim, M. Basic Anthocyanin Chemistry and Dietary Sources. In Anthocyanins in Health and Disease; Wallace, T.C., Giusti, M.M., Eds.; Taylor & Francis Inc.: Abingdon, UK; CRC Press: New York, NY, USA, 2013; pp. 13–90. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Bomser, J.; Schwartz, S.J.; He, J.; Magnuson, B.A.; Giusti, M.M. Structure-function relationships of anthocyanins from various anthocyanin-rich extracts on the inhibition of colon cancer cell growth. J. Agric. Food Chem. 2008, 56, 9391–9398. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Chen, C.-Y.; Jin, X.; Mi, M.-T.; Yu, B.; Chang, H.; Ling, W.-H.; Zhang, T. Structural requirements of anthocyanins in relation to inhibition of endothelial injury induced by oxidized low-density lipoprotein and correlation with radical scavenging activity. FEBS Lett. 2010, 584, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Jhin, C.; Hwang, K.T. Prediction of radical scavenging activities of anthocyanins applying adaptive neuro-fuzzy interference system (ANFIS) with quantum chemical descriptors. Int. J. Mol. Sci. 2014, 15, 14715–14727. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.H.; Almagribi, W.; Al-Rashidi, M.N. Antiradical and reductant activities of anthocyanidins and anthocyanins, structure-activity relationship and synthesis. Food Chem. 2016, 194, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-L.; Yu, Y.-Q.; Chen, Z.-J.; Wen, G.-S.; Wei, F.-G.; Zheng, Q.; Wang, C.-D.; Xiao, X.-L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2017, 214, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.; Fernandes, I.; Faria, A.; Oliveira, J.; Fernandes, A.; de Freitas, V.; Mateus, N. Antioxidant properties of anthocyanidins, anthocyanidins 3-glucosides and respective portisins. Food Chem. 2010, 119, 518–523. [Google Scholar] [CrossRef]
- Cassidy, A.; O’Reilly, E.J.; Kay, C.; Sampson, L.; Franz, M.; Forman, J.P.; Curhan, G.; Rimm, E.B. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011, 93, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.P.; Nettleton, J.A.; Jacobs, D.R. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar] [PubMed]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P.E. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I.; Koli, R.; Alfthan, G.; Marniemi, J.; Puukka, P.; Mustonen, P.; Mattila, P.; Jula, A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am. J. Clin. Nutr. 2008, 87, 323–331. [Google Scholar] [PubMed]
- Miyazaki, K.; Makino, K.; Iwadate, E.; Deguchi, Y.; Ishikawa, F. Anthocyanins from purple sweet potato ipomoea batatas cultivar Ayamurasaki suppress the development of atherosclerotic lesions and both enhancements of oxidative stress and soluble vascular cell adhesion molecule-1 in Apolipoprotein E-Deficient Mice. J. Agric. Food Chem. 2008, 56, 11485–11492. [Google Scholar] [CrossRef] [PubMed]
- Mauray, A.; Milenkovic, D.; Besson, C.; Caccia, N.; Morand, C.; Michel, F.; Mazur, A.; Scalbert, A.; Felgines, C. Atheroprotective effects of bilberry extracts in Apo E-Deficient Mice. J. Agric. Food Chem. 2009, 57, 11106–11111. [Google Scholar] [CrossRef] [PubMed]
- Mauray, A.; Felgines, C.; Morand, C.; Mazur, A.; Scalbert, A.; Milenkovic, D. Bilberry anthocyanin-rich extract alters expression of genes related to atherosclerosis development in aorta of apo E-deficient mice. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Osterud, B.; Bjorklid, E. Role of monocytes in atherogenesis. Physiol. Rev. 2003, 83, 1069–1112. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Asseburg, H.; Dold, S.; Rompp, A.; Frohling, B.; Kunz, C.; Rudloff, S. Inhibition of low-grade inflammation by anthocyanins from grape extract in an in vitro epithelial-endothelial co-culture model. Food Funct. 2015, 6, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Medda, R.; Lyros, O.; Schmidt, J.L.; Jovanovic, N.; Nie, L.; Link, B.J.; Otterson, M.F.; Stoner, G.D.; Shaker, R.; Rafiee, P. Anti-inflammatory and anti-angiogenic effect of black raspberry extract on human esophageal and intestinal microvascular endothelial cells. Microvasc. Res. 2015, 97, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, C.; Roursgaard, M.; Porrini, M.; Loft, S.; Moller, P.; Riso, P. Different effects of anthocyanins and phenolic acids from wild blueberry (Vaccinium angustifolium) on monocytes adhesion to endothelial cells in a TNF-alpha stimulated proinflammatory environment. Mol. Nutr. Food Res. 2016, 60, 2355–2366. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Antioxidant capacity: Which capacity and how to assess it? J. Berry Res. 2011, 1, 169–176. [Google Scholar]
- Wu, X.; Prior, R.L. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: Fruits and berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; de la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin 3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A (13) C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, H.P.; Czank, C.; Raheem, S.; Zhang, Q.Z.; Botting, N.P.; Cassidy, A.; Kay, C.D. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol. Nutr. Food Res. 2015, 59, 1095–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overall, J.; Bonney, S.A.; Wilson, M.; Beermann, A., III; Grace, M.H.; Esposito, D.; Lila, M.A.; Komarnytsky, S. Metabolic effects of berries with structurally diverse anthocyanins. Int. J. Mol. Sci. 2017, 18, 422. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Blackcurrant anthocyanins attenuate weight gain and improve glucose metabolism in diet-induced obese mice with intact, but not disrupted, gut microbiome. J. Agric. Food Chem. 2015, 63, 6172–6180. [Google Scholar] [CrossRef] [PubMed]
- Tani, T.; Nishikawa, S.; Kato, M.; Tsuda, T. Delphinidin 3-rutinoside-rich blackcurrant extract ameliorates glucose tolerance by increasing the release of glucagon-like peptide-1 secretion. Food Sci. Nutr. 2017, 5, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ohyama, A.; Ippoushi, K.; Ichiyanagi, T.; Takeuchi, A.; Saito, T.; Fukuoka, H. Structures and antioxidant activity of anthocyanins in many accessions of eggplant and its related species. J. Agric. Food Chem. 2008, 56, 10154–10159. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, S.; Vrhovsek, U.; Mattivi, F. The interaction of anthocyanins with bilitranslocase. Biochem. Biophys. Res. Commun. 2002, 296, 631–636. [Google Scholar] [CrossRef]
- Kurilich, A.; Clevidence, B.A.; Britz, S.J.; Simon, P.W.; Novotny, J.A. Plasma and urine responses are lower for acylated vs. non-acylated anthocyanins from raw and cooked purple carrots. J. Agric. Food Chem. 2005, 53, 6537–6542. [Google Scholar] [CrossRef] [PubMed]
- Gonzali, S.; Mazzucato, A.; Perata, P. Purple as a tomato: Towards high anthocyanin tomatoes. Trends Plant Sci. 2009, 14, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakowska-Barczak, A. Acylated anthocyanins as stable, natural food colorants—A review. Pol. J. Food Nutr. Sci. 2005, 14/55, 107–116. [Google Scholar]
- Yi, L.; Chen, C.Y.; Jin, X.; Zhang, T.; Zhou, Y.; Zhang, Q.Y.; Zhu, J.D.; Mi, M.T. Differential suppression of intracellular reactive oxygen species-mediated signaling pathway in vascular endothelial cells by several subclasses of flavonoids. Biochimie 2012, 94, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Canali, R.; Chirafisi, J.; Saija, A.; Virgili, F.; Cimino, F. Cyanidin-3-O-glucoside protection against TNF-alpha-induced endothelial dysfunction: Involvement of nuclear factor-kappa B signaling. J. Agric. Food Chem. 2010, 58, 12048–12054. [Google Scholar] [CrossRef] [PubMed]
- Krga, I.; Monfoulet, L.E.; Konic-Ristic, A.; Mercier, S.; Glibetic, M.; Morand, C.; Milenkovic, D. Anthocyanins and their gut metabolites reduce the adhesion of monocyte to TNF alpha-activated endothelial cells at physiologically relevant concentrations. Arch. Biochem. Biophys. 2016, 599, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, C.; Frassinetti, S.; Caltavuturo, L.; Leone, A.; Lecci, R.; Calabriso, N.; Carluccio, M.A.; Blando, F.; Mita, G. Anti-proliferative, anti-inflammatory and anti-mutagenic activities of a Prunus mahaleb L. anthocyanin-rich fruit extract. J. Funct. Food 2016, 27, 537–548. [Google Scholar] [CrossRef]
- Blando, F.; Albano, C.; Liu, Y.Z.; Nicoletti, I.; Corradini, D.; Tommasi, N.; Gerardi, C.; Mita, G.; Kitts, D.D. Polyphenolic composition and antioxidant activity of the under-utilised Prunus mahaleb L. fruit. J. Sci. Food Agric. 2016, 96, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Frøytlog, C.; Slimestad, R.; Andersen, Ø.M. Combination of chromatographic techniques for the preparative isolation of anthocyanins—Applied on blackcurrant (Ribes nigrum) fruits. J. Chromatogr. A 1998, 825, 89–95. [Google Scholar] [CrossRef]
- Glässgen, W.E.; Wray, V.; Strack, D.; Metzger, J.W.; Seitz, H.U. Anthocyanins from cell suspension cultures of Daucus carota. Phytochemistry 1992, 31, 1593–1601. [Google Scholar] [CrossRef]
- Andersen, Ø.M.; Opheim, S.; Aksnes, D.W.; Frøystein, N.Å. Structure of petanin, an acylated anthocyanin isolated from Solanum tuberosum, using homo- and hetero-nuclear two-dimensional nuclear magnetic resonance techniques. Phytochem. Anal. 1991, 2, 230–236. [Google Scholar] [CrossRef]
- Slimestad, R.; Aaberg, A.; Andersen, Ø.M. Acylated anthocyanins from petunia flowers. Phytochemistry 1999, 50, 1081–1086. [Google Scholar] [CrossRef]
- Chandra, A.; Rana, J.; Li, Y. Separation, identification, quantification and method validation of anthocyanins in botanical row materials by HPLC and HPLC/MS. J. Agric. Food Chem. 2001, 49, 3515–3521. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, C.; Tommasi, N.; Albano, C.; Pinthus, E.; Rescio, L.; Blando, F.; Mita, G. Prunus mahaleb L. fruit extracts: A novel source for natural pigments. Eur. Food Res. Technol. 2015, 241, 683–695. [Google Scholar] [CrossRef]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, M.A.; Siculella, L.; Ancora, M.A.; Massaro, M.; Scoditti, E.; Storelli, C.; Visioli, F.; Distante, A.; De Caterina, R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation—Antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 622–629. [Google Scholar] [CrossRef] [PubMed]



| Source | % |
|---|---|
| Mahaleb Cherry | |
| Cyanidin 3-(6-(rhamnosyl)glucoside) (4) | 34.3 |
| Cyanidin 3-glucoside (2) | 33.4 |
| Cyanidin 3-(6-(rhamnosyl)-2-(xylosyl)glucoside) (3) | 21.3 |
| Cyanidin 3-(2-(xylosyl)glucoside) (1) | 10.9 |
| Blackcurrant | |
| Delphinidin 3-(6-(rhamnosyl)glucoside) (6) | 56.1 |
| Cyanidin 3-(6-(rhamnosyl)glucoside) (4) | 32.4 |
| Delphinidin 3-glucoside (5) | 5.9 |
| Cyanidin 3-glucoside (2) | 3.3 |
| Black Carrot | |
| Cyanidin 3-(6-(6-(feruloyl)glucosyl)-2-(xylosyl)galactoside) (10) | 77.1 |
| Cyanidin 3-(6-(6-(sinapoyl)glucosyl)-2-(xylosyl)galactoside) (9) | 9.9 |
| Cyanidin 3-(2-(xylosyl)galactoside) (8) | 4.9 |
| Cyanidin 3-(6-(glucosyl)-2-(xylosyl)galactoside) (7) | 4.8 |
| Cyanidin 3-(6-(6-(p-coumaroyl)glucosyl)-2-(xylosyl)galactoside) (11) | 3.1 |
| “Sun Black” Tomato | |
| Petunidin 3-(6-(4-(E-p-coumaroyl)rhamnosyl)glucoside)-5-glucoside (petanin) (12) | 56.6 |
| Malvidin 3-(6-(4-(E-p-coumaroyl)rhamnosyl)glucoside)-5-glucoside (13) | 21.4 |
| Unknown | 22.0 |
| Source | TA | TEAC | TEAC | ORAC | ORAC |
|---|---|---|---|---|---|
| mg AntE /g DW | μmol TE/mg PAS | μmol TE/μmol PAS | μmol TE/mg PAS | μmol TE/μmol PAS | |
| Mahaleb cherry | 38.5 ± 1.50 a | 6.01 ± 0.46 a | 3.44 ± 0.31 a | 15.32 ± 1.73 a b | 8.77 ± 0.63 b |
| Blackcurrant | 32.2 ± 2.33 b | 6.44 ± 0.51 a | 3.89 ± 0.32 a | 17.88 ± 1.87 a | 11.02 ± 0.62 a |
| Black carrot | 12.1 ± 0.48 c | 2.53 ± 0.59 b | 2.24 ± 0.28 b | 12.66 ± 1.86 b | 11.20 ± 0.87 a |
| “Sun Black” tomato | 4.9 ± 0.26 d | 1.30 ± 0.13 c | 1.26 ± 0.22 c | 11.44 ± 1.56 b | 10.68 ± 0.38 a |
| Significance | *** | *** | *** | ** | ** |
| PAS | μmol/L | |||
|---|---|---|---|---|
| Mahaleb cherry | 1.7 | 17.5 | 43.6 | 87.3 |
| Blackcurrant | 1.6 | 16.2 | 40.5 | 81.1 |
| Black carrot | 1.1 | 11.3 | 28.2 | 56.5 |
| “Sun Black” tomato | 1.1 | 10.7 | 26.8 | 53.5 |
| μg/mL | 1 | 10 | 25 | 50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blando, F.; Calabriso, N.; Berland, H.; Maiorano, G.; Gerardi, C.; Carluccio, M.A.; Andersen, Ø.M. Radical Scavenging and Anti-Inflammatory Activities of Representative Anthocyanin Groupings from Pigment-Rich Fruits and Vegetables. Int. J. Mol. Sci. 2018, 19, 169. https://doi.org/10.3390/ijms19010169
Blando F, Calabriso N, Berland H, Maiorano G, Gerardi C, Carluccio MA, Andersen ØM. Radical Scavenging and Anti-Inflammatory Activities of Representative Anthocyanin Groupings from Pigment-Rich Fruits and Vegetables. International Journal of Molecular Sciences. 2018; 19(1):169. https://doi.org/10.3390/ijms19010169
Chicago/Turabian StyleBlando, Federica, Nadia Calabriso, Helge Berland, Gabriele Maiorano, Carmela Gerardi, Maria Annunziata Carluccio, and Øyvind M. Andersen. 2018. "Radical Scavenging and Anti-Inflammatory Activities of Representative Anthocyanin Groupings from Pigment-Rich Fruits and Vegetables" International Journal of Molecular Sciences 19, no. 1: 169. https://doi.org/10.3390/ijms19010169
APA StyleBlando, F., Calabriso, N., Berland, H., Maiorano, G., Gerardi, C., Carluccio, M. A., & Andersen, Ø. M. (2018). Radical Scavenging and Anti-Inflammatory Activities of Representative Anthocyanin Groupings from Pigment-Rich Fruits and Vegetables. International Journal of Molecular Sciences, 19(1), 169. https://doi.org/10.3390/ijms19010169