Inhibition of Aquaporin-4 Improves the Outcome of Ischaemic Stroke and Modulates Brain Paravascular Drainage Pathways
Abstract
:1. Introduction
2. Results
2.1. Motor Testing for Treated/Untreated Animals after MCAO
2.2. Aquaporin-4 Expression in the Stroke Model
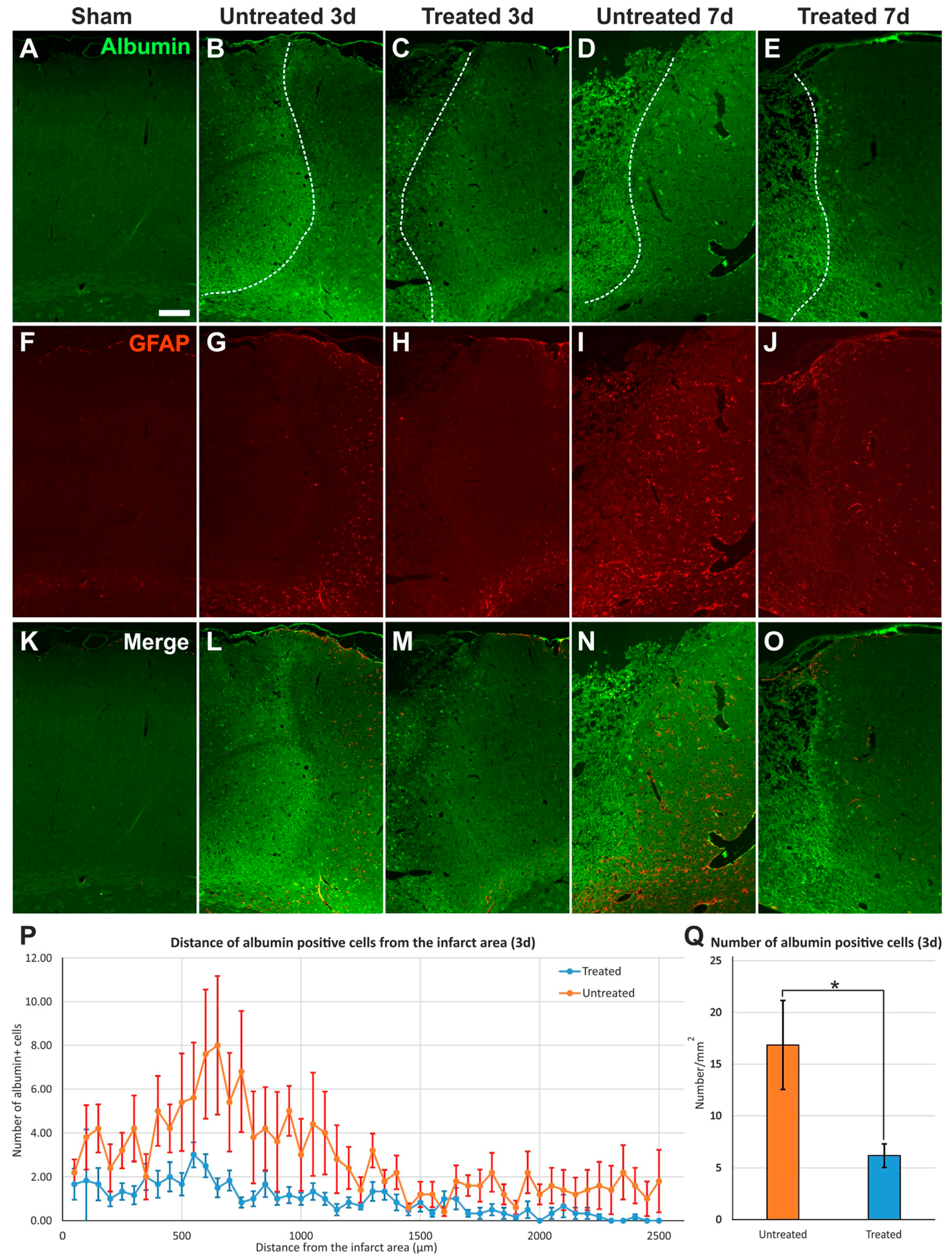
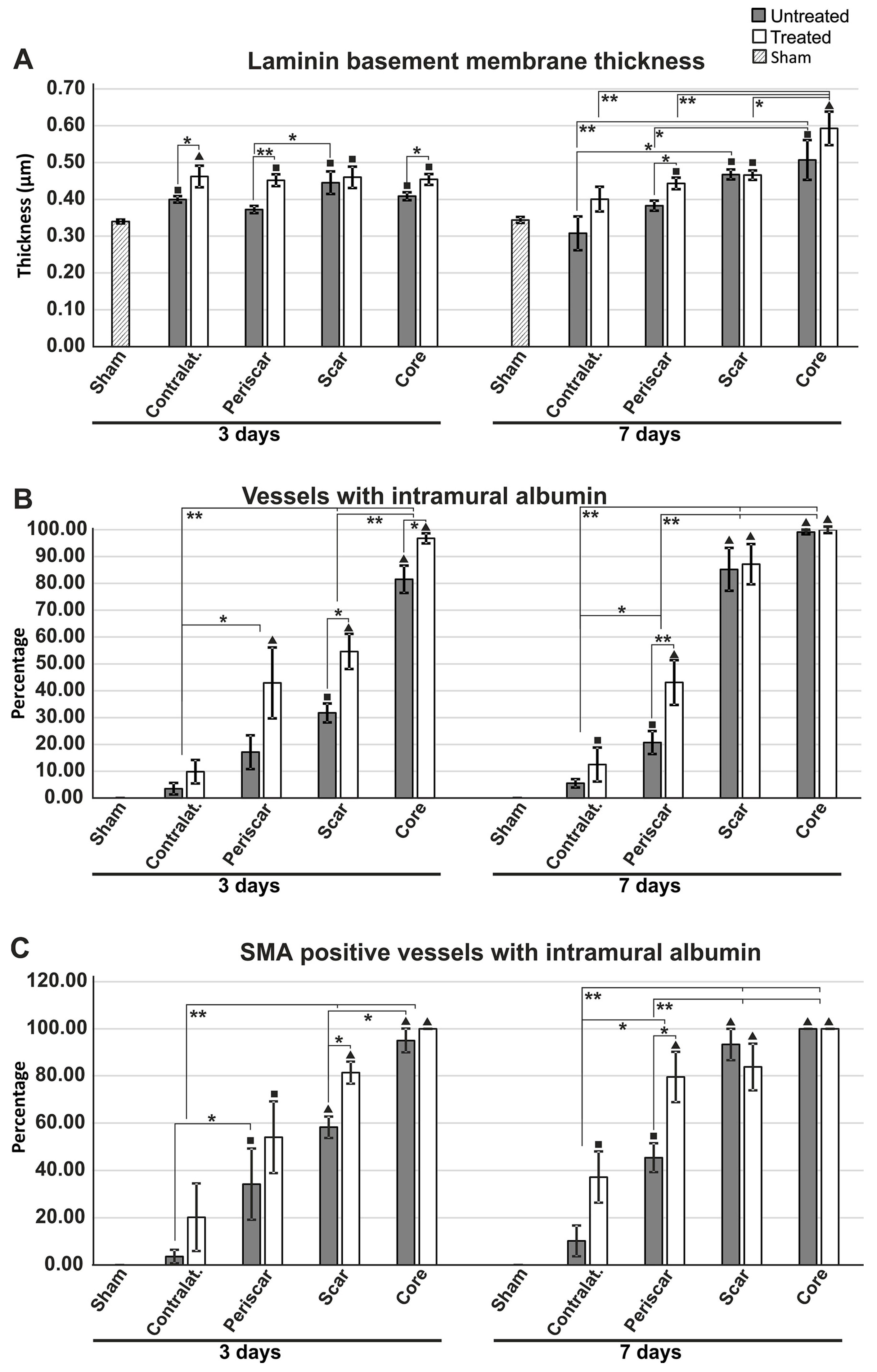
2.3. Reduced Albumin Extravasation in TGN-020-Treated Animals
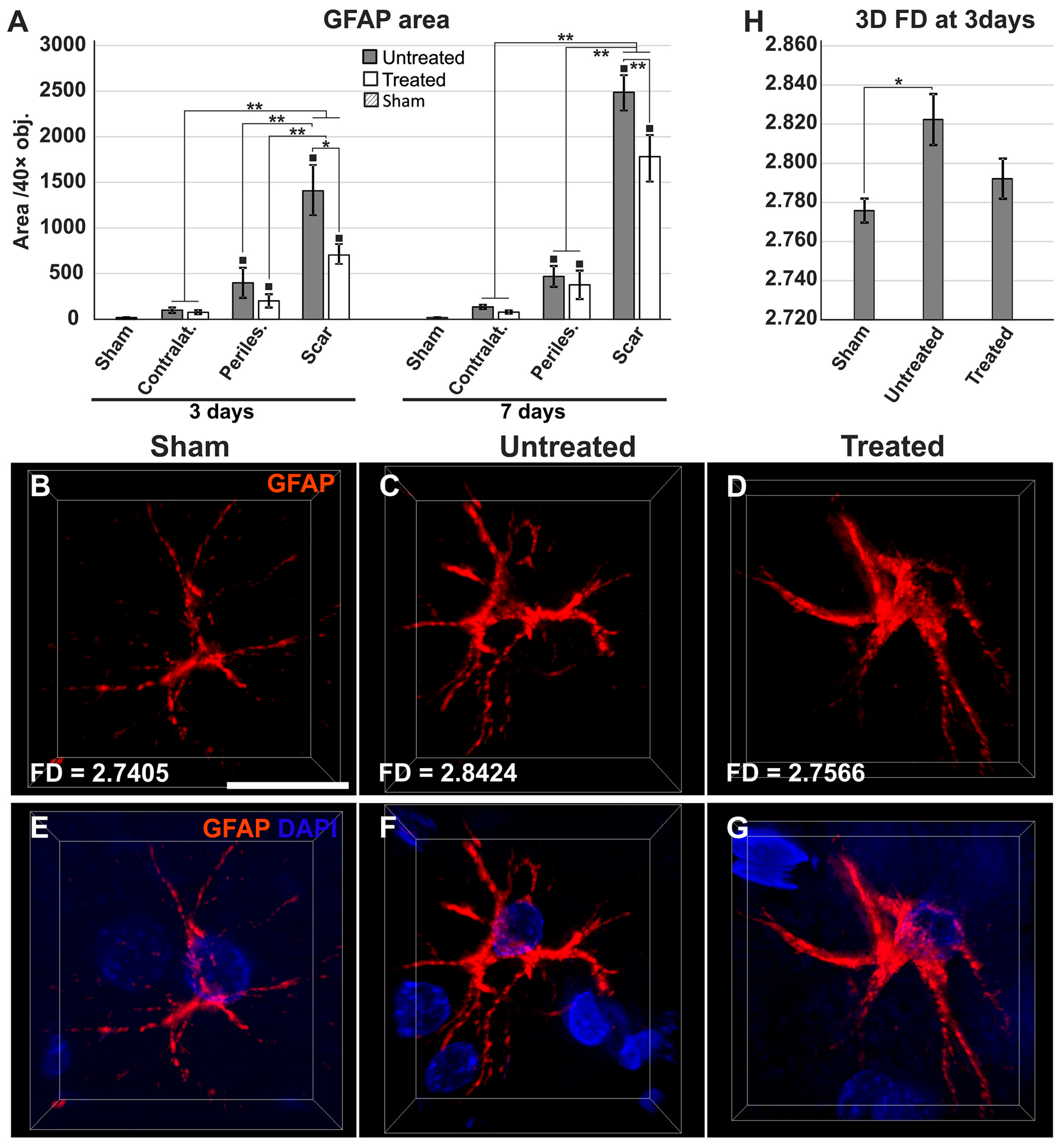
2.4. Reduced Gliosis and Different Glial Morphology in TGN-020-Treated Animals
2.5. Reduced Oedema in TGN-020-Treated Animals
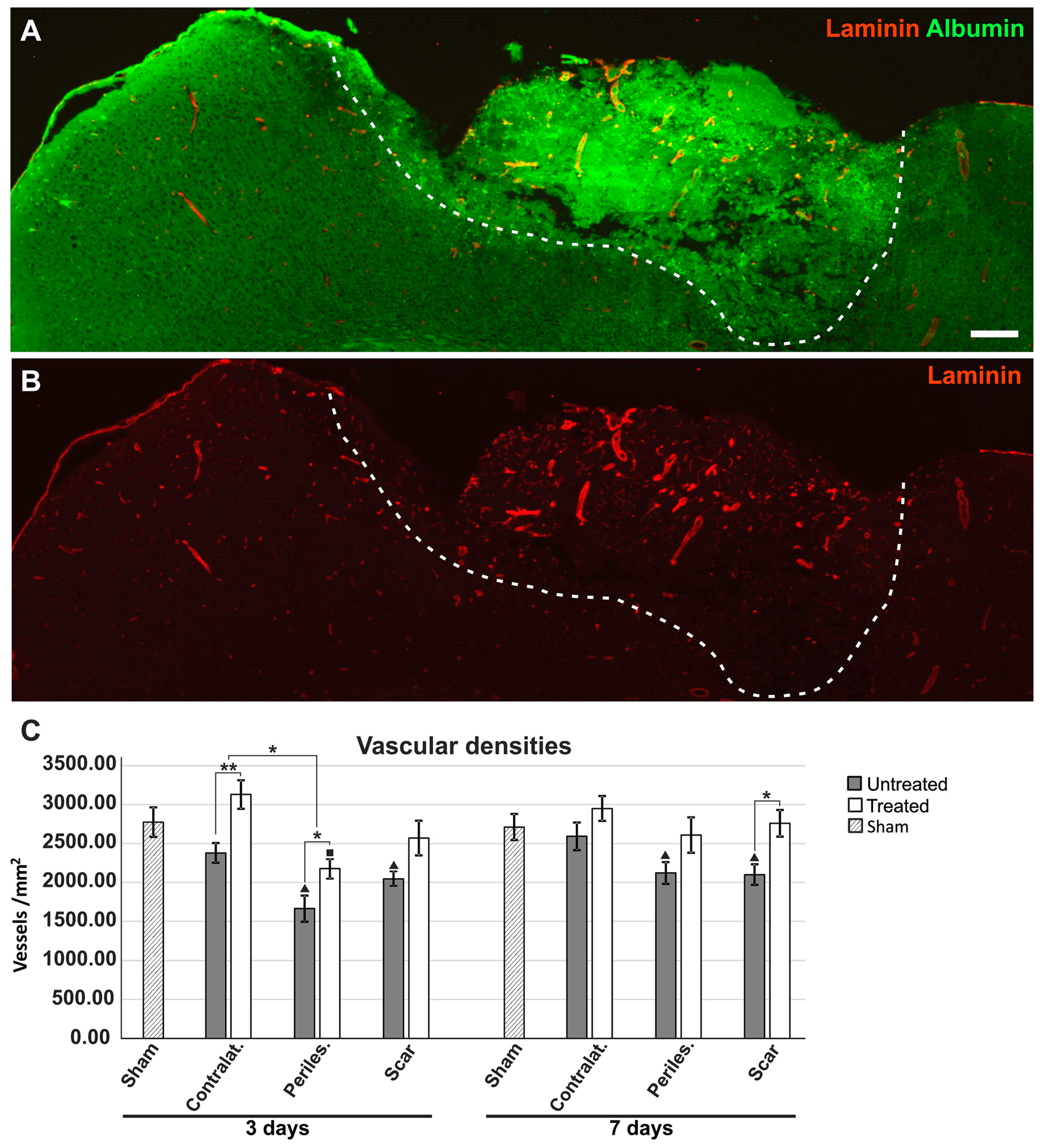
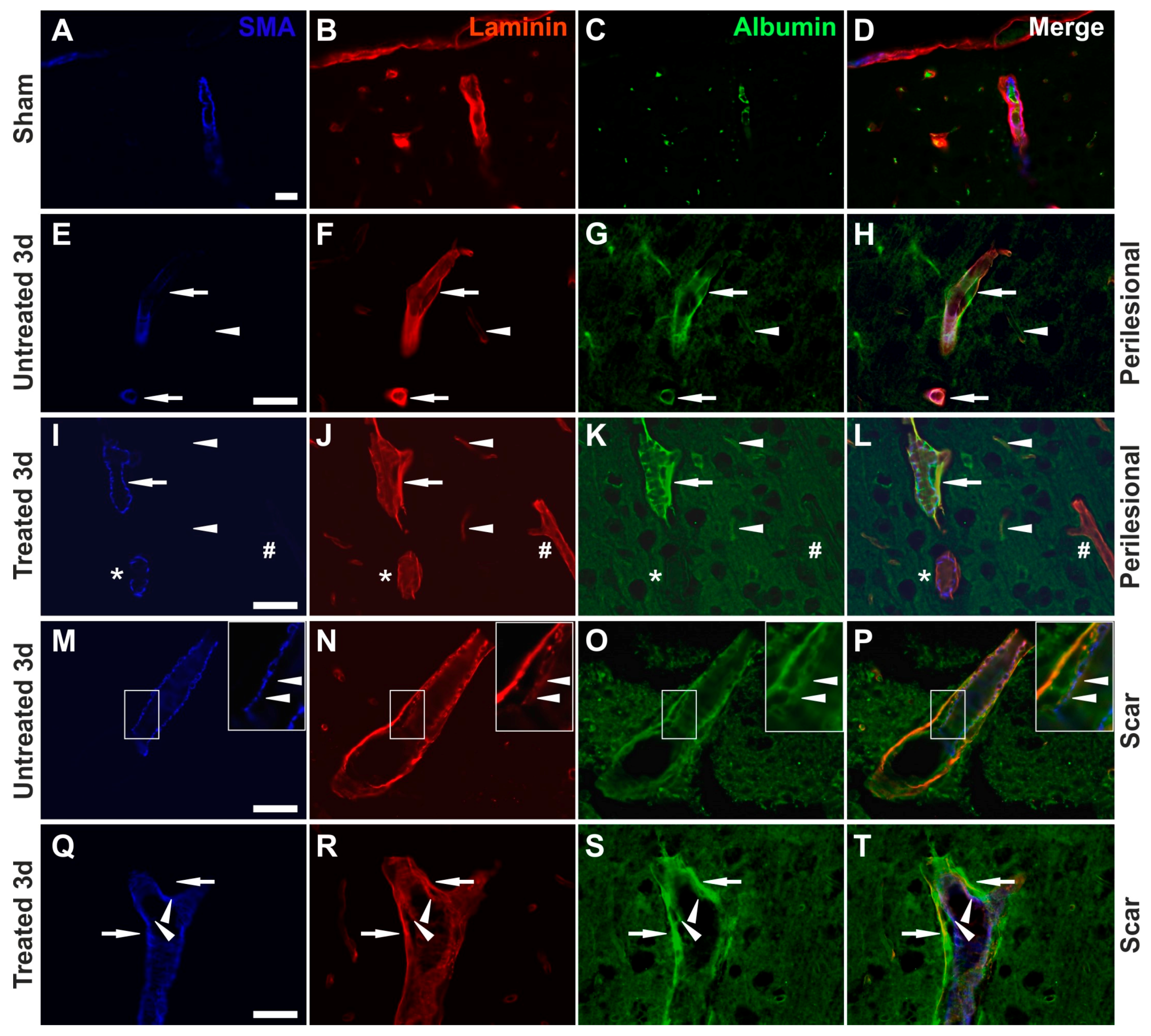
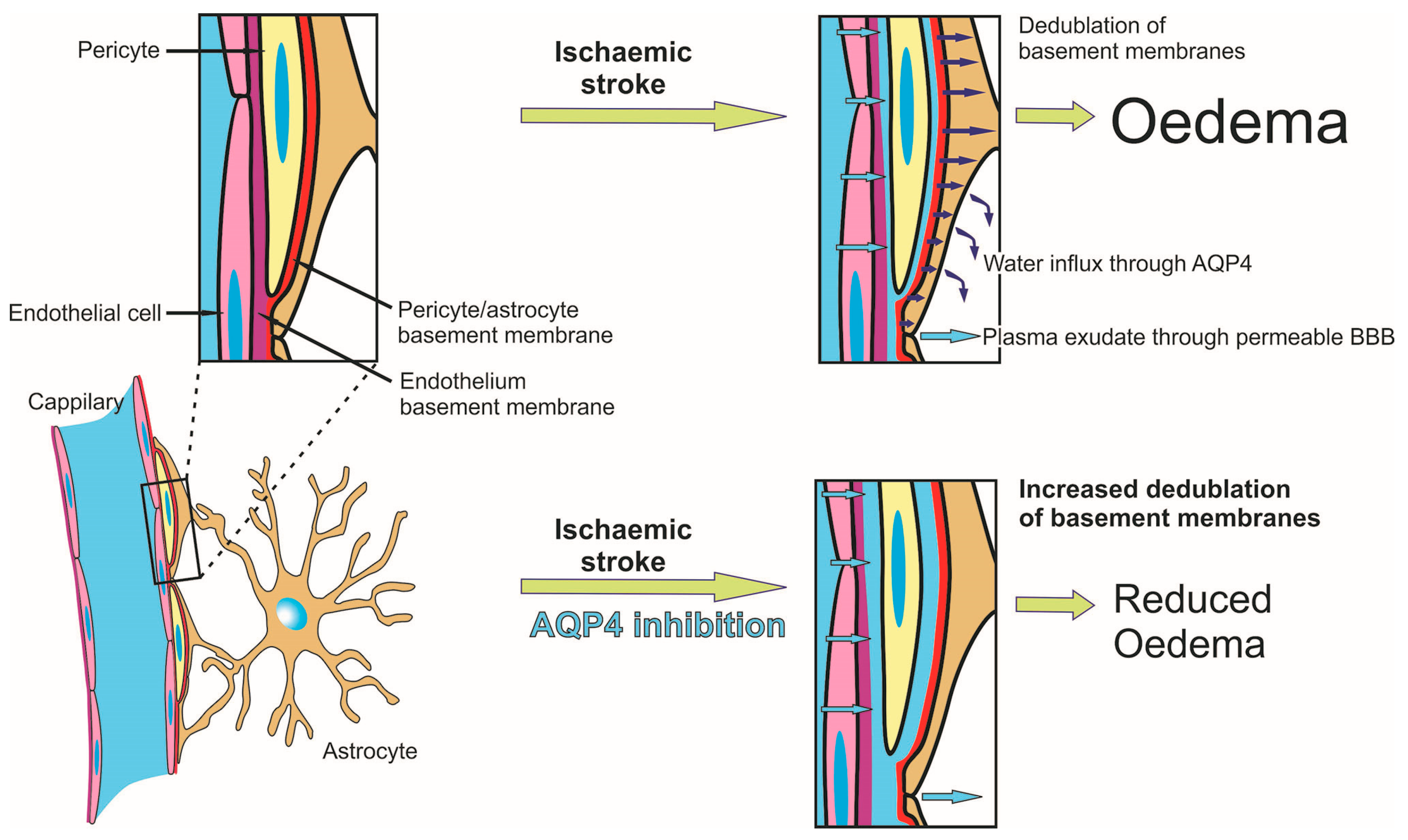
2.6. Effects of AQP4 Inhibition on the Pathways for Intramural Periarterial Drainage
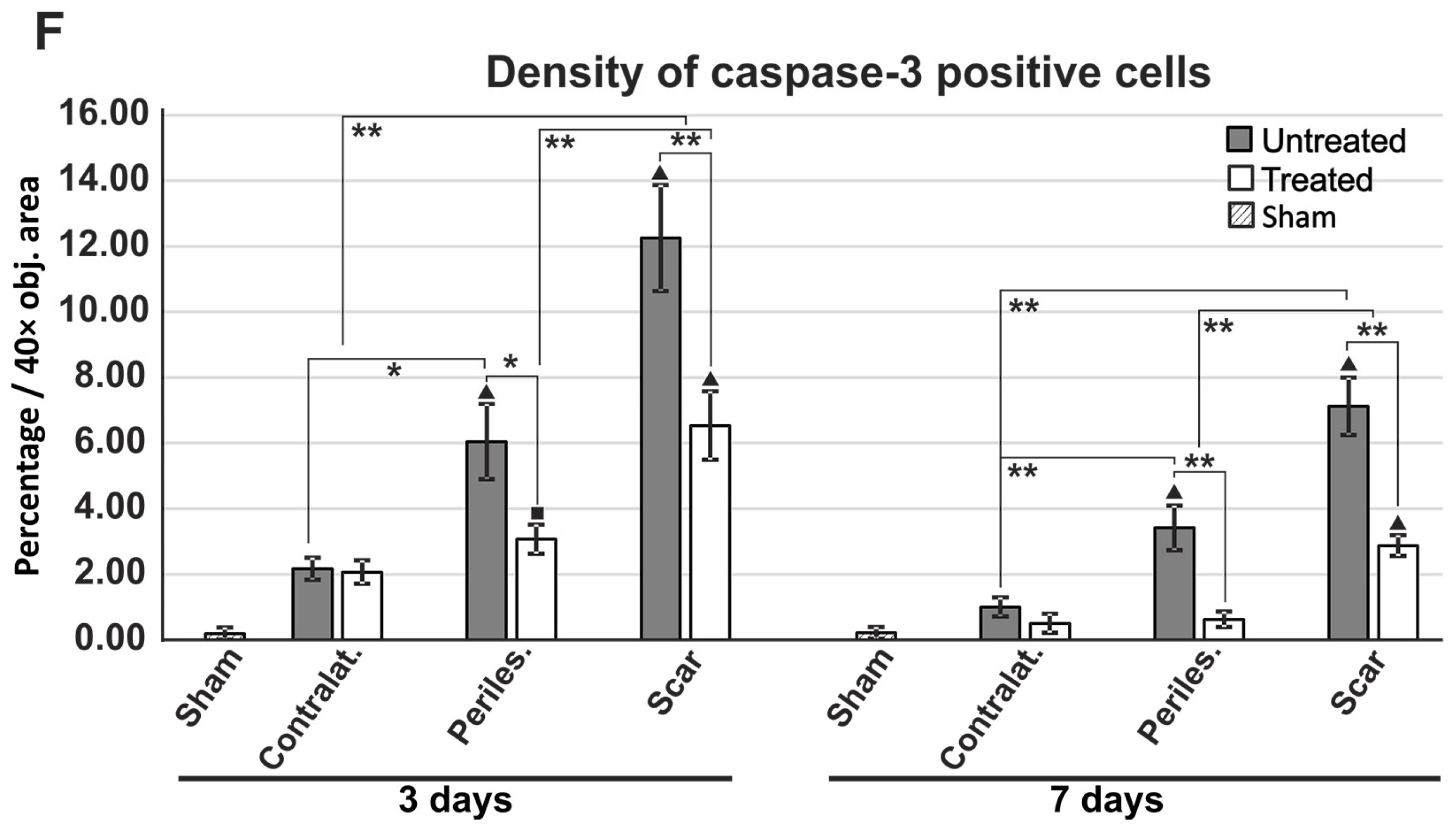
2.7. Reduced Apoptosis in TGN-020-Treated Animals
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgery and Sham Animals
4.3. TGN-020 Preparation and Treatment
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Aβ | Amyloid beta peptide |
| AQP4 | Aquaporin-4 |
| BBB | Blood-brain barrier |
| IOD | Integrated optical density |
| MCA | Linear dichroism |
| MCAO | Medial cerebral artery occlusion |
| siRNA | Small interfering RNA |
| SMA | Smooth Muscle Actin |
| TGN-020 | 2-(Nicotinamide)-1,3,4-thiadiazole AQP4 inhibitor |
| tPA | Tissue plasminogen activator |
References
- Mackay, J.; Mensah, G.; Mendis, S.; Greenlund, K. The Atlas of Heart Disease and Stroke; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Adams, R.J.; Berry, J.D.; Brown, T.M.; Carnethon, M.R.; Dai, S.; de Simone, G.; Ford, E.S.; et al. Heart disease and stroke statistics—2011 update: A report from the American Heart Association. Circulation 2011, 123, e18–e209. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, S.; Patkar, S.; Ogunshola, O.O. Cell-specific blood-brain barrier regulation in health and disease: A focus on hypoxia. Br. J. Pharmacol. 2014, 171, 1210–1230. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Suarez, J.I.; Yahia, A.M.; Mohammad, Y.; Uzun, G.; Suri, M.F.; Zaidat, O.O.; Ayata, C.; Ali, Z.; Wityk, R.J. Timing of neurologic deterioration in massive middle cerebral artery infarction: A multicenter review. Crit. Care Med. 2003, 31, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, T.; Szczudlik, A.; Klimkowicz, A.; Rog, T.M.; Slowik, A. Is mannitol safe for patients with intracerebral hemorrhages? Renal considerations. Clin. Neurol. Neurosurg. 2003, 105, 87–89. [Google Scholar] [CrossRef]
- Benga, G.; Popescu, O.; Pop, V.I. Water exchange through erythrocyte membranes: P-chloromercuribenzene sulfonate inhibition of water diffusion in ghosts studied by a nuclear magnetic resonance technique. Biosci. Rep. 1985, 5, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Lasbennes, F.; Magistretti, P.J.; Regli, L. Aquaporins in brain: Distribution, physiology, and pathophysiology. J. Cereb. Blood Flow Metab. 2002, 22, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin water channels in the nervous system. Nat. Rev. Neurosci. 2013, 14, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Nagelhus, E.A.; Amiry-Moghaddam, M.; Bourque, C.; Agre, P.; Ottersen, O.P. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997, 17, 171–180. [Google Scholar] [PubMed]
- Nagelhus, E.A.; Mathiisen, T.M.; Ottersen, O.P. Aquaporin-4 in the central nervous system: Cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience 2004, 129, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Mogoanta, L.; Ciurea, M.; Pirici, I.; Margaritescu, C.; Simionescu, C.; Ion, D.A.; Pirici, D. Different dynamics of aquaporin 4 and glutamate transporter-1 distribution in the perineuronal and perivascular compartments during ischemic stroke. Brain Pathol. 2014, 24, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J. Biol. Chem. 2005, 280, 13906–13912. [Google Scholar] [CrossRef] [PubMed]
- Bloch, O.; Papadopoulos, M.C.; Manley, G.T.; Verkman, A.S. Aquaporin-4 gene deletion in mice increases focal edema associated with staphylococcal brain abscess. J. Neurochem. 2005, 95, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Bloch, O.; Auguste, K.I.; Manley, G.T.; Verkman, A.S. Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J. Cereb. Blood Flow Metab. 2006, 26, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W.; Chan, P.; Verkman, A.S. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000, 6, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zador, Z.; Verkman, A.S. Glial cell aquaporin-4 overexpression in transgenic mice accelerates cytotoxic brain swelling. J. Biol. Chem. 2008, 283, 15280–15286. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.J.; Tsujita, M.; Nakada, T. Identification of aquaporin 4 inhibitors using in vitro and in silico methods. Bioorg. Med. Chem. 2009, 17, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Huber, V.J.; Tsujita, M.; Nakada, T. Pretreatment with a novel aquaporin 4 inhibitor, TGN-020, significantly reduces ischemic cerebral edema. Neurol. Sci. 2011, 32, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Suzuki, Y.; Tsujita, M.; Huber, V.J.; Yamada, K.; Nakada, T. Development of a Novel Ligand, [C]TGN-020, for Aquaporin 4 Positron Emission Tomography Imaging. ACS Chem. Neurosci. 2011, 2, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Eidsvaag, V.A.; Enger, R.; Hansson, H.A.; Eide, P.K.; Nagelhus, E.A. Human and mouse cortical astrocytes differ in aquaporin-4 polarization toward microvessels. Glia 2017, 65, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Smith, B.L.; Christensen, E.I.; Agre, P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc. Natl. Acad. Sci. USA 1993, 90, 7275–7279. [Google Scholar] [CrossRef] [PubMed]
- Haj-Yasein, N.N.; Vindedal, G.F.; Eilert-Olsen, M.; Gundersen, G.A.; Skare, O.; Laake, P.; Klungland, A.; Thoren, A.E.; Burkhardt, J.M.; Ottersen, O.P.; et al. Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc. Natl. Acad. Sci. USA 2011, 108, 17815–17820. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nakamura, Y.; Yamada, K.; Huber, V.J.; Tsujita, M.; Nakada, T. Aquaporin-4 positron emission tomography imaging of the human brain: First report. J. Neuroimaging 2013, 23, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Miyazaki, M.; Iwase, H.; Maeda, M. Temporal profile of changes in brain tissue extracellular space and extracellular ion (Na(+), K(+)) concentrations after cerebral ischemia and the effects of mild cerebral hypothermia. J. Neurotrauma 2002, 19, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Ashwal, S.; Adami, A.; Tone, B.; Recker, R.; Spagnoli, D.; Ternon, B.; Obenaus, A. Brain water mobility decreases after astrocytic aquaporin-4 inhibition using RNA interference. J. Cereb. Blood Flow Metab. 2011, 31, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Nour, M.; Scalzo, F.; Liebeskind, D.S. Ischemia-reperfusion injury in stroke. Interv. Neurol. 2013, 1, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Yang, G.Y.; Zhou, L.F.; Stern, J.D.; Betz, A.L. Focal cerebral ischemia in the mouse: Description of a model and effects of permanent and temporary occlusion. Brain Res. Mol. Brain Res. 1999, 63, 366–370. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Hirt, L.; Bogousslavsky, J.; Regli, L.; Badaut, J. Time course of aquaporin expression after transient focal cerebral ischemia in mice. J. Neurosci. Res. 2006, 83, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, K.E.; Witt, K.A. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 2008, 32, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Braganza, O.; Bedner, P.; Huttmann, K.; von Staden, E.; Friedman, A.; Seifert, G.; Steinhauser, C. Albumin is taken up by hippocampal NG2 cells and astrocytes and decreases gap junction coupling. Epilepsia 2012, 53, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Goldshmit, Y.; Frisca, F.; Pinto, A.R.; Pebay, A.; Tang, J.K.; Siegel, A.L.; Kaslin, J.; Currie, P.D. Fgf2 improves functional recovery-decreasing gliosis and increasing radial glia and neural progenitor cells after spinal cord injury. Brain Behav. 2014, 4, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Goldshmit, Y.; Galea, M.P.; Wise, G.; Bartlett, P.F.; Turnley, A.M. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J. Neurosci. 2004, 24, 10064–10073. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.T.; Gu, Y.H.; Izawa, Y.; Del Zoppo, G.J. Disruption of dystroglycan-laminin interactions modulates water uptake by astrocytes. Brain Res. 2013, 1503, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Popescu, E.S.; Pirici, I.; Ciurea, R.N.; Balseanu, T.A.; Catalin, B.; Margaritescu, C.; Mogoanta, L.; Hostiuc, S.; Pirici, D. Three-dimensional organ scanning reveals brain edema reduction in a rat model of stroke treated with an aquaporin 4 inhibitor. Rom. J. Morphol. Embryol. 2017, 58, 59–66. [Google Scholar] [PubMed]
- Igarashi, H.; Tsujita, M.; Suzuki, Y.; Kwee, I.L.; Nakada, T. Inhibition of aquaporin-4 significantly increases regional cerebral blood flow. Neuroreport 2013, 24, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Tsirka, S.E. Inflammation modulates expression of laminin in the central nervous system following ischemic injury. J. Neuroinflamm. 2012, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.A.; Michalski, D.; Anders, R.; Nissel, S.; Grosche, J.; Bechmann, I.; Carare, R.O.; Hartig, W. Stroke-induced opposite and age-dependent changes of vessel-associated markers in co-morbid transgenic mice with Alzheimer-like alterations. Exp. Neurol. 2013, 250, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Sixt, M.; Engelhardt, B.; Pausch, F.; Hallmann, R.; Wendler, O.; Sorokin, L.M. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 2001, 153, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.W.; Sharp, M.M.; Albargothy, N.J.; Fernandes, R.; Hawkes, C.A.; Verma, A.; Weller, R.O.; Carare, R.O. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. 2016, 131, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Castejon, O.J. Ultrastructural alterations of human cortical capillary basement membrane in human brain oedema. Folia Neuropathol. 2014, 52, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B.; Carare, R.O.; Bechmann, I.; Flugel, A.; Laman, J.D.; Weller, R.O. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol. 2016, 132, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xiao, N.; Chen, Y.; Huang, H.; Marshall, C.; Gao, J.; Cai, Z.; Wu, T.; Hu, G.; Xiao, M. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol. Neurodegener. 2015, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.O.; Massey, A.; Newman, T.A.; Hutchings, M.; Kuo, Y.M.; Roher, A.E. Cerebral amyloid angiopathy: Amyloid β accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am. J. Pathol. 1998, 153, 725–733. [Google Scholar] [CrossRef]
- Weller, R.O.; Massey, A.; Kuo, Y.M.; Roher, A.E. Cerebral amyloid angiopathy: accumulation of A beta in interstitial fluid drainage pathways in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2000, 903, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Nishi, J.; Minamino, T.; Miyauchi, H.; Nojima, A.; Tateno, K.; Okada, S.; Orimo, M.; Moriya, J.; Fong, G.H.; Sunagawa, K.; et al. Vascular endothelial growth factor receptor-1 regulates postnatal angiogenesis through inhibition of the excessive activation of Akt. Circ. Res. 2008, 103, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Hara-Chikuma, M.; Verkman, A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 2005, 434, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.M.; Adami, A.; Pop, V.; Bellone, J.A.; Coats, J.S.; Hartman, R.E.; Ashwal, S.; Obenaus, A.; Badaut, J. Posttraumatic reduction of edema with aquaporin-4 RNA interference improves acute and chronic functional recovery. J. Cereb. Blood Flow Metab. 2013, 33, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Michinaga, S.; Koyama, Y. Pathogenesis of brain edema and investigation into anti-edema drugs. Int. J. Mol. Sci. 2015, 16, 9949–9975. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S.; Byrnes, K.R.; Symes, A.J. Temporal dynamics of cerebral blood flow, cortical damage, apoptosis, astrocyte-vasculature interaction and astrogliosis in the pericontusional region after traumatic brain injury. Front. Neurol. 2014, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Burda, J.E.; Sofroniew, M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Popa-Wagner, A.; Stocker, K.; Balseanu, A.T.; Rogalewski, A.; Diederich, K.; Minnerup, J.; Margaritescu, C.; Schabitz, W.R. Effects of granulocyte-colony stimulating factor after stroke in aged rats. Stroke 2010, 41, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Llovera, G.; Roth, S.; Plesnila, N.; Veltkamp, R.; Liesz, A. Modeling stroke in mice: Permanent coagulation of the distal middle cerebral artery. J. Vis. Exp. 2014, e51729. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirici, I.; Balsanu, T.A.; Bogdan, C.; Margaritescu, C.; Divan, T.; Vitalie, V.; Mogoanta, L.; Pirici, D.; Carare, R.O.; Muresanu, D.F. Inhibition of Aquaporin-4 Improves the Outcome of Ischaemic Stroke and Modulates Brain Paravascular Drainage Pathways. Int. J. Mol. Sci. 2018, 19, 46. https://doi.org/10.3390/ijms19010046
Pirici I, Balsanu TA, Bogdan C, Margaritescu C, Divan T, Vitalie V, Mogoanta L, Pirici D, Carare RO, Muresanu DF. Inhibition of Aquaporin-4 Improves the Outcome of Ischaemic Stroke and Modulates Brain Paravascular Drainage Pathways. International Journal of Molecular Sciences. 2018; 19(1):46. https://doi.org/10.3390/ijms19010046
Chicago/Turabian StylePirici, Ionica, Tudor Adrian Balsanu, Catalin Bogdan, Claudiu Margaritescu, Tamir Divan, Vacaras Vitalie, Laurentiu Mogoanta, Daniel Pirici, Roxana Octavia Carare, and Dafin Fior Muresanu. 2018. "Inhibition of Aquaporin-4 Improves the Outcome of Ischaemic Stroke and Modulates Brain Paravascular Drainage Pathways" International Journal of Molecular Sciences 19, no. 1: 46. https://doi.org/10.3390/ijms19010046
APA StylePirici, I., Balsanu, T. A., Bogdan, C., Margaritescu, C., Divan, T., Vitalie, V., Mogoanta, L., Pirici, D., Carare, R. O., & Muresanu, D. F. (2018). Inhibition of Aquaporin-4 Improves the Outcome of Ischaemic Stroke and Modulates Brain Paravascular Drainage Pathways. International Journal of Molecular Sciences, 19(1), 46. https://doi.org/10.3390/ijms19010046





