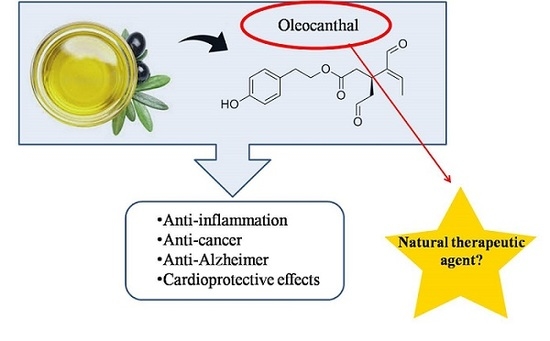
Current Disease-Targets for Oleocanthal as Promising Natural Therapeutic Agent
Abstract
:1. Introduction
2. Oleocanthal
3. Biological Effects of Oleocanthal
3.1. Anti-Inflammatory Properties of Oleocanthal
3.2. Oleocanthal and Inflammatory Arthropathies
3.3. Oleocanthal as Anti-Alzheimer Agent
3.4. Oleocanthal as Anticarcinogenic Agent
3.5. Cardioprotective Effects of Oleocanthal
4. Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | β-amyloid peptides oligomerization |
| ADDLs | Amyloid-derived diffusible ligands |
| EVOO | Extra virgin olive oil |
| HGF | Hepatocyte growth factor |
| LMP | Lysosomal membrane permeabilization |
| mTOR | Mammalian target of rapamycin |
| MUFA | Monounsaturated fatty acid |
| NO | Nitric oxide |
| NSAID | Non-steroidal anti-inflammatory drug |
| OA | Osteoarthritis |
| OC | Oleocanthal |
| PG | Prostaglandin |
| PUFA | Polyunsaturated fatty acids |
| SFA | Saturated fat acids |
| TLRs | Toll-like receptors |
References
- Covas, M.I.; Konstantinidou, V.; Fitó, M. Olive Oil and Cardiovascular Health. J. Cardiovasc. Pharmacol. 2009, 54, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Peyrot des Gachons, C.; Uchida, K.; Bryant, B.; Shima, A.; Sperry, J.B.; Dankulich-Nagrudny, L.; Tominaga, M.; Smith III, A.B.; Beauchamp, G.K.; Breslin, P.A.S. Unusual Pungency from Extra-Virgin Olive Oil Is Attributable to Restricted Spatial Expression of the Receptor of Oleocanthal. J. Neurosci. 2011, 31, 999–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.; Casini, A. Adherence to Mediterranean diet and health status: meta-analysis. Br. Med. J. 2008, 337, a1344.3. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Zhang, H.Y. Multipotent natural agents to combat Alzheimer’s disease. Functional spectrum and structural features. Acta Pharmacol. Sin. 2008, 29, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sperry, J.N.; Crowe, A.; Trojanoswki, J.Q.; Smith III, A.B.; Lee, V.M.Y. Inhibition of tau fibrillization by oleocanthal via reaction with the amino groups of tau. J. Neurochem. 2009, 110, 1339–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith III, A.B.; Sperry, J.B.; Han, Q. Syntheses of (−)-Oleocanthal, a Natural NSAID Found in Extra Virgin Olive Oil, the (−)-Deacetoxy-OleuropeinAglycone, and Related Analogues. J. Org. Chem. 2007, 72, 6891–6900. [Google Scholar] [CrossRef] [PubMed]
- Covas, M.I.; Ruiz-Gutiérrez, V.; de la Torre, R.; Kafatos, A.; Lamuela-Raventós, R.M. Minor components of olive oil: evidence to date of health benefits in humans. Nutr Rev. 2006, 64, 20–30. [Google Scholar] [CrossRef]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, C.M.; Mesa, M.D.; Ramirez-Tortosa, M.C.; Nestares, M.T.; Ros, E.; Gil, A. Sunflower oil does not protect against LDL oxidation as virgin olive oil does in patients with peripheral vascular disease. Clin. Nutr. 2004, 23, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.R.; Edwards, M.C.; Jacobson, T.A. Flaxseed oil supplementation does not affect plasma lipoprotein concentration or particle size in human subjects. J. Nutr. 2006, 136, 2844–2848. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.; Keast, R. Biological Activities of Phenolic Compounds Present in Virgin Olive Oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cioffi, G.; Pesca, M.S.; De Capraiis, P.; Braca, A.; Severin, L.; De Tommasi, N. Phenolic compounds in olive oil and olive pomace from Cilento (Campania, Italy) and their antioxidant activity. Food Chem. 2010, 121, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Gallina-Toschi, T.; Fernandez-Gutierrez, A. Analytical determination of polyphenols in olive oils. J. Sep. Sci. 2005, 28, 837–858. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, R.; Ruiz, C.; Milia, E.; Lorenzo, M.L.; Jimenez, B.; Sánchez-Ortiz, A.; Rivas, A. Phenolic compounds in Extra Virgin Olive Oil stimulate human osteoblastic cell proliferation. PLoS ONE 2016, 11, e0150045. [Google Scholar] [CrossRef] [PubMed]
- Lavelli, V.; Bondesan, L. Secoiridoids, tocopherols, and antioxidant activity of monovarietal extra virgin olive oils extracted from destoned fruits. J. Agric. Food Chem. 2005, 53, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Gallina-Toschi, T.; Cerretani, L.; Bendini, A.; Bonoli-Carbognin, M.; Lercker, G. Oxidative stability and phenolic content of virgin olive oil: an analytical approach by traditional and high resolution techniques. J. Sep. Sci. 2005, 28, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Ferri, C. Flavonoids: Antioxidants against atherosclerosis. Nutrients 2010, 2, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.; Hidalgo, F.J.; Garcia, A.; Rios, J.; Garcia, P.; Zamora, R.; Garrido-Fernández, A. Pinoresinol and 1-acetoxypinoresinol, two new phenolic compounds identified in olive oil. J. Am. Oil Chem. Soc. 2000, 77, 715–720. [Google Scholar] [CrossRef]
- Montedoro, G.; Servili, M.; Baldioli, M.; Selvaggini, R.; Miniati, E.; Macchioni, A. Simple and hydrolyzable compounds in virgin olive oil. 3. Spectroscopic characterizations of the secoiridoid derivatives. J. Agric. Food Chem. 1993, 41, 2228–2234. [Google Scholar] [CrossRef]
- Andrewes, P.; Busch, J.L.H.C.; De Joode, T.; Groenewegen, A.; Alexandre, H. Sensory properties of Virgin Olive Oil polyphenols: Identification of deacetoxy-ligstrosideaglycon as a key contributor to pungency. J. Agric. Food Chem. 2003, 51, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.M.; Ritieni, A.; Sacchi, R.; Skog, K.; Borgen, E.; Fogliano, V. Characterization of phenolic compounds in Virgin Olive Oil and their effect on the formation of carcinogenic/mutagenic heterocyclic amines in a model system. J. Agric. Food Chem. 2001, 49, 3969–3975. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, R. Bioavailability of olive oil phenolic compounds in humans. Inflammopharmacology 2008, 16, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.; Keast, R.; Morel, D.; Liu, J.; Pika, J.; Han, Q.; Lee, C.; Smith III, A.B.; Breslin, P. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, J.; Lin, J. A simple High-Performance Liquid Chromatography Method for the determination of throat-burning oleocanthal with probated antiinflammatory activity in Extra Virgin Olive Oils. J. Agric. Food Chem. 2006, 54, 3204–3208. [Google Scholar] [CrossRef] [PubMed]
- Fogliano, V.; Sacchi, R. Oleocanthal in olive oil: Between myth and reality. Mol. Nutr. Food Res. 2006, 50, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Alarcón-de-la-Lastra, C. An up-date of Olive Oil Phenols in inflammation and cancer: Molecular mechanisms and clinical implications. Curr. Med. Chem. 2013, 20, 4758–4776. [Google Scholar] [CrossRef] [PubMed]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Direct measurement of oleocanthal and oleacein levels in olive oil by quantitative 1H NMR. Establishment of a new index for the characterization of extra virgin olive oils. J. Agric. Food Chem. 2012, 60, 11696–11703. [Google Scholar] [CrossRef] [PubMed]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Quantitative measurement of major secoiridoid derivatives in olive oil using qNMR. Proof of the artificial formation of aldehydicoleuropein and ligstrosideaglycon isomers. J. Agric. Food Chem. 2014, 62, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Kotsiou, A.; Tesseromatis, C. Oleocanthal an extra-virgin olive oil bioactive component. J. Med. Plants Stud. 2017, 5, 95–100. [Google Scholar]
- Boyd, L.A.; McCann, M.J.; Hashim, Y.; Bennett, R.N.; Gill, C.I.; Rowland, I.R. Assessment of the anti-genotoxic, anti-proliferative, and anti-metastatic potential of crude watercress extract in human colon cancer cells. Nutr. Cancer 2006, 55, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Y.J. The vanilloid receptor TRPV1: role in cardiovascular and gastrointestinal protection. Eur. J. Pharmacol. 2010, 627, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Caruso, D.; Plasmati, E.; Patelli, R.; Mulinacci, N.; Romani, A.; Galli, G.; Galli, C. Hydroxytyrosol, as a component of olive mill waste water, is dose- dependently absorbed and increases the antioxidant capacity of rat plasma. Free Radical Res. 2001, 34, 301–305. [Google Scholar] [CrossRef]
- Breslin, P.A.S.; Gingerich, T.N.; Green, B.G. Ibuprofen as a chemesthetic stimulus: evidence of a novel mechanism of throat irritation. Chem. Sens. 2001, 26, 55–66. [Google Scholar] [CrossRef]
- Lucas, L.; Russell, A.; Keast, R. Molecular Mechanisms of Inflammation. Anti-Inflammatory Benefits of Virgin Olive Oil and the Phenolic Compound Oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Scotece, M.; Gómez, R.; Conde, J.; Lopez, V.; Gómez-Reino, J.J.; Lago, F.; Smith, A.B., III; Gualillo, O. Further evidence for the anti-inflammatory activity of oleocanthal: Inhibition of MIP-1α and IL-6 in J774 macrophages and in ATDC5 chondrocytes. Life Sci. 2012, 91, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, C.; Lin, C.; Chen, L.C.; Chang, W.C.; Tsai, S.J. Distinct regulation of cyclooxygenase-2 by interleukin-1beta in normal and endometriotic stromal cells. J. Clin. Endocrinol. Metab. 2005, 90, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Howe, L. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res. 2007, 9, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Müller-Decker, K.; Fürstenberger, G. The cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesis. Mol. Carcinog. 2007, 46, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pergola, C.; Werz, O. 5-Lipoxygenase inhibitors: a review of recent developments and patents. Expert Opin. Ther. Pat. 2010, 20, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Vougogiannopoulou, K.; Lemus, C.; Halabalaki, M.; Pergola, C.; Werz, O.; Smith III, A.B.; Michel, S.; Skaltsounis, L.; Deguin, B. One-Step semisynthesis of oleacein and the determination as a 5-Lipoxygenase inhibitor. J. Nat. Prod. 2014, 77, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Werz, O. Inhibition of 5-lipoxygenase product synthesis by natural compounds of plant origin. Planta Med. 2007, 73, 1331–1357. [Google Scholar] [CrossRef] [PubMed]
- Abramson, S.B. Osteoarthritis and nitric oxide. Osteoarthr. Cartilage 2008, 16, S15–S20. [Google Scholar] [CrossRef]
- Gómez, R.; Villalvilla, A.; Largo, R.; Gualillo, O.; Herrero-Beaumont, G. TLR4 signalling in osteoarthritis-finding targets for candidate DMOADs. Nat. Rev. Rheumatol. 2015, 11, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Iacono, A.; Gómez, R.; Sperry, J.; Conde, J.; Bianco, G.; Meli, R.; Gómez-Reino, J.J.; Smith, A.B., III; Gualillo, O. Effect of oleocanthal and its derivatives on inflammatory response induced by lipopolysaccharide in a murine chondrocyte cell line. Arthritis Rheum. 2010, 62, 1675–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvinge, H.; Bertone, P. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr. Cartilage 2005, 13, 769–781. [Google Scholar] [Green Version]
- Gómez, R.; Conde, J.; Scotece, M.; Gómez-Reino, J.J.; Lago, F.; Gualillo, O. What’s new in our understanding of the role of adipokines in rheumatic diseases? Nat. Rev. Rheumatol. 2011, 7, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Beaumont, G.; Roman-Blas, J.A. Osteoarthritis: Osteoporotic OA: a reasonable target for bone-acting agents. Nat. Rev. Rheumatol. 2013, 9, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Cho, M.L.; Choi, H.Y.; Yoon, C.S.; Jhun, J.Y.; Oh, H.J.; Kim, H.Y. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006, 54, 2152–2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, L.A. TLRs: Professor Mechnikov, sit on your hat. Trends Immunol. 2004, 25, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.; O’Neill, L.A. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J. Leukoc. Biol. 2000, 67, 508–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Abramson, S.B.; Attur, M.; Amin, M.R.; Clancy, R. Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis. Curr. Rheumatol. Rep. 2001, 3, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Hepler, R.W.; Grimm, K.M.; Nahas, D.D.; Breese, R.; Dodson, E.C.; Acton, P.; Keller, P.M.; Yeager, M.; Wang, H.; Shughrue, P.; et al. Solution state characterization of amyloid beta-derived diffusible ligands. Biochemistry 2006, 45, 15157–15167. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.X.; Iqbal, K. Hyperphosphorylation of microtubule-associated protein Tau: A promising therapeutic target for alzheimer disease. Curr. Med. Chem. 2008, 15, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Szekely, C.A.; Breitner, J.C.; Fitzpatrick, A.L.; Rea, T.D.; Psaty, B.M.; Kuller, L.H.; Zandi, P.P. NSAID use and dementia risk in the Cardiovascular Health Study: role of APOE and NSAID type. Neurology 2008, 70, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, D.; Coen, K.; De Deyn, P. Ibuprofen modifies cognitive disease progression in an Alzheimer’s mouse model. J. Psychopharmacol. 2010, 24, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Vlad, S.C.; Miller, D.R.; Kowall, N.W.; Felson, D.T. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 2008, 70, 1672–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.A.; Carreras, I.; Hossain, L.; Ryu, H.; Kelin, W.L.; Oddo, S.; LaFerla, F.M.; Jenkins, J.G.; Kowal, N.W.; Dedeoglu, A. Ibuprofen reduces Abeta, hyperphosphorylated tau and memory deficits in Alzheimer mice. Brain Res. 2008, 1207, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zhang, K.; Liu, H.; Babu-Khan, S.; Vassar, R.; Biere, A.L.; Citron, M.; Landreth, G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. J. Neurosci. 2003, 23, 7504–7509. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.; Roth, W.; Lacor, P.; Smith III, A.B.; Blankenship, M.; Velasco, P.; De Felice, F.; Breslin, P.; Klein, W.L. Alzheimer’s-associated Abeta oligomers show altered structure, immunoreactivity and synaptotoxicity with low doses of oleocanthal. Toxicol. Appl. Pharmacol. 2009, 240, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Abuznait, A.H.; Qosa, H.; Busnena, B.A.; El Sayed, K.A.; Kaddoumi, A. Olive-oil-derived oleocanthal enhances β-amyloid clearance as a potential neuroprotective mechanism against Alzheimer’s disease: in vitro and in vivo studies. ACS Chem. Neurosci. 2013, 4, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Qosa, H.; Batarseh, Y.S.; Mohyeldin, M.M.; El Sayed, K.A.; Keller, J.N.; Kaddoumi, A. Oleocanthal enhances amyloid-β clearance from the brains of TgSwDI mice and in vitro across a human blood-brain barrier model. ACS Chem. Neurosci. 2015, 6, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Batarseh, Y.S.; Kaddoumi, A. Oleocanthal-rich extra-virgin olive oil enhances donepezil effect by reducing amyloid-β load and related toxicity in a mouse model of Alzheimer’s disease. J. Nutr. Biochem. 2017, 55, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.C.; Margarucci, L.; Riccio, R.; Casapullo, A. Modulation of Tau protein fibrillization by oleocanthal. Nat. Prod. 2012, 75, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.C.; Margarucci, L.; Tosco, A.; Riccio, R.; Casapullo, A. New insights on the interaction mechanism between tau protein and oleocanthal, an extra-virgin olive-oil bioactive component. Food Funct. 2011, 2, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Akl, M.R.; Ayoub, N.M.; Mohyeldin, M.M.; Busnena, B.A.; Foudah, A.I.; Liu, Y.Y.; EI Sayed, K.A. (−)-Oleocanthal attenuates cell proliferation, invasiveness, and tumor growth in breast cancer models. PLoS ONE 2014, 9, e97622. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Meng, Q.; Han, J.; Sun, H.; Li, L.; Song, R.; Sun, B.; Pan, S.; Liang, D.; Liu, L. (−)-Oleocanthal inhibits growth and metastasis by blocking activation of STAT3 in human hepatocellular carcinoma. Oncotarget 2016, 7, 43475–43491. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A.; Shanmugam, M.K.; Perumal, E.; Li, F.; Nachiyappan, A.; Dai, X.; Swamy, S.N.; Ahn, K.S.; Kumar, A.P.; Tan, B.K.; et al. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim. Biophys. Acta. 2013, 1835, 46–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polini, B.; Digiacomo, M.; Carpi, S.; Bertini, S.; Gado, F.; Saccomanni, G.; Macchia, M.; Nieri, P.; Manera, C.; Fogli, S. Oleocanthal and oleacein contribute to the in vitro therapeutic potential of extra virgin oil-derived extracts in non-melanoma skin cancer. Toxicol. In Vitro 2018, 52, 243–250. [Google Scholar] [CrossRef] [PubMed]
- LeGendre, O.; Breslin, P.A.S.; Foster, D.A. (−)-Oleocanthal rapidly and selectively induces cancer cell death via lysosomal membrane permeabilization. Mol. Cell Oncol. 2015, 2, e1006077. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.M.; Siddiqueb, A.B.; Ebrahimb, H.Y.; Mohyeldinb, M.M.; El Sayedb, K.A. The olive oil phenolic (−)-oleocanthal modulates estrogen receptor expression in luminal breast cancer in vitro and in vivo and synergizes with tamoxifen treatment. Eur. J. Pharmacol 2017, 810, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, J.; Peng, L. (−)-Oleocanthal exerts anti-melanoma activities and inhibits STAT3 signaling pathway. Oncol. Rep. 2017, 37, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Khanal, P.; Oh, W.K.; Yun, H.J.; Namgoong, G.M.; Ahn, S.G.; Kwon, S.M.; Choi, H.K.; Choi, H.S. p-HPEA-EDA, a phenolic compound of virgin olive oil, activates AMP-activated protein kinase to inhibit carcinogenesis. Carcinogenesis 2011, 32, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Borowiak, M.; Garratt, A.N.; Wustefeld, T.; Strehle, M.; Trautwein, C.; Birchmeier, C. Met provides essential signals for liver regeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 10608–10613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, C.G.; Factor, V.M.; Sanchez, A.; Uchida, K.; Conner, E.A.; Thorgeirsson, S.S. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc. Natl. Acad. Sci. USA 2004, 101, 4477–4482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra, J.R.; Tsao, M.S. c-MET as a potential therapeutic target and biomarker in cáncer. Ther. Adv. Med. Oncol. 2011, 3, S21–S35. [Google Scholar] [CrossRef] [PubMed]
- Bellon, S.; Kaplan-Lefko, P.; Yang, Y.; Zhang, Y.; Moriguchi, J.; Rex, K.; Johnson, C.; Rose, P.; Long, A.; OʼConnor, A.; et al. c-Met inhibitors with novel binding mode show activity against several hereditary papillary renal cell carcinoma-related mutations. J. Biol. Chem. 2008, 283, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Phromnoi, K.; Yadav, V.R.; Chaturvedi, M.M.; Aggarwal, B.B. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med. 2010, 76, 1044–1063. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, S.; Comoglio, P.M. The MET receptor tyrosine kinase in invasion and metastasis. J. Cell Physiol. 2007, 213, 316–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elnagar, A.Y.; Sylvester, P.W.; El Sayed, K.A. (−)-Oleocanthal as a c-Met inhibitor for the control of metastatic breast and prostate cancers. Planta Med. 2011, 77, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.X.; Huang, F.J.; Aldape, K.D.; Kang, S.H.; Liu, M.; Gershenwald, J.E.; Xie, K.; Sawaya, R.; Huang, S. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006, 66, 3188–3196. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, A.; Balasus, D.; Azzolina, A.; Augello, G.; Emma, M.R.; Di Sano, C.; Gramignoli, R.; Strom, S.C.; McCubrey, J.A.; Montalto, G.; et al. Oleocanthal exerts antitumor effects on human liver and colon cancer cells through ROS generation. Int. J. Oncol. 2017, 51, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Voiculescu, V.; Calenic, B.; Ghita, M.; Lupu, M.; Caruntu, A.; Moraru, L.; Voiculescu, S.; Ion, A.; Greabu, M.; Ishkitiev, N.; et al. From normal skin to squamous cell carcinoma: a quest for novel biomarkers. Dis. Markers 2016, 4517492. [Google Scholar] [CrossRef] [PubMed]
- Boya, P.; Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 2008, 27, 6434–6451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holowatyj, A.N.; Ruterbusch, J.J.; Ratnam, M.; Gorski, D.H.; Cote, M.L. HER2 status and disparities in luminal breast cancers. Cancer Med. 2016, 5, 2109–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.H.; Hu, P.H.; Tu, J.H.; Yu, N.S. Luminal B breast cancer: patterns of recurrence and clinical outcome. Oncotarget 2016, 7, 65024–65033. [Google Scholar] [CrossRef] [PubMed]
- Khanfar, M.A.; Bardaweel, S.K.; Akl, M.R.; El Sayed, K.A. Olive oil-derived oleocanthal as potent inhibitor of mammalian target of rapamycin: Biological evaluation and molecular modeling studies. Phytother. Res. 2015, 29, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, A.; Majumder, S.; Richardson, A.; Strong, R.; Oddo, S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J. Biol. Chem. 2010, 285, 13107–13120. [Google Scholar] [CrossRef] [PubMed]
- Don, A.S.; Zheng, X.F. Recent clinical trials of mTOR-targeted cancer therapies. Rev. Recent. Clin. Trials 2011, 6, 24–35. [Google Scholar] [PubMed]
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, K.; McCarthy, A.; Smith, D.; Green, K.A.; Grahame Hardie, D.; Ashworth, A.; Alessi, D.R. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005, 24, 1810–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.T.; Park, I.J.; Shin, J.I.; Lee, Y.K.; Lee, S.K.; Baik, H.W.; Ha, J.; Park, O.J. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2005, 338, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Margarucci, L.; Monti, M.C.; Cassiano, C.; Mozzicafreddo, M.; Angeletti, M.; Riccio, R.; Tosco, A.; Casapullo, A. Chemical proteomics-driven discovery of oleocanthal as an Hsp90 inhibitor. Chem. Commun. 2013, 49, 5844–5846. [Google Scholar] [CrossRef] [PubMed]
- Mbofung, R.M.; McKenzie, J.A.; Malu, S.; Zhang, M.; Peng, W.; Liu, C.; Kuiatse, I.; Tieu, T.; Williams, L.; Devi, S.; et al. HSP90 inhibition enhances cancer immunotherapy by upregulating interferon response genes. Nat. Commun. 2017, 8, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, S.C.; Burlison, J.A.; Blagg, B.S.J. Hsp90: A novel target for the disruption of multiple signaling cascades. Curr. Cancer Drug Targets 2007, 4, 369–388. [Google Scholar] [CrossRef]
- May, A.E.; Seizer, P.; Gawaz, M. Platelets: inflammatory firebugs of vascular walls. Arterioscler. Thromb. Vasc. Biol. 2008, 28, S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Melliou, E.; Li, X.; Pedersen, T.L.; Wang, S.C.; Magiatis, P.; Newmana, J.W.; Holt, R.R. Oleocanthal-rich extra virgin olive oil demonstrates acute anti-platelet effects in healthy men in a randomized trial. J. Funct. Foods 2017, 36, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Adhami, H.R.; Zehl, M.; Dangl, C.; Dorfmeister, D.; Stadler, M.; Urban, E.; Hewitson, P.; Ignatova, S.; Krenn, L. Preparative isolation of oleocanthal, tyrosol, and hydroxytyrosol from olive oil by HPCCC. Food Chem. 2015, 170, 154–159. [Google Scholar] [CrossRef] [PubMed]



| Polyphenolic Groups | Characteristics | Phenolic Compounds | References |
|---|---|---|---|
| Phenolic acids | Based on the chemical structure of C6–C1 for benzoic acids and C6–C3 for cinnamic acids derivatives | gallic acid, vanillic acid, caffeic acid, syringic acid, o-coumaric acid, protocatechuic acid, p-hydroxybenzoic acid, sinapic acid | [13] |
| Phenolic alcohols | Showing a hydroxyl group attached to an aromatic hydrocarbon group | hydroxytyrosol, tyrosol | [14] |
| Secoiridoids | Characterized by the presence of either elenolic acid or elenolic acid derivatives | Oleuropeinaglycone, demethyloleuropein, ligstrosideaglycone, nuzenide | [13,15] |
| Hydroxy-isocromans | Constituted by 3,4-dihydro-1H-benzo[c]pyran derivatives | 1-(3-methoxy-4-hydroxy)phenyl-6,7-dihydroxyisochroman, 1,phenyl-6,7-dihydroxy-isochroman | [13,16,17] |
| Flavonoids | Characterized by two benzene rings joined by a linear three carbon chain. Sometimes glycosilated. They can be further divided into flavones and flavanols | apigenine, luteoline, (+)-taxifoline, rutin, luteolin-7-glucoside, glycosides of delphinidin | [16] |
| Lignans | The structure is based on aromatic aldehydes condensation | pinoresinol (P), 1-acetoxypinoresinol, hydroxypinoresinol | [18] |
| Animal Model | Damaging Agent | Treatment | Duration | Oleocanthal Cancer Target | Effects | Reference |
|---|---|---|---|---|---|---|
| Nude mice | Injection of 5 × 106 A375 cells in 200 μl of PBS. Human melanoma | Oleocanthal or DMSO 15 mg/kg/day | 1 week | Signal transducerand activator of transcription 3 (STAT3) | Significant decrease of tumor size. Ki-67 and CD31, markers of proliferation and angiogenesis respectively, were significantly decreased | [74] |
| Athymic nude mice | Injection of 1 × 106 MDA-MB-231/GFP cells Human breast cancer | Oleocanthal or DMSO 5 mg/kg/day | 4 weeks | HGF and c-Met | Reduction of 60% in tumor growth. Ki-67 and CD31 markers were significantly decreased | [68] |
| Male BALB/c athymic nude mice | Injection of 4 × 106 HCCLM3-luc cells in 150 μL of PBS Human hepatocellular Carcinoma | Oleocanthal or DMSO 5 or 10 mg/kg/day | 5 weeks | Signal transducerand activator of transcription 3 (STAT3) | Tumor gross reduction Ki-67 marker was decreased Increasing of apoptotic cells in a dose dependent manner | [69] |
| Fertilized chicken eggs | Injection of 2 × 106 HT-29 cells Human colon carcinoma | Oleocanthal or saline solution (50 µg/mL) | 3 days | Cyclooxygenase-2 (COX-2) and Adenosine Monophosphate-activated Protein Kinase(AMPK) | HT-29 cells inhibition AMPK significantly induced | [75] |
| Female athymic nude mice | Injection of 1 × 107 BT-474 cells Human luminal breast cancer | Oleocanthal or DMSO 5 or 10 mg/kg/day | >8 weeks | Estrogen receptors α (ERα) | Significant reduction in tumor growth and volume | [73] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segura-Carretero, A.; Curiel, J.A. Current Disease-Targets for Oleocanthal as Promising Natural Therapeutic Agent. Int. J. Mol. Sci. 2018, 19, 2899. https://doi.org/10.3390/ijms19102899
Segura-Carretero A, Curiel JA. Current Disease-Targets for Oleocanthal as Promising Natural Therapeutic Agent. International Journal of Molecular Sciences. 2018; 19(10):2899. https://doi.org/10.3390/ijms19102899
Chicago/Turabian StyleSegura-Carretero, Antonio, and Jose Antonio Curiel. 2018. "Current Disease-Targets for Oleocanthal as Promising Natural Therapeutic Agent" International Journal of Molecular Sciences 19, no. 10: 2899. https://doi.org/10.3390/ijms19102899
APA StyleSegura-Carretero, A., & Curiel, J. A. (2018). Current Disease-Targets for Oleocanthal as Promising Natural Therapeutic Agent. International Journal of Molecular Sciences, 19(10), 2899. https://doi.org/10.3390/ijms19102899