The Glutamatergic System in Primary Somatosensory Neurons and Its Involvement in Sensory Input-Dependent Plasticity
Abstract
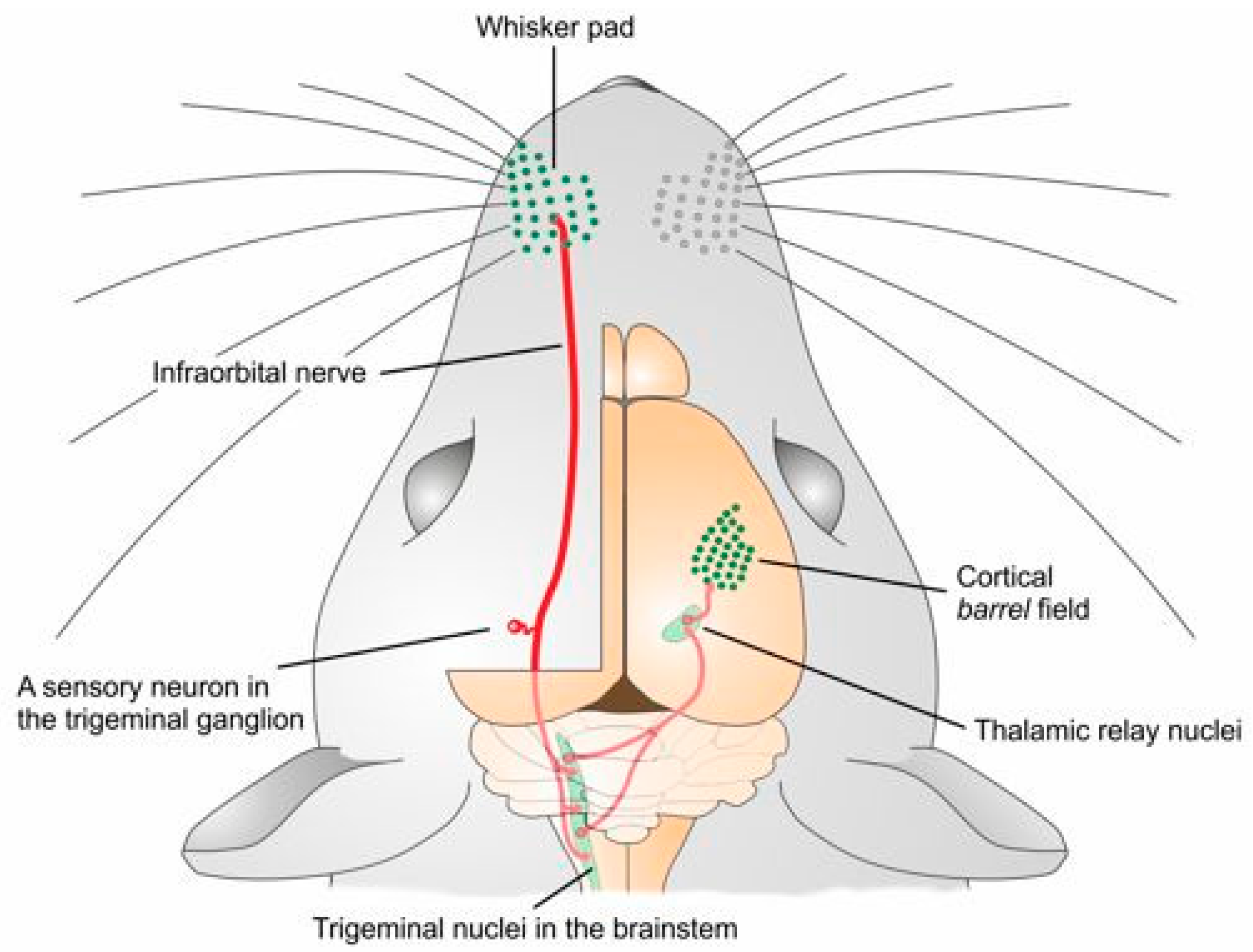
:1. Introduction
2. All Primary Sensory Neurons Use Glutamate and Express Glutamate Receptors and Transporters
2.1. Ionotropic Receptors
2.1.1. Kainate
2.1.2. AMPA
2.1.3. NMDA
2.1.4. Delta
2.2. Metabotropic Receptors
2.2.1. Group I
2.2.2. Group II
2.2.3. Group III
2.3. Glutamate Transporters
2.3.1. Vesicular Glutamate Transporters
2.3.2. Excitatory Amino Acid Transporters (EAATs)
3. Glutamate Receptors and Transporters in Non-Neuronal Cells in DRG and TG
3.1. Satellite Glial Cells
3.2. Schwann Cells
3.3. T-Cells, Macrophages and Dendritic Cells
3.4. Fibroblasts
3.5. Pericytes, Endothelial Cells, Smooth Muscle Cells, and Mast Cells
4. Glutamatergic Receptors and Transporters in Sensory Ganglia Mobilize in Response to Damage of Peripheral Tissues and Sensory Nerves
5. The Glutamatergic System Reacts to Innocuous Manipulation of the Input: Beyond Lesion-Driven and Pain-Related Effects to Experience-Dependent Plasticity in the Brain
6. The Glutamatergic System and Experience-Dependent Plasticity in the Sensory Ganglia
7. Concluding Note
Acknowledgments
Author Contributions
Conflicts of Interest
References
- DeFelipe, J. Brain plasticity and mental processes: Cajal again. Nat. Rev. Neurosci. 2006, 7, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Will, B.; Dalrymple-Alford, J.; Wolff, M.; Cassel, J.C. Reflections on the use of the concept of plasticity in neurobiology. Translation and adaptation by Bruno Will, John Dalrymple-Alford, Mathieu Wolff and Jean-Christophe Cassel from J. Paillard, J Psychol 1976;1:33–47. Behav. Brain Res. 2008, 192, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Neurotransmitters in the regulation of neuronal cytoarchitecture. Brain Res. 1988, 472, 179–212. [Google Scholar] [CrossRef]
- Harris, K.M. Structure, development, and plasticity of dendritic spines. Curr. Opin. Neurobiol. 1999, 9, 343–348. [Google Scholar] [CrossRef]
- Cline, H.; Haas, K. The regulation of dendritic arbor development and plasticity by glutamatergic synaptic input: A review of the synaptotrophic hypothesis. J. Physiol. 2008, 586, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Fedder, K.N.; Sabo, S.L. On the Role of Glutamate in Presynaptic Development: Possible Contributions of Presynaptic NMDA Receptors. Biomolecules 2015, 5, 3448–3466. [Google Scholar] [CrossRef] [PubMed]
- Kossut, M.; Hand, P. Early development of changes in cortical representation of C3 vibrissa following neonatal denervation of surrounding vibrissa receptors: A 2-deoxyglucose study in the rat. Neurosci. Lett. 1984, 46, 7–12. [Google Scholar] [CrossRef]
- Simons, D.J.; Land, P.W. Early experience of tactile stimulation influences organization of somatic sensory cortex. Nature 1987, 326, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Fox, K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J. Neurosci. 1992, 12, 1826–1838. [Google Scholar] [PubMed]
- Wright, N.; Glazewski, S.; Hardingham, N.; Phillips, K.; Pervolaraki, E.; Fox, K. Laminar analysis of the role of GluR1 in experience-dependent and synaptic depression in barrel cortex. Nat. Neurosci. 2008, 11, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Glazewski, S.; Fox, K. Time course of experience-dependent synaptic potentiation and depression in barrel cortex of adolescent rats. J. Neurophysiol. 1996, 75, 1714–1729. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M. Contribution of synaptic plasticity in the insular cortex to chronic pain. Neuroscience 2016, 338, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Luscher, C.; Malenka, R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.E.; Younts, T.J.; Chavez, A.E.; Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.E. Synaptic mechanisms for plasticity in neocortex. Annu. Rev. Neurosci. 2009, 32, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Rema, V.; Armstrong-James, M.; Ebner, F.F. Experience-dependent plasticity of adult rat S1 cortex requires local NMDA receptor activation. J. Neurosci. 1998, 18, 10196–10206. [Google Scholar] [PubMed]
- Tropea, D.; Van Wart, A.; Sur, M. Molecular mechanisms of experience-dependent plasticity in visual cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.S.; Smith, I.T.; Miriyala, J.; Han, J.E.; Corlew, R.J.; Smith, S.L.; Philpot, B.D. Synapse-specific control of experience-dependent plasticity by presynaptic NMDA receptors. Neuron 2014, 83, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.F.; Lotufo, C.M.; Araldi, D.; Rodrigues, M.A.; Macedo, L.P.; Ferreira, S.H.; Parada, C.A. Inflammatory sensitization of nociceptors depends on activation of NMDA receptors in DRG satellite cells. Proc. Natl. Acad. Sci. USA 2014, 111, 18363–18368. [Google Scholar] [CrossRef] [PubMed]
- Carozzi, V.; Marmiroli, P.; Cavaletti, G. Focus on the role of Glutamate in the pathology of the peripheral nervous system. CNS Neurol. Disord. Drug Targets 2008, 7, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.; Broman, J. Synaptic plasticity and pain: Role of ionotropic glutamate receptors. Neuroscientist 2011, 17, 256–273. [Google Scholar] [CrossRef] [PubMed]
- Lawson, S.N. The Peripheral Sensory Nervous System: Dorsal Root Ganglion Neurons. In Peripheral Neuropathy; Dyck, P.J., Thomas, P.K., Saunders, W.B., Eds.; Elsevier Inc.: New York, NY, USA, 2005; pp. 163–202. [Google Scholar]
- Bae, Y.C.; Ihn, H.J.; Park, M.J.; Ottersen, O.P.; Moritani, M.; Yoshida, A.; Shigenaga, Y. Identification of signal substances in synapses made between primary afferents and their associated axon terminals in the rat trigeminal sensory nuclei. J. Comp. Neurol. 2000, 418, 299–309. [Google Scholar] [CrossRef]
- DeGroot, J.; Zhou, S.; Carlton, S.M. Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuroreport 2000, 11, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.E.; Hoffman, E.M.; Sutharshan, M.; Schechter, R. Glutamate pharmacology and metabolism in peripheral primary afferents: Physiological and pathophysiological mechanisms. Pharmacol. Ther. 2011, 130, 283–309. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.H.; Gong, K.; Adedoyin, M.; Ng, J.; Bhargava, A.; Ohara, P.T.; Jasmin, L. Evidence for glutamate as a neuroglial transmitter within sensory ganglia. PLoS ONE 2013, 8, e68312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanaka, A.; Shiotani, Y.; Kiyama, H.; Matsuyama, T.; Kamada, T.; Shiosaka, S.; Tohyama, M. Glutamate-like immunoreactive structures in primary sensory neurons in the rat detected by a specific antiserum against glutamate. Exp. Brain Res. 1987, 65, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, G.; Rustioni, A. Coexistence of glutamate and substance P in dorsal root ganglion neurons of the rat and monkey. J. Comp. Neurol. 1988, 277, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Kai-Kai, M.A. Cytochemistry of the trigeminal and dorsal root ganglia and spinal cord of the rat. Comp. Biochem. Physiol. A Comp. Physiol. 1989, 93, 183–193. [Google Scholar] [CrossRef]
- Stoyanova, I.; Dandov, A.; Lazarov, N.; Chouchkov, C. GABA- and glutamate-immunoreactivity in sensory ganglia of cat: A quantitative analysis. Arch. Physiol. Biochem. 1998, 106, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Brumovsky, P.R. VGLUTs in Peripheral Neurons and the Spinal Cord: Time for a Review. ISRN Neurol. 2013, 2013, 829753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carozzi, V.A.; Zoia, C.P.; Maggioni, D.; Verga, E.; Marmiroli, P.; Ferrarese, C.; Cavaletti, G. Expression, distribution and glutamate uptake activity of high affinity-excitatory aminoacid transporters in in vitro cultures of embryonic rat dorsal root ganglia. Neuroscience 2011, 192, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, G.; Bidoret, C.; Casado, M.; Paoletti, P. Presynaptic NMDA receptors: Roles and rules. Neuroscience 2015, 311, 322–340. [Google Scholar] [CrossRef] [PubMed]
- Cahusac, P.M.; Mavulati, S.C. Non-competitive metabotropic glutamate 1 receptor antagonists block activity of slowly adapting type I mechanoreceptor units in the rat sinus hair follicle. Neuroscience 2009, 163, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Carlton, S.M.; Hargett, G.L. Colocalization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J. Comp. Neurol. 2007, 501, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.R.; Hwang, S.J.; Phend, K.D.; Rustioni, A.; Valtschanoff, J.G. Primary afferent terminals in spinal cord express presynaptic AMPA receptors. J. Neurosci. 2002, 22, 9522–9529. [Google Scholar] [PubMed]
- Marvizon, J.C.; McRoberts, J.A.; Ennes, H.S.; Song, B.; Wang, X.; Jinton, L.; Corneliussen, B.; Mayer, E.A. Two N-methyl-d-aspartate receptors in rat dorsal root ganglia with different subunit composition and localization. J. Comp. Neurol. 2002, 446, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Willcockson, H.; Valtschanoff, J. AMPA and NMDA glutamate receptors are found in both peptidergic and non-peptidergic primary afferent neurons in the rat. Cell Tissue Res. 2008, 334, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Collingridge, G.L.; Olsen, R.W.; Peters, J.; Spedding, M. A nomenclature for ligand-gated ion channels. Neuropharmacology 2009, 56, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.J.; Lodge, D. New developments in the molecular pharmacology of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate and kainate receptors. Pharmacol. Ther. 1996, 70, 65–89. [Google Scholar] [CrossRef]
- Kerchner, G.A.; Wilding, T.J.; Huettner, J.E.; Zhuo, M. Kainate receptor subunits underlying presynaptic regulation of transmitter release in the dorsal horn. J. Neurosci. 2002, 22, 8010–8017. [Google Scholar] [PubMed]
- Hwang, S.J.; Rustioni, A.; Valtschanoff, J.G. Kainate receptors in primary afferents to the rat gracile nucleus. Neurosci. Lett. 2001, 312, 137–140. [Google Scholar] [CrossRef]
- Sahara, Y.; Noro, N.; Iida, Y.; Soma, K.; Nakamura, Y. Glutamate receptor subunits GluR5 and KA-2 are coexpressed in rat trigeminal ganglion neurons. J. Neurosci. 1997, 17, 6611–6620. [Google Scholar] [PubMed]
- Bardoni, R. Role of presynaptic glutamate receptors in pain transmission at the spinal cord level. Curr. Neuropharmacol. 2013, 11, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Kerchner, G.A.; Wilding, T.J.; Li, P.; Zhuo, M.; Huettner, J.E. Presynaptic kainate receptors regulate spinal sensory transmission. J. Neurosci. 2001, 21, 59–66. [Google Scholar] [PubMed]
- Sato, K.; Kiyama, H.; Park, H.T.; Tohyama, M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. Neuroreport 1993, 4, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.L. Metabotropic actions of kainate receptors in dorsal root ganglion cells. Adv. Exp. Med. Biol. 2011, 717, 69–80. [Google Scholar] [PubMed]
- Munoz, A.; Woods, T.M.; Jones, E.G. Laminar and cellular distribution of AMPA, kainate, and NMDA receptor subunits in monkey sensory-motor cortex. J. Comp. Neurol. 1999, 407, 472–490. [Google Scholar] [CrossRef]
- Paternain, A.V.; Herrera, M.T.; Nieto, M.A.; Lerma, J. GluR5 and GluR6 kainate receptor subunits coexist in hippocampal neurons and coassemble to form functional receptors. J. Neurosci. 2000, 20, 196–205. [Google Scholar] [PubMed]
- Rustioni, A. Modulation of sensory input to the spinal cord by presynaptic ionotropic glutamate receptors. Arch. Ital. Biol. 2005, 143, 103–112. [Google Scholar] [PubMed]
- Lucifora, S.; Willcockson, H.H.; Lu, C.R.; Darstein, M.; Phend, K.D.; Valtschanoff, J.G.; Rustioni, A. Presynaptic low- and high-affinity kainate receptors in nociceptive spinal afferents. Pain 2006, 120, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Lerma, J.; Marques, J.M. Kainate receptors in health and disease. Neuron 2013, 80, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ro, J.Y. Differential regulation of glutamate receptors in trigeminal ganglia following masseter inflammation. Neurosci. Lett. 2007, 421, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Wenthold, R.J.; Morioka, H.; Petralia, R.S. Light and electron microscopic immunocytochemical localization of AMPA-selective glutamate receptors in the rat spinal cord. J. Comp. Neurol. 1994, 344, 431–454. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Bardoni, R.; Tong, C.K.; Engelman, H.S.; Joseph, D.J.; Magherini, P.C.; MacDermott, A.B. Functional expression of AMPA receptors on central terminals of rat dorsal root ganglion neurons and presynaptic inhibition of glutamate release. Neuron 2002, 35, 135–146. [Google Scholar] [CrossRef]
- Santos, S.D.; Carvalho, A.L.; Caldeira, M.V.; Duarte, C.B. Regulation of AMPA receptors and synaptic plasticity. Neuroscience 2009, 158, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Carlton, S.M.; Hargett, G.L.; Coggeshall, R.E. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci. Lett. 1995, 197, 25–28. [Google Scholar] [CrossRef]
- Coggeshall, R.E.; Carlton, S.M. Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. J. Comp. Neurol. 1998, 391, 78–86. [Google Scholar] [CrossRef]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999, 51, 7–61. [Google Scholar] [PubMed]
- Lu, C.R.; Hwang, S.J.; Phend, K.D.; Rustioni, A.; Valtschanoff, J.G. Primary afferent terminals that express presynaptic NR1 in rats are mainly from myelinated, mechanosensitive fibers. J. Comp. Neurol. 2003, 460, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Sheng, M.; Jan, L.Y.; Jan, Y.N.; Basbaum, A.I. Evidence for presynaptic N-methyl-d-aspartate autoreceptors in the spinal cord dorsal horn. Proc. Natl. Acad. Sci. USA 1994, 91, 8383–8387. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, I.S.; Genever, P.G.; Cahusac, P.M. Essential components for a glutamatergic synapse between Merkel cell and nerve terminal in rats. Neurosci. Lett. 2004, 362, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Cull-Candy, S.G.; Leszkiewicz, D.N. Role of distinct NMDA receptor subtypes at central synapses. Sci. STKE 2004, 2004, re16. [Google Scholar] [CrossRef] [PubMed]
- Kohr, G. NMDA receptor function: Subunit composition versus spatial distribution. Cell Tissue Res. 2006, 326, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Villmann, C.; Strutz, N.; Morth, T.; Hollmann, M. Investigation by ion channel domain transplantation of rat glutamate receptor subunits, orphan receptors and a putative NMDA receptor subunit. Eur. J. Neurosci. 1999, 11, 1765–1778. [Google Scholar] [CrossRef] [PubMed]
- Collingridge, G.L.; Olsen, R.; Peters, J.A.; Spedding, M. Ligand gated ion channels. Neuropharmacology 2009, 56, 1. [Google Scholar] [CrossRef] [PubMed]
- Kohda, K.; Kakegawa, W.; Yuzaki, M. Unlocking the secrets of the delta2 glutamate receptor: A gatekeeper for synaptic plasticity in the cerebellum. Commun. Integr. Biol. 2013, 6, e26466. [Google Scholar] [CrossRef] [PubMed]
- Safieddine, S.; Wenthold, R.J. The glutamate receptor subunit delta1 is highly expressed in hair cells of the auditory and vestibular systems. J. Neurosci. 1997, 17, 7523–7531. [Google Scholar] [PubMed]
- Palazzo, E.; de Novellis, V.; Rossi, F.; Maione, S. Supraspinal metabotropic glutamate receptor subtype 8: A switch to turn off pain. Amino Acids 2014, 46, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, E.; Marabese, I.; de Novellis, V.; Rossi, F.; Maione, S. Supraspinal metabotropic glutamate receptors: A target for pain relief and beyond. Eur. J. Neurosci. 2014, 39, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Valerio, A.; Rizzonelli, P.; Paterlini, M.; Moretto, G.; Knopfel, T.; Kuhn, R.; Memo, M.; Spano, P. mGluR5 metabotropic glutamate receptor distribution in rat and human spinal cord: A developmental study. Neurosci. Res. 1997, 28, 49–57. [Google Scholar] [CrossRef]
- Araki, T.; Kenimer, J.G.; Nishimune, A.; Sugiyama, H.; Yoshimura, R.; Kiyama, H. Identification of the metabotropic glutamate receptor-1 protein in the rat trigeminal ganglion. Brain Res. 1993, 627, 341–344. [Google Scholar] [CrossRef]
- Ohishi, H.; Akazawa, C.; Shigemoto, R.; Nakanishi, S.; Mizuno, N. Distributions of the mRNAs for L-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J. Comp. Neurol. 1995, 360, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.; Reeve, A.; Bowes, M.; Winter, J.; Wotherspoon, G.; Davis, A.; Schmid, P.; Gasparini, F.; Kuhn, R.; Urban, L. mGlu5 receptors and nociceptive function II. mGlu5 receptors functionally expressed on peripheral sensory neurones mediate inflammatory hyperalgesia. Neuropharmacology 2001, 40, 10–19. [Google Scholar] [CrossRef]
- Maksimovic, S.; Baba, Y.; Lumpkin, E.A. Neurotransmitters and synaptic components in the Merkel cell-neurite complex, a gentle-touch receptor. Ann. N. Y. Acad. Sci. 2013, 1279, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Carlton, S.M.; Hargett, G.L.; Coggeshall, R.E. Localization of metabotropic glutamate receptors 2/3 on primary afferent axons in the rat. Neuroscience 2001, 105, 957–969. [Google Scholar] [CrossRef]
- Azkue, J.J.; Liu, X.G.; Zimmermann, M.; Sandkuhler, J. Induction of long-term potentiation of C fibre-evoked spinal field potentials requires recruitment of group I, but not group II/III metabotropic glutamate receptors. Pain 2003, 106, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Ohishi, H.; Kaneko, T.; Shigemoto, R.; Neki, A.; Nakanishi, S.; Mizuno, N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR7, in ganglion neurons of the rat; with special reference to the presence in glutamatergic ganglion neurons. Neurosci. Lett. 1996, 204, 9–12. [Google Scholar] [CrossRef]
- Li, J.Y.; Wang, X.; Ji, P.T.; Li, X.F.; Guan, G.H.; Jiang, X.S.; Zhou, G.S.; Hua, F.; Wang, N. Peripheral nerve injury decreases the expression of metabolic glutamate receptor 7 in dorsal root ganglion neurons. Neurosci. Lett. 2012, 531, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Boye Larsen, D.; Ingemann Kristensen, G.; Panchalingam, V.; Laursen, J.C.; Norgaard Poulsen, J.; Skallerup Andersen, M.; Kandiah, A.; Gazerani, P. Investigating the expression of metabotropic glutamate receptors in trigeminal ganglion neurons and satellite glial cells: Implications for craniofacial pain. J. Recept. Signal. Transduct. Res. 2014, 34, 261–269. [Google Scholar] [CrossRef] [PubMed]
- El Mestikawy, S.; Wallen-Mackenzie, A.; Fortin, G.M.; Descarries, L.; Trudeau, L.E. From glutamate co-release to vesicular synergy: Vesicular glutamate transporters. Nat. Rev. Neurosci. 2011, 12, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Fujiyama, F.; Kaneko, T.; Mizuno, N. Expression of vesicular glutamate transporters, VGluT1 and VGluT2, in axon terminals of nociceptive primary afferent fibers in the superficial layers of the medullary and spinal dorsal horns of the rat. J. Comp. Neurol. 2003, 457, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; Hydling, F.; Olsson, E.; Shi, T.; Edwards, R.H.; Fujiyama, F.; Kaneko, T.; Hokfelt, T.; Cullheim, S.; Meister, B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse 2003, 50, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Malet, M.; Vieytes, C.A.; Lundgren, K.H.; Seal, R.P.; Tomasella, E.; Seroogy, K.B.; Hokfelt, T.; Gebhart, G.F.; Brumovsky, P.R. Transcript expression of vesicular glutamate transporters in lumbar dorsal root ganglia and the spinal cord of mice—Effects of peripheral axotomy or hindpaw inflammation. Neuroscience 2013, 248, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.L.; Konig, P.; Shimizu, T.; Jobling, P.; Gibbins, I.L. Most peptide-containing sensory neurons lack proteins for exocytotic release and vesicular transport of glutamate. J. Comp. Neurol. 2005, 483, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Honma, S.; Kato, A.; Shi, L.; Yatani, H.; Wakisaka, S. Vesicular glutamate transporter immunoreactivity in the periodontal ligament of the rat incisor. Anat. Rec. 2012, 295, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Lim, G.; Mao, J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J. Neurosci. 2003, 23, 2899–2910. [Google Scholar] [PubMed]
- Carozzi, V.A.; Canta, A.; Oggioni, N.; Ceresa, C.; Marmiroli, P.; Konvalinka, J.; Zoia, C.; Bossi, M.; Ferrarese, C.; Tredici, G.; et al. Expression and distribution of ‘high affinity’ glutamate transporters GLT1, GLAST, EAAC1 and of GCPII in the rat peripheral nervous system. J. Anat. 2008, 213, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Avendano, C. El Concierto Celular del Dolor Neuropático in Sociedad Española de Bioquímica y Biología Molecular 2010; Sociedad Española de Bioquímica y Biología Molecular: Madrid, Spain, 2010. [Google Scholar]
- Castillo, C.; Norcini, M.; Martin Hernandez, L.A.; Correa, G.; Blanck, T.J.; Recio-Pinto, E. Satellite glia cells in dorsal root ganglia express functional NMDA receptors. Neuroscience 2013, 240, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.E.; Richards, B.A.; Kriebel, R.M. Glutamine-, glutamine synthetase-, glutamate dehydrogenase- and pyruvate carboxylase-immunoreactivities in the rat dorsal root ganglion and peripheral nerve. Brain Res. 2002, 945, 202–211. [Google Scholar] [CrossRef]
- Wagner, L.; Warwick, R.A.; Pannicke, T.; Reichenbach, A.; Grosche, A.; Hanani, M. Glutamate release from satellite glial cells of the murine trigeminal ganglion. Neurosci. Lett. 2014, 578, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Kinkelin, I.; Brocker, E.B.; Koltzenburg, M.; Carlton, S.M. Localization of ionotropic glutamate receptors in peripheral axons of human skin. Neurosci. Lett. 2000, 283, 149–152. [Google Scholar] [CrossRef]
- Campana, W.M.; Mantuano, E.; Azmoon, P.; Henry, K.; Banki, M.A.; Kim, J.H.; Pizzo, D.P.; Gonias, S.L. Ionotropic glutamate receptors activate cell signaling in response to glutamate in Schwann cells. FASEB J. 2017, 31, 1744–1755. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Montoya, J.; Buendia, I.; Martin, Y.B.; Egea, J.; Negredo, P.; Avendano, C. Sensory Input-Dependent Changes in Glutamatergic Neurotransmission- Related Genes and Proteins in the Adult Rat Trigeminal Ganglion. Front. Mol. Neurosci. 2016, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.; Wyatt, A.; Webb, J.K.; O’Donnell, R.; Mason, G.; Rigby, M.; Sirinathsinghji, D.; Hill, R.G.; Rupniak, N.M. Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: Correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology 1999, 38, 611–623. [Google Scholar] [CrossRef]
- Fernández-Montoya, J. Mecanismos Moleculares y Celulares Implicados en la Respuesta del Sistema Trigeminal Periférico a Alteraciones Inducidas en la Entrada Sensorial. Ph.D. Thesis, Neuroscience, Universidad Autonoma de Madrid, Madrid, Spain, 2017. [Google Scholar]
- Azkue, J.J.; Murga, M.; Fernandez-Capetillo, O.; Mateos, J.M.; Elezgarai, I.; Benitez, R.; Osorio, A.; Diez, J.; Puente, N.; Bilbao, A.; et al. Immunoreactivity for the group III metabotropic glutamate receptor subtype mGluR4a in the superficial laminae of the rat spinal dorsal horn. J. Comp. Neurol. 2001, 430, 448–457. [Google Scholar] [CrossRef]
- Brumovsky, P.; Watanabe, M.; Hokfelt, T. Expression of the vesicular glutamate transporters-1 and -2 in adult mouse dorsal root ganglia and spinal cord and their regulation by nerve injury. Neuroscience 2007, 147, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Rozanski, G.M.; Li, Q.; Stanley, E.F. Transglial transmission at the dorsal root ganglion sandwich synapse: Glial cell to postsynaptic neuron communication. Eur. J. Neurosci. 2013, 37, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Mantuano, E.; Lam, M.S.; Shibayama, M.; Campana, W.M.; Gonias, S.L. The NMDA receptor functions independently and as an LRP1 co-receptor to promote Schwann cell survival and migration. J. Cell Sci. 2015, 128, 3478–3488. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.J.; Frohlich, N.; Kula, B.; Barzan, R.; Kukley, M. Glutamate activates AMPA receptor conductance in the developing Schwann cells of the mammalian peripheral nerves. J. Neurosci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Micu, I.; Plemel, J.R.; Lachance, C.; Proft, J.; Jansen, A.J.; Cummins, K.; van Minnen, J.; Stys, P.K. The molecular physiology of the axo-myelinic synapse. Exp. Neurol. 2016, 276, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Kazey, V.I.; Leinsoo, T.A.; Mashkina, A.P.; Tyulina, O.V.; Johnson, P.; Tuneva, J.O.; Chittur, S.; Carpenter, D.O. Rodent lymphocytes express functionally active glutamate receptors. Biochem. Biophys. Res. Commun. 2004, 324, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.; Ciruela, F.; Casado, V.; Mallol, J.; Gallart, T.; Lluis, C.; Franco, R. Group I metabotropic glutamate receptors mediate a dual role of glutamate in T cell activation. J. Biol. Chem. 2004, 279, 33352–33358. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.; Gallart, T.; Lluis, C.; Franco, R. Role of glutamate on T-cell mediated immunity. J. Neuroimmunol. 2007, 185, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Fallarino, F.; Volpi, C.; Fazio, F.; Notartomaso, S.; Vacca, C.; Busceti, C.; Bicciato, S.; Battaglia, G.; Bruno, V.; Puccetti, P.; et al. Metabotropic glutamate receptor-4 modulates adaptive immunity and restrains neuroinflammation. Nat. Med. 2010, 16, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Rimaniol, A.C.; Haik, S.; Martin, M.; Le Grand, R.; Boussin, F.D.; Dereuddre-Bosquet, N.; Gras, G.; Dormont, D. Na+-dependent high-affinity glutamate transport in macrophages. J. Immunol. 2000, 164, 5430–5438. [Google Scholar] [CrossRef] [PubMed]
- Shanshiashvili, L.; Tsitsilashvili, E.; Dabrundashvili, N.; Kalandadze, I.; Mikeladze, D. Metabotropic glutamate receptor 5 may be involved in macrophage plasticity. Biol. Res. 2017, 50, 4. [Google Scholar] [CrossRef] [PubMed]
- Levite, M. Glutamate, T cells and multiple sclerosis. J. Neural Transm. 2017, 124, 775–798. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; McLachlan, E.M. Distinct functional types of macrophage in dorsal root ganglia and spinal nerves proximal to sciatic and spinal nerve transections in the rat. Exp. Neurol. 2003, 184, 590–605. [Google Scholar] [CrossRef]
- Austin, P.J.; Berglund, A.M.; Siu, S.; Fiore, N.T.; Gerke-Duncan, M.B.; Ollerenshaw, S.L.; Leigh, S.J.; Kunjan, P.A.; Kang, J.W.; Keay, K.A. Evidence for a distinct neuro-immune signature in rats that develop behavioural disability after nerve injury. J. Neuroinflamm. 2015, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Lv, X.; Zeng, S.; Shi, J. Activity-dependent neuronal control of gap-junctional communication in fibroblasts. Brain Res. 2009, 1280, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Busija, D.W.; Bari, F.; Domoki, F.; Horiguchi, T.; Shimizu, K. Mechanisms involved in the cerebrovascular dilator effects of cortical spreading depression. Prog. Neurobiol. 2008, 86, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; Macvicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Collard, C.D.; Park, K.A.; Montalto, M.C.; Alapati, S.; Buras, J.A.; Stahl, G.L.; Colgan, S.P. Neutrophil-derived glutamate regulates vascular endothelial barrier function. J. Biol. Chem. 2002, 277, 14801–14811. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.S.; Huang, Q.H.; Zhang, F.X.; Bao, L.; Lu, Y.J.; Guo, C.; Yang, L.; Huang, W.J.; Fu, G.; Xu, S.H.; et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc. Natl. Acad. Sci. USA 2002, 99, 8360–8365. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, F.X.; Huang, F.; Lu, Y.J.; Li, G.D.; Bao, L.; Xiao, H.S.; Zhang, X. Peripheral nerve injury induces trans-synaptic modification of channels, receptors and signal pathways in rat dorsal spinal cord. Eur. J. Neurosci. 2004, 19, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Pathobiology of neuropathic pain. Eur. J. Pharmacol. 2001, 429, 23–37. [Google Scholar] [CrossRef]
- Zhou, S.; Bonasera, L.; Carlton, S.M. Peripheral administration of NMDA, AMPA or KA results in pain behaviors in rats. Neuroreport 1996, 7, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Yamaki, F.; Takemura, M.; Koike, Y.; Furuyama, A.; Yonehara, N. Capsaicin-induced glutamate release is implicated in nociceptive processing through activation of ionotropic glutamate receptors and group I metabotropic glutamate receptor in primary afferent fibers. J. Pharmacol. Sci. 2009, 109, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M. Ionotropic glutamate receptors contribute to pain transmission and chronic pain. Neuropharmacology 2017, 112, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Malet, M.; Brumovsky, P.R. VGLUTs and Glutamate Synthesis-Focus on DRG Neurons and Pain. Biomolecules 2015, 5, 3416–3437. [Google Scholar] [CrossRef] [PubMed]
- Christoph, T.; Reissmuller, E.; Schiene, K.; Englberger, W.; Chizh, B.A. Antiallodynic effects of NMDA glycine(B) antagonists in neuropathic pain: Possible peripheral mechanisms. Brain Res. 2005, 1048, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Chia, Y.Y.; Chow, L.H.; Chen, J.J.; Yang, L.C.; Hung, K.C.; Chen, H.S.; Kuo, C.H. Gene knockdown of the N-methyl-d-aspartate receptor NR1 subunit with subcutaneous small interfering RNA reduces inflammation-induced nociception in rats. Anesthesiology 2010, 112, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.E.; Knudsen, E.I. Experience-dependent plasticity and the maturation of glutamatergic synapses. Neuron 1998, 20, 1067–1071. [Google Scholar] [CrossRef]
- Crawford, D.C.; Mennerick, S. Presynaptically silent synapses: Dormancy and awakening of presynaptic vesicle release. Neuroscientist 2012, 18, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Hanse, E.; Seth, H.; Riebe, I. AMPA-silent synapses in brain development and pathology. Nat. Rev. Neurosci. 2013, 14, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Jeong, J.H.; Ko, S.; Yu, X.; Kim, Y.H.; Isaac, J.T.R.; Koretsky, A.P. Peripheral Sensory Deprivation Restores Critical-Period-like Plasticity to Adult Somatosensory Thalamocortical Inputs. Cell Rep. 2017, 19, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.; Li, J.; Cline, H.T. AMPA receptors regulate experience-dependent dendritic arbor growth in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 12127–12131. [Google Scholar] [CrossRef] [PubMed]
- Hickmott, P.W.; Ethell, I.M. Dendritic plasticity in the adult neocortex. Neuroscientist 2006, 12, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Esteban, J.A.; Hayashi, Y.; Malinow, R. Postnatal synaptic potentiation: Delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat. Neurosci. 2000, 3, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Holtmaat, A.J.; Trachtenberg, J.T.; Wilbrecht, L.; Shepherd, G.M.; Zhang, X.; Knott, G.W.; Svoboda, K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron 2005, 45, 279–291. [Google Scholar] [CrossRef] [PubMed]
- De Paola, V.; Holtmaat, A.; Knott, G.; Song, S.; Wilbrecht, L.; Caroni, P.; Svoboda, K. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron 2006, 49, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Ma, L.; Li, W.; Tsai, J.W.; Yang, G.; Gan, W.B. Long-term stability of axonal boutons in the mouse barrel cortex. Dev. Neurobiol. 2016, 76, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.E.; Armstrong-James, M.; Ebner, F.F. Experience-dependent plasticity in adult rat barrel cortex. Proc. Natl. Acad. Sci. USA 1993, 90, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Rema, V.; Armstrong-James, M.; Jenkinson, N.; Ebner, F.F. Short exposure to an enriched environment accelerates plasticity in the barrel cortex of adult rats. Neuroscience 2006, 140, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Kolb, B.; Gorny, G.; Soderpalm, A.H.; Robinson, T.E. Environmental complexity has different effects on the structure of neurons in the prefrontal cortex versus the parietal cortex or nucleus accumbens. Synapse 2003, 48, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Machin, R.; Perez-Cejuela, C.G.; Bjugn, R.; Avendano, C. Effects of long-term sensory deprivation on asymmetric synapses in the whisker barrel field of the adult rat. Brain Res. 2006, 1107, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Gelfo, F.; De Bartolo, P.; Giovine, A.; Petrosini, L.; Leggio, M.G. Layer and regional effects of environmental enrichment on the pyramidal neuron morphology of the rat. Neurobiol. Learn. Mem. 2009, 91, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Negredo, P.; Martin, Y.B.; Lagares, A.; Castro, J.; Villacorta, J.A.; Avendano, C. Trigeminothalamic barrelette neurons: Natural structural side asymmetries and sensory input-dependent plasticity in adult rats. Neuroscience 2009, 163, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Landers, M.S.; Knott, G.W.; Lipp, H.P.; Poletaeva, I.; Welker, E. Synapse formation in adult barrel cortex following naturalistic environmental enrichment. Neuroscience 2011, 199, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Oberlaender, M.; Ramirez, A.; Bruno, R.M. Sensory experience restructures thalamocortical axons during adulthood. Neuron 2012, 74, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Schubert, V.; Lebrecht, D.; Holtmaat, A. Peripheral deafferentation-driven functional somatosensory map shifts are associated with local, not large-scale dendritic structural plasticity. J. Neurosci. 2013, 33, 9474–9487. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, C.E.; Barnes, S.J.; Albieri, G.; Knott, G.W.; Finnerty, G.T. Pansynaptic enlargement at adult cortical connections strengthened by experience. Cereb. Cortex 2014, 24, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Martin, Y.B.; Negredo, P.; Villacorta-Atienza, J.A.; Avendano, C. Trigeminal intersubnuclear neurons: Morphometry and input-dependent structural plasticity in adult rats. J. Comp. Neurol. 2014, 522, 1597–1617. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.B.; Celikel, T.; Feldman, D.E. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat. Neurosci. 2003, 6, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Svoboda, K.; Malinow, R. Experience strengthening transmission by driving AMPA receptors into synapses. Science 2003, 299, 1585–1588. [Google Scholar] [CrossRef] [PubMed]
- Dachtler, J.; Hardingham, N.R.; Glazewski, S.; Wright, N.F.; Blain, E.J.; Fox, K. Experience-dependent plasticity acts via GluR1 and a novel neuronal nitric oxide synthase-dependent synaptic mechanism in adult cortex. J. Neurosci. 2011, 31, 11220–11230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gierdalski, M.; Jablonska, B.; Smith, A.; Skangiel-Kramska, J.; Kossut, M. Deafferentation induced changes in GAD67 and GluR2 mRNA expression in mouse somatosensory cortex. Brain Res. Mol. Brain Res. 1999, 71, 111–119. [Google Scholar] [CrossRef]
- Hebb, D.O. The Organization of Behavior. A Neuropsychological Theory; Wiley: New York, NY, USA, 1949. [Google Scholar]
- Diamond, M.C.; Krech, D.; Rosenzweig, M.R. The Effects of an Enriched Environment on the Histology of the Rat Cerebral Cortex. J. Comp. Neurol. 1964, 123, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Andin, J.; Hallbeck, M.; Mohammed, A.H.; Marcusson, J. Influence of environmental enrichment on steady-state mRNA levels for EAAC1, AMPA1 and NMDA2A receptor subunits in rat hippocampus. Brain Res. 2007, 1174, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Valles, A.; Boender, A.J.; Gijsbers, S.; Haast, R.A.; Martens, G.J.; de Weerd, P. Genomewide analysis of rat barrel cortex reveals time- and layer-specific mRNA expression changes related to experience-dependent plasticity. J. Neurosci. 2011, 31, 6140–6158. [Google Scholar] [CrossRef] [PubMed]
- Staiger, J.F.; Masanneck, C.; Bisler, S.; Schleicher, A.; Zuschratter, W.; Zilles, K. Excitatory and inhibitory neurons express c-Fos in barrel-related columns after exploration of a novel environment. Neuroscience 2002, 109, 687–699. [Google Scholar] [CrossRef]
- Rampon, C.; Jiang, C.H.; Dong, H.; Tang, Y.P.; Lockhart, D.J.; Schultz, P.G.; Tsien, J.Z.; Hu, Y. Effects of environmental enrichment on gene expression in the brain. Proc. Natl. Acad. Sci. USA 2000, 97, 12880–12884. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.P.; Wang, H.; Feng, R.; Kyin, M.; Tsien, J.Z. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology 2001, 41, 779–790. [Google Scholar] [CrossRef]
- Bender, K.J.; Allen, C.B.; Bender, V.A.; Feldman, D.E. Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex. J. Neurosci. 2006, 26, 4155–4165. [Google Scholar] [CrossRef] [PubMed]
- Kubota, J.; Mikami, Y.; Kanemaru, K.; Sekiya, H.; Okubo, Y.; Iino, M. Whisker experience-dependent mGluR signaling maintains synaptic strength in the mouse adolescent cortex. Eur. J. Neurosci. 2016, 44, 2004–2014. [Google Scholar] [CrossRef] [PubMed]
- Turrigiano, G.G.; Leslie, K.R.; Desai, N.S.; Rutherford, L.C.; Nelson, S.B. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 1998, 391, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Urban-Ciecko, J.; Wen, J.A.; Parekh, P.K.; Barth, A.L. Experience-dependent regulation of presynaptic NMDARs enhances neurotransmitter release at neocortical synapses. Learn. Mem. 2014, 22, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Larsen, R.S.; Philpot, B.D.; Paulsen, O. Roles of Presynaptic NMDA Receptors in Neurotransmission and Plasticity. Trends Neurosci. 2016, 39, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Bragina, L.; Bonifacino, T.; Bassi, S.; Milanese, M.; Bonanno, G.; Conti, F. Differential expression of metabotropic glutamate and GABA receptors at neocortical glutamatergic and GABAergic axon terminals. Front. Cell. Neurosci. 2015, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Cahusac, P.M.; Wan, H. Group II metabotropic glutamate receptors reduce excitatory but not inhibitory neurotransmission in rat barrel cortex in vivo. Neuroscience 2007, 146, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Hokfelt, T.; Zhang, X.; Wiesenfeld-Hallin, Z. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci. 1994, 17, 22–30. [Google Scholar] [CrossRef]
- Fu, S.Y.; Gordon, T. The cellular and molecular basis of peripheral nerve regeneration. Mol. Neurobiol. 1997, 14, 67–116. [Google Scholar] [CrossRef] [PubMed]
- Vogelaar, C.F.; Hoekman, M.F.; Gispen, W.H.; Burbach, J.P. Homeobox gene expression in adult dorsal root ganglia during sciatic nerve regeneration: Is regeneration a recapitulation of development? Eur. J. Pharmacol. 2003, 480, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Krames, E.S. The dorsal root ganglion in chronic pain and as a target for neuromodulation: A review. Neuromodulation 2015, 18, 24–32; discussion 32. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Montoya, J.; Martin, Y.B.; Negredo, P.; Avendano, C. Changes in the axon terminals of primary afferents from a single vibrissa in the rat trigeminal nuclei after active touch deprivation or exposure to an enriched environment. Brain Struct. Funct. 2017. [Google Scholar] [CrossRef] [PubMed]


| Gene Protein | Neuron | Satellite Glial Cell | Schwann Cell | Whole Ganglia | ||
|---|---|---|---|---|---|---|
| Body | Central Projection | Peripheral Projection | ||||
| Grik1 Gluk1 | +[46,47] | +[47] +[42] | +[58,94] | +[94] | −[95] * | +[44] |
| Grik2 Gluk2 | +[47] −[26] | +[47] +[42] | +[58,94] | +[26,94] | +[95] * | +[44] |
| Grik3 Gluk3 | +[47] | +[47] +[42] | +[58,94] | +[94] | +[95] * | +[44] |
| Grik4 Gluk4 | +[47] | +[51] | +[95] * | +[44] | ||
| Grik5 Gluk5 | +[47] | +[51] | +[95]* | +[44] | ||
| Gria1 GluA1 | +[46,55] | +[58] | −[54] | +[95] * | +[96] | |
| Gria2/3 GluA2/3 | +[38,46,54,55] | +[36] | +[94] | + [94] | +[95] * | +[96] +[96] |
| Gria4 GluA4 | +[38,54] | +[26,36] | +[26,54] | +[95] * | +[96] | |
| Grin1 GluN1 | +[37,46] +[37,38] | +[58,94] | +[91,94] | +[95] * +[95] * | +[96] +[37] | |
| Grin2A GluN2A | +[26,37] | −[97] | +[26] | +[95] * | +[37,96] −[37] | |
| Grin2B GluN2B | +[37] | +[97] | +[91] | +[95] * +[95] * | +[37,96] +[37,96] | |
| Grin2C GluN2C | +[37] | +[95] * | +[37,98] +[37] | |||
| Grin2D GluN2D | +[37] | +[95] * | +[37,98] +[37] | |||
| Grin3A GluN3A | +[95] * | |||||
| Grin3B GluN3B | +[95] * | |||||
| Grid1 GluD1 | ||||||
| Grid2 GluD2 | ||||||
| Grm1 mGluR1 | +[35,81] | +[81] | +[98] | |||
| Grm2/3 mGluR2/3 | +[35,77,81] | +[77] | +[77] | −[81] | +[96] +[96] | |
| Grm4 mGluR4 | +[99] | +[72] | +[96] | |||
| Grm5 mGluR5 | +[72] | +[72] | +[75] | +[96] | ||
| Grm6 mGluR6 | ||||||
| Grm7 mGluR7 | +[74,77] | +[74,77] | +[96] | |||
| Grm8 mGluR8 | +[26,35,81] | +[26,35,81] | +[96] | |||
| Slc1a3 EAAT1 | +[89] | +[89] | +[89] | +[89] | +[89] | |
| Slc1a2 EAAT2 | −[89] | +[89] | +[89] | +[89] | +[89] | |
| Slc1a1 EAAT3 | +[89] | +[89] | −[89] | +[89] | +[89] | |
| Slc1a6 EAAT4 | ||||||
| Slc1a7 EAAT5 | ||||||
| Slc17a7 VGLUT1 | +[85] +[87,100] | +[84,100] | +[100] | +[85,87] +[84] | ||
| Slc17a6 VGLUT2 | +[85] +[87,100] | +[84,100] | +[100] | +[85,87] | ||
| Slc17a8 VGLUT3 | +[85] +[87] | +[84] | +[31] | +[85,87] +[87] | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Montoya, J.; Avendaño, C.; Negredo, P. The Glutamatergic System in Primary Somatosensory Neurons and Its Involvement in Sensory Input-Dependent Plasticity. Int. J. Mol. Sci. 2018, 19, 69. https://doi.org/10.3390/ijms19010069
Fernández-Montoya J, Avendaño C, Negredo P. The Glutamatergic System in Primary Somatosensory Neurons and Its Involvement in Sensory Input-Dependent Plasticity. International Journal of Molecular Sciences. 2018; 19(1):69. https://doi.org/10.3390/ijms19010069
Chicago/Turabian StyleFernández-Montoya, Julia, Carlos Avendaño, and Pilar Negredo. 2018. "The Glutamatergic System in Primary Somatosensory Neurons and Its Involvement in Sensory Input-Dependent Plasticity" International Journal of Molecular Sciences 19, no. 1: 69. https://doi.org/10.3390/ijms19010069
APA StyleFernández-Montoya, J., Avendaño, C., & Negredo, P. (2018). The Glutamatergic System in Primary Somatosensory Neurons and Its Involvement in Sensory Input-Dependent Plasticity. International Journal of Molecular Sciences, 19(1), 69. https://doi.org/10.3390/ijms19010069




