Inhibitory and Inductive Effects of Opuntia ficus indica Extract and Its Flavonoid Constituents on Cytochrome P450s and UDP-Glucuronosyltransferases
Abstract
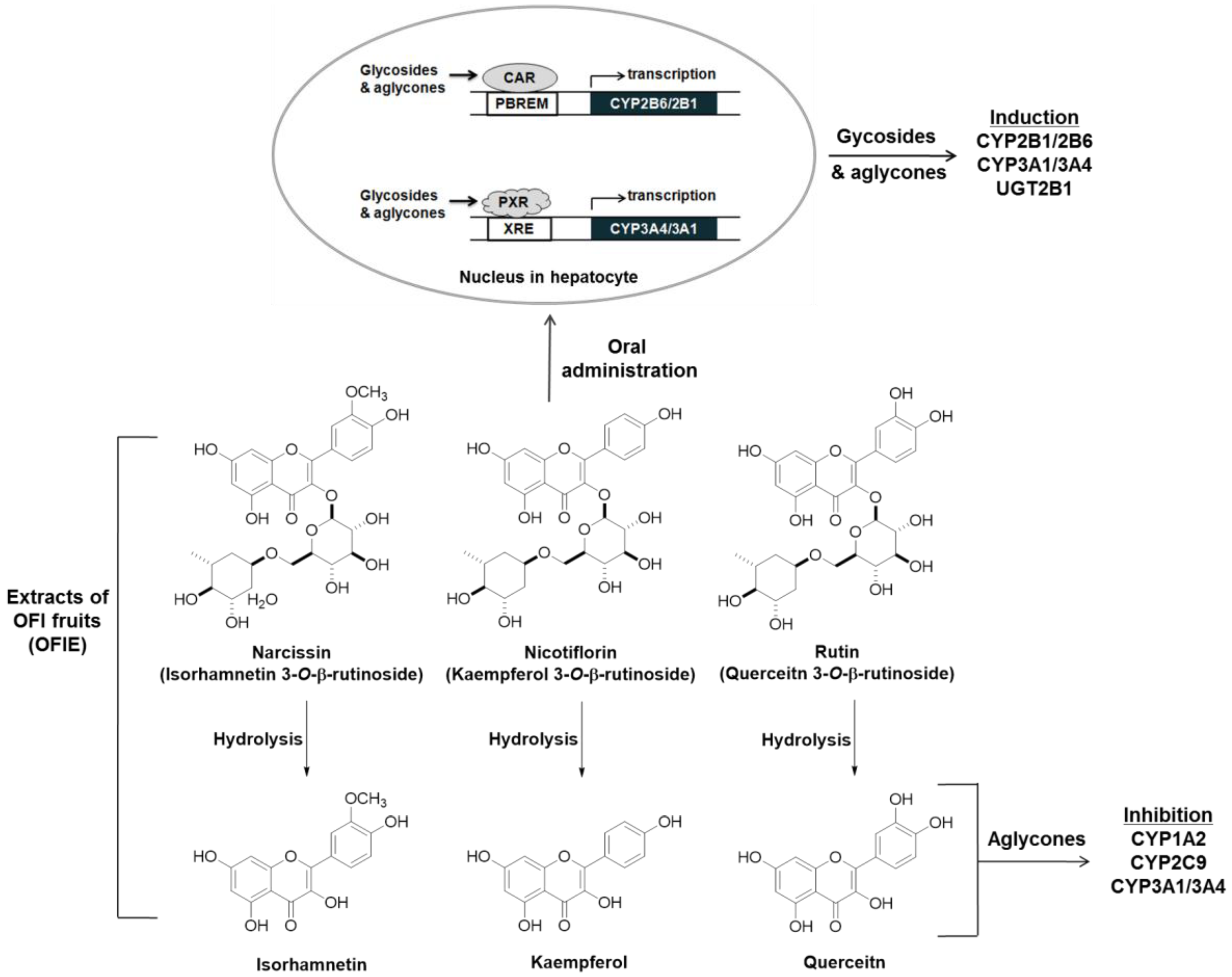
:1. Introduction
2. Results
2.1. Inductive Effects of Oral Administration of OFIE on the Hepatic CYPs and UGTs Genes
2.2. Induction of Catalytic Activities of Liver Microsomal CYPs and UGTs by the Oral Administration of OFIE
2.3. Effects of OFIE on CYP2B6 and CYP3A4 Promoter Activities via CAR and PXR Transactivation
2.4. Inhibition of Activities of CYPs and UGTs in Liver Microsomes by OFIE
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagent
4.2. Animals
4.3. Ovariectomy, Oral Administration of Opuntia ficus indica Extract, and Preparation of Liver Microsomes
4.4. Quantitative Polymerase Chain Reaction (qPCR) Analysis
4.5. CYP and UGT Activity and Inhibition Assays
4.6. HPLC Instrumentation and Tandem Mass Spectrometry Analysis
4.7. Cell Culture
4.8. Plasmid Transfection and Luciferase Reporter Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fasinu, P.S.; Bouic, P.J.; Rosenkranz, B. An overview of the evidence and mechanisms of herb–drug interactions. Front Pharmacol. 2012, 3, 69. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-X.; Yi, X.-L.; Si, D.-Y.; Xiao, X.-F.; He, X.; Li, Y.-Z. Herb-drug interactions involving drug metabolizing enzymes and transporters. Curr. Drug Metab. 2011, 12, 835–849. [Google Scholar] [CrossRef] [PubMed]
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug metabolism in the liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.G.; McLachlan, A.J.; de Cabo, R. Aging, drugs, and drug metabolism. J. Gerontol. 2012, 67, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Dijk, D.J.; James, L.M.; Peters, S.; Walsh, J.K.; Deacon, S. Sex differences and the effect of gaboxadol and zolpidem on eeg power spectra in nrem and rem sleep. J. Psychopharmacol. 2010, 24, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.H.; Jurkovic, S.; Yang, K.; Choi, S.Y.; Jung, J.W.; Kim, K.P.; Zhang, W.; Jeong, H. Estradiol induces cytochrome p450 2b6 expression at high concentrations: Implication in estrogen-mediated gene regulation in pregnancy. Biochem. Pharmacol. 2012, 84, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.A.; Avery, A.J.; Duerden, M.; Saunders, C.L.; Simpson, C.R.; Abel, G.A. Prevalence of polypharmacy in a scottish primary care population. Eur. J. Clin. Pharmacol. 2014, 70, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Schieber, A.; Carle, R. Amino acid composition and betaxanthin formation in fruits from Opuntia ficus-indica. Planta Med. 1999, 65, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Hwang, K.H.; Han, D.H.; Kim, S.D. Compositions of Opuntia ficus-indica. Korean J. Food Sci. Technol. 1997, 29, 847–853. [Google Scholar]
- Hwang, J.H.; Lim, S.B. Immunostimulatory activity of Opuntia ficus-indica var. Saboten cladodes fermented by Lactobacillus plantarum and Bacillus subtilis in raw 264.7 macrophages. J. Med. Food 2017, 20, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Gonzalez, A.; Gabriel-Ortiz, G.; Puebla-Perez, A.M.; Huizar-Contreras, M.D.; Munguia-Mazariegos, M.R.; Mejia-Arreguin, S.; Calva, E. A purified extract from prickly pear cactus (Opuntia fuliginosa) controls experimentally induced diabetes in rats. J. Ethnopharmacol. 1996, 55, 27–33. [Google Scholar] [CrossRef]
- An, B.H.; Jeong, H.; Zhou, W.; Liu, X.; Kim, S.; Jang, C.Y.; Kim, H.-S.; Sohn, J.; Park, H.-J.; Sung, N.-H.; et al. Evaluation of the biological activity of Opuntia ficus indica as a tissue- and estrogen receptor subtype-selective modulator. Phytother. Res. 2016, 30, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; An, B.H.; Zhou, W.; Liu, X.; Song, Y.S.; Chang, M. Enzymatic deglycosylation of Opuntia ficus indica improves its estrogen receptor-subtype selective transcriptional and anti-inflammatory activities. J. Nutr. Food Sci. 2016, 6, 1000568. [Google Scholar] [CrossRef]
- Moussa-Ayoub, T.E.; El-Samahy, S.K.; Kroh, L.W.; Rohn, S. Identification and quantification of flavonol aglycons in cactus pear (Opuntia ficus indica) fruit using a commercial pectinase and cellulase preparation. Food Chem. 2011, 124, 1177–1184. [Google Scholar] [CrossRef]
- Ginestra, G.; Parker, M.L.; Bennett, R.N.; Robertson, J.; Mandalari, G.; Narbad, A.; Lo Curto, R.B.; Bisignano, G.; Faulds, C.B.; Waldron, K.W. Anatomical, chemical, and biochemical characterization of cladodes from prickly pear [Opuntia ficus-indica (L.) Mill.]. J. Agri. Food Chem. 2009, 57, 10323–10330. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, J.A.; Almela, L.; Obon, J.M.; Castellar, R. Determination of antioxidant constituents in cactus pear fruits. Plant Foods Hum. Nutr. 2010, 65, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Krizkova, J.; Burdova, K.; Stiborova, M.; Kren, V.; Hodek, P. The effects of selected flavonoids on cytochromes p450 in rat liver and small intestine. Interdisc. Toxicol. 2009, 2, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.K.; Chen, J.; Teng, X.W. Distinct role of bilobalide and ginkgolide a in the modulation of rat cyp2b1 and cyp3a23 gene expression by ginkgo biloba extract in cultured hepatocytes. Drug Metab. Dispos. 2006, 34, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Bi, H.C.; Zhao, L.Z.; He, F.; Liu, Y.Q.; Yu, J.J.; Ou, Z.M.; Ding, L.; Chen, X.; Huang, Z.Y.; et al. Induction of cytochrome p450s by terpene trilactones and flavonoids of the Ginkgo biloba extract Egb 761 in rats. Xenobiotica 2008, 38, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Willson, T.M.; Kliewer, S.A. PXR and CAR and drug metabolism. Nat. Rev. Drug Discov. 2002, 1, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Swales, K.; Kakizaki, S.; Yamamoto, Y.; Inoue, K.; Kobayashi, K.; Negishi, M. Novel CAR-mediated mechanism for synergistic activation of two distinct elements within the human cytochrome P450 2B6 gene in HepG2 cells. J. Biol. Chem. 2005, 280, 3458–3466. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Luo, M.; Jiang, H.; Yu, L.; Zeng, S. Involvement of CAR and PXR in the transcriptional regulation of CYP2B6 gene expression by ingredients from herbal medicines. Xenobiotica 2015, 45, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.Y.; Sueyoshi, T.; Negishi, M.; Chang, T.K. Identification of Ginkgo biloba as a novel activator of pregnane X receptor. Drug Metab. Dispos. 2008, 36, 2270–2276. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Stanton, J.D.; Tolson, A.H.; Luo, Y.; Wang, H. Bioactive terpenoids and flavonoids from Ginkgo biloba extract induce the expression of hepatic drug-metabolizing enzymes through pregnane X receptor, constitutive androstane receptor, and aryl hydrocarbon receptor-mediated pathways. Pharm. Res. 2009, 26, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, T.A.; Montoro, P. Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J. Chromatogr. A 2009, 1216, 2002–2032. [Google Scholar] [CrossRef] [PubMed]
- Umegaki, K.; Saito, K.; Kubota, Y.; Sanada, H.; Yamada, K.; Shinozuka, K. Ginkgo biloba extract markedly induces pentoxyresorufin O-dealkylase activity in rats. Jpn. J. Pharmacol. 2002, 90, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Von Moltke, L.L.; Weemhoff, J.L.; Bedir, E.; Khan, I.A.; Harmatz, J.S.; Goldman, P.; Greenblatt, D.J. Inhibition of human cytochromes P450 by components of Ginkgo biloba. J. Pharm. Pharmacol. 2004, 56, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.F.; Connick, J.P.; Reed, J.R.; Backes, W.L.; Desai, M.C.; Xu, L.; Estrada, D.F.; Laurence, J.S.; Scott, E.E. Correlating structure and function of drug-metabolizing enzymes: Progress and ongoing challenges. Drug Metab. Dispos. 2014, 42, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kim, S.; Lee, J.; Park, J.Y.; Zhou, W.; Liu, X.; Kim, S.D.; Song, Y.S.; Jang, C.Y.; Oh, S.R.; et al. Characterization of phase I and phase II hepatic metabolism and reactive intermediates of Larrea nitida cav. And its lignan compounds. Phytother. Res. 2017, 31, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Obach, R.S. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: An examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab. Dispos. 1999, 27, 1350–1359. [Google Scholar] [PubMed]





| CYP Isozyme (Rat/Human) | Phenotyping Reaction | % Inhibition in OVX Rat Hepatic Microsomes a | % Inhibition in Human Hepatic Microsomes | ||
|---|---|---|---|---|---|
| OFIE | Hydrolyzed (hdl) OFIE b | OFIE | Hydrolyzed (hdl) OFIE | ||
| 1A2/1A2 | PCOD c | 16 ± 1.4 d | 22 ± 0.49 | 19 ± 2.3 | 41 ± 0.93 |
| 2B1/2B6 | BPHY | 4.3 ± 0.53 | 1.2 ± 0.44 | 5.8 ± 0.84 | 1.7 ± 1.4 |
| 2C11/2C9 | TOLHY | 0.7 ± 0.69 | 16 ± 0.55 | 6.8 ± 1.3 | 27 ± 1.4 |
| 2D1/2D6 | DEXOD | 16 ± 0.25 | 18 ± 0.53 | 5.5 ± 5.0 | 3.7 ± 6.8 |
| 3A1/3A4 | TSTHY | 0.70 ± 0.25 | 40 ± 0.43 | 19 ± 1.8 | 49 ± 2.0 |
| UGT Isozyme (Rat/Human) | Phenotyping Reaction | % Inhibition in OVX Rat Hepatic Microsomes | % Inhibition in Human Hepatic Microsomes | ||
|---|---|---|---|---|---|
| OFIE | Hydrolyzed (hdl) OFIE | OFIE | Hydrolyzed (hdl) OFIE | ||
| 1A1/1A1 | ESG a | −1.6 ± 1.9 b | −7.2 ± 1.9 | 16 ± 2.3 | 21 ± 2.9 |
| 1A3/1A3 | CDCAG | 2.9 ± 2.7 | −5.0 ± 1.2 | 11 ± 0.9 | 16 ± 6.0 |
| 1A6/1A6 | NPG | −4.7 ± 1.8 | −9.7 ± 0.50 | 8.9 ± 1.6 | 7.5 ± 1.2 |
| 1A7/1A9 | MPAG | 7.7 ± 0.26 | 11 ± 2.8 | 8.2 ± 2.1 | 3.1 ± 4.9 |
| Rat 2B1 | TSTG | 0.2 ± 1.9 | −15 ± 0.97 | N.A. c | N.A. |
| Human 2B7 | AZTG | N.A. | N.A. | 11 ± 2.2 | 7.9 ± 3.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.; Kim, S.; Kim, M.-y.; Lee, J.; An, B.H.; Kim, H.-D.; Jeong, H.; Song, Y.S.; Chang, M. Inhibitory and Inductive Effects of Opuntia ficus indica Extract and Its Flavonoid Constituents on Cytochrome P450s and UDP-Glucuronosyltransferases. Int. J. Mol. Sci. 2018, 19, 3400. https://doi.org/10.3390/ijms19113400
Jeong H, Kim S, Kim M-y, Lee J, An BH, Kim H-D, Jeong H, Song YS, Chang M. Inhibitory and Inductive Effects of Opuntia ficus indica Extract and Its Flavonoid Constituents on Cytochrome P450s and UDP-Glucuronosyltransferases. International Journal of Molecular Sciences. 2018; 19(11):3400. https://doi.org/10.3390/ijms19113400
Chicago/Turabian StyleJeong, Hyesoo, Soolin Kim, Mi-yeon Kim, Jimin Lee, Byoung Ha An, Hee-Doo Kim, Hyunyoung Jeong, Yun Seon Song, and Minsun Chang. 2018. "Inhibitory and Inductive Effects of Opuntia ficus indica Extract and Its Flavonoid Constituents on Cytochrome P450s and UDP-Glucuronosyltransferases" International Journal of Molecular Sciences 19, no. 11: 3400. https://doi.org/10.3390/ijms19113400
APA StyleJeong, H., Kim, S., Kim, M.-y., Lee, J., An, B. H., Kim, H.-D., Jeong, H., Song, Y. S., & Chang, M. (2018). Inhibitory and Inductive Effects of Opuntia ficus indica Extract and Its Flavonoid Constituents on Cytochrome P450s and UDP-Glucuronosyltransferases. International Journal of Molecular Sciences, 19(11), 3400. https://doi.org/10.3390/ijms19113400






