The Role of CD38 on the Function of Regulatory B Cells in a Murine Model of Lupus
Abstract
:1. Introduction
2. Results
2.1. Similar Proportion of Splenic CD1dhiCD5+ B Cells in Naïve Cd38−/− and WT Mice
2.2. Increased Surface Expression of CD38 in WT CD1dhiCD5+ B Cell Subset
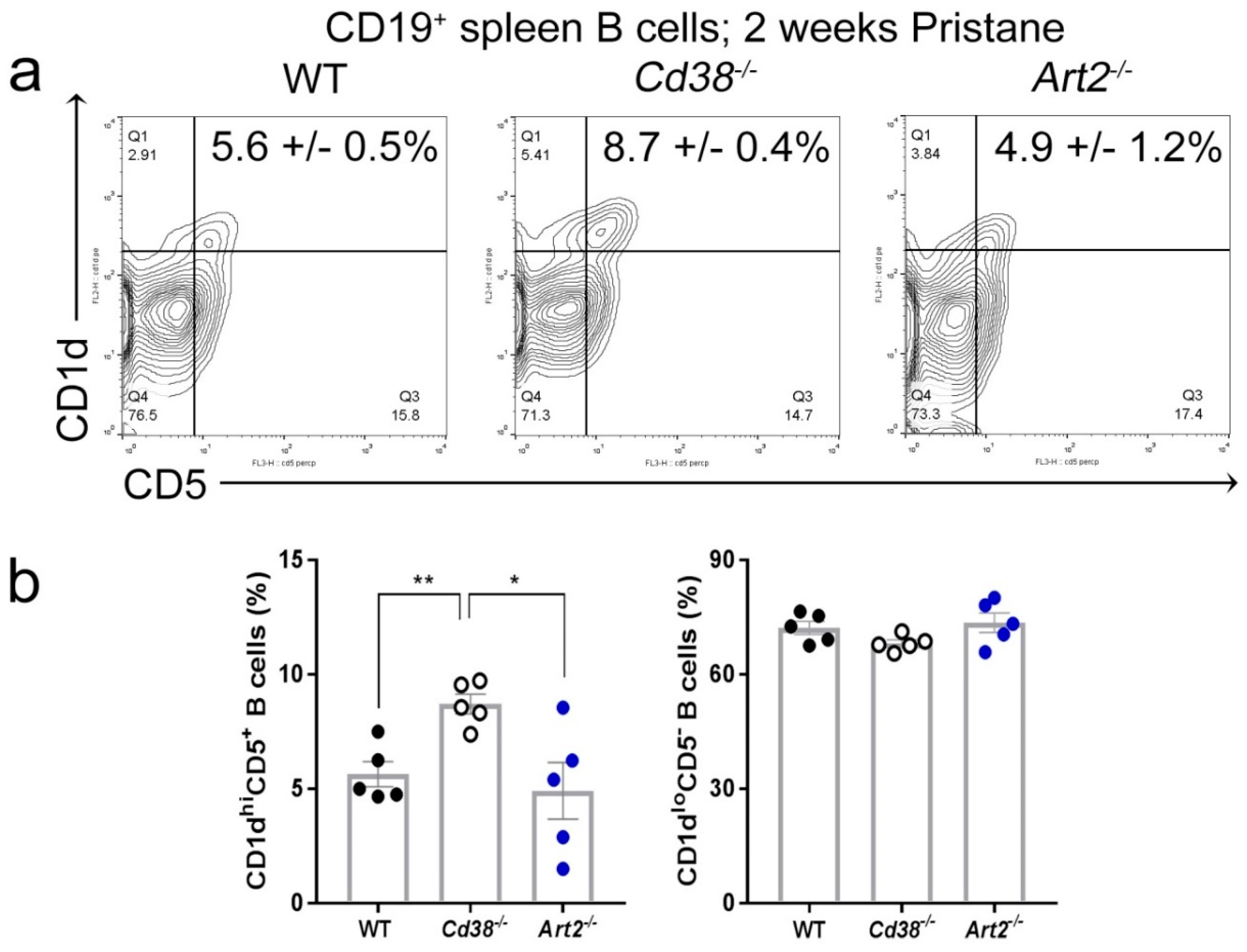
2.3. Increased Frequency of CD1dhiCD5+ B Cells in Spleen from Pristane-treated Cd38−/− Mice
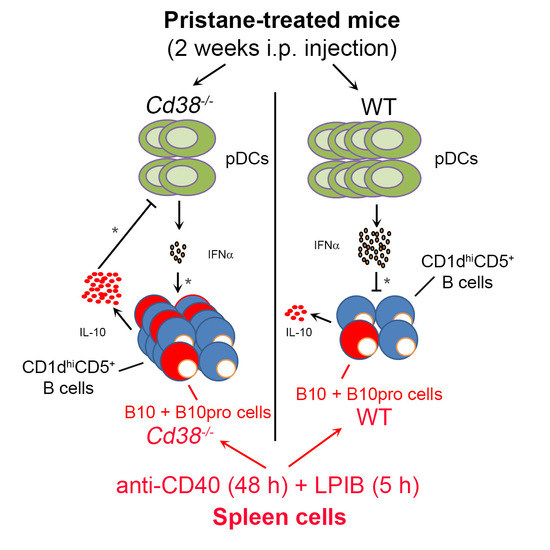
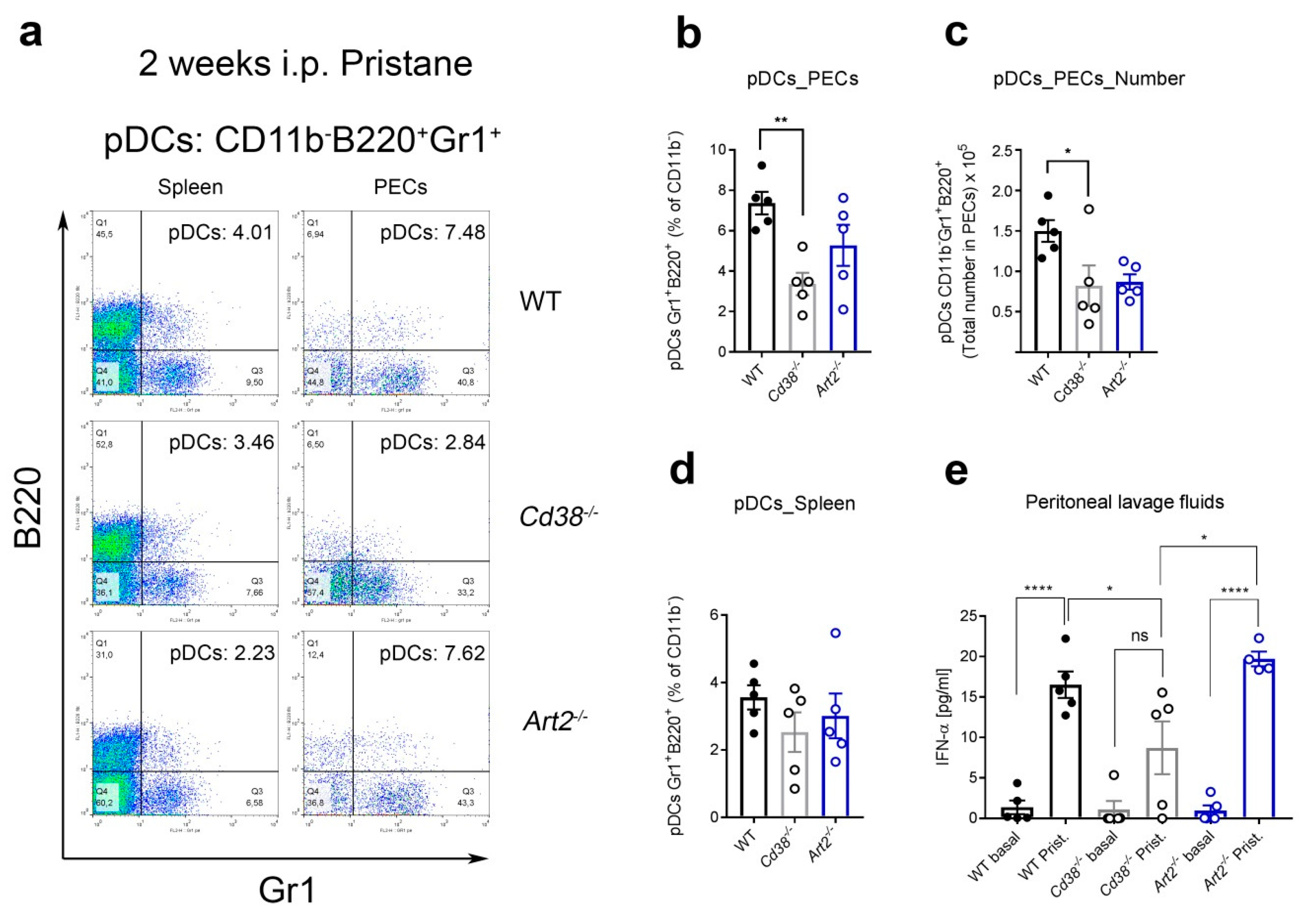
2.4. Decreased Percentages of Plasmacytoid Dendritic Cells (pDCs) and Diminished IFN-α Secretion in Peritoneal Lavage Fluids of Pristane-Treated Cd38−/− Mice
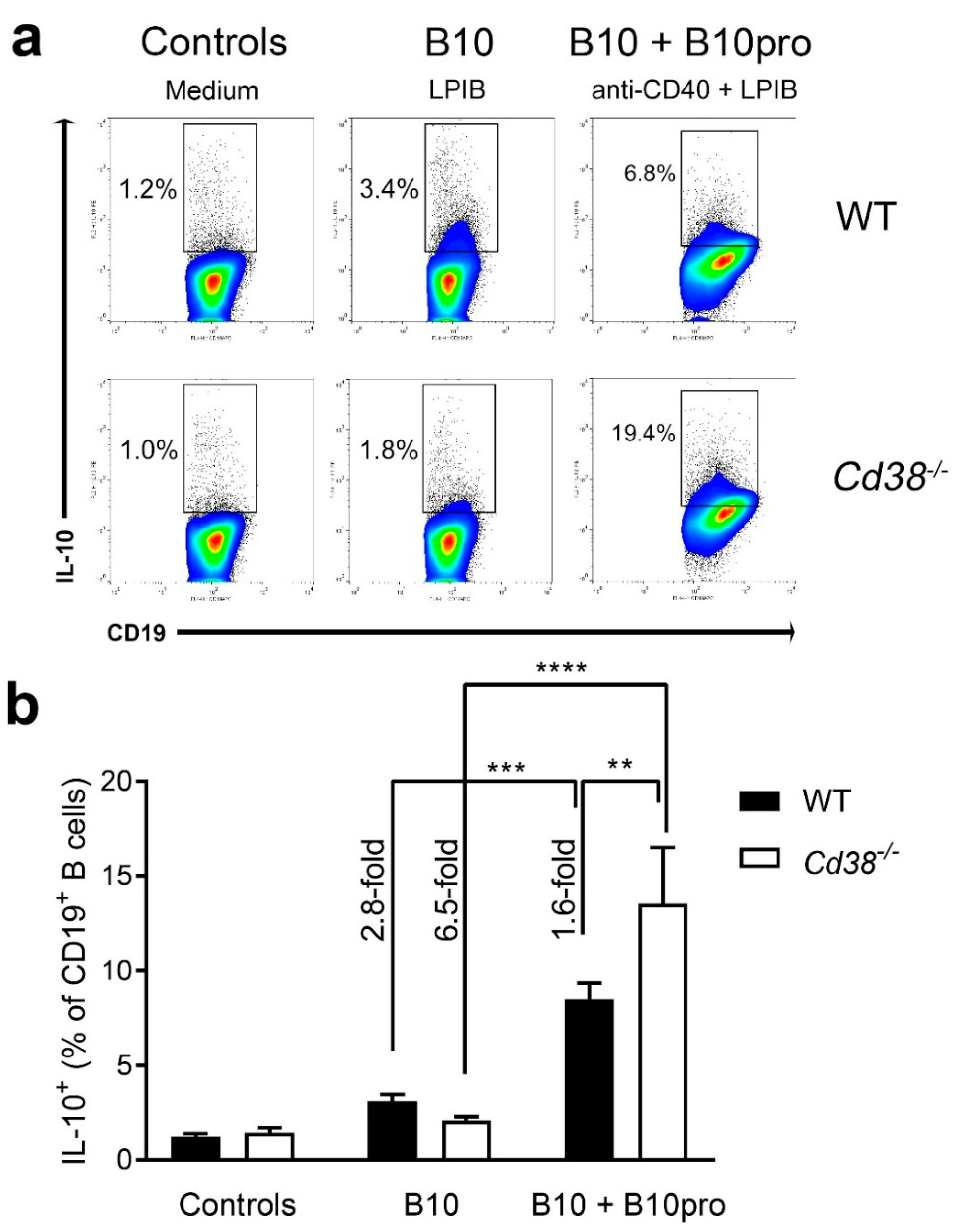
2.5. Increased Frequencies of B10 + B10pro Cells in Spleen from Cd38−/− Mice
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Splenocytes
4.3. Counting
4.4. Flow Cytometry
4.4.1. B Cell Subsets and pDCs
4.4.2. Intracellular IL-10 Staining
4.4.3. Data Acquisition and Analysis
4.5. Functional Analysis of Regulatory B (B10) Cells
4.6. IFN-α ELISA
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Art2 | T-cell ecto-ADP-ribosyltransferase 2 |
| B10 | IL-10-producing B cells |
| B10pro | B10 progenitor cells |
| Bregs | Regulatory B cells |
| LPIB | LPS + PMA + Ionomycin + Brefeldin A |
| LPS | Lipopolysaccharide |
| pDCs | Plasmacytoid Dendritic Cells |
| PECs | Peritoneal Exudate Cells |
| PIM | PMA + Ionomycin + Monensin |
| PMA | Phorbol 12-Myristate 13-Acetate |
| Pristane | 2,6,10,14-Tetramethylpentadecane |
| SLE | Systemic Lupus Erythematosus |
| TRPM2 | Transient Receptor Potential Melastatin 2 |
| WT | Wild-Type |
References
- Pavon, E.J.; Zumaquero, E.; Rosal-Vela, A.; Khoo, K.M.; Cerezo-Wallis, D.; Garcia-Rodriguez, S.; Carrascal, M.; Abian, J.; Graeff, R.; Callejas-Rubio, J.L.; et al. Increased CD38 expression in T cells and circulating anti-CD38 IgG autoantibodies differentially correlate with distinct cytokine profiles and disease activity in systemic lupus erythematosus patients. Cytokine 2013, 62, 232–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Rodriguez, S.; Callejas-Rubio, J.L.; Ortego-Centeno, N.; Zumaquero, E.; Rios-Fernandez, R.; Arias-Santiago, S.; Navarro, P.; Sancho, J.; Zubiaur, M. Altered AKT1 and MAPK1 gene expression on peripheral blood mononuclear cells and correlation with T-helper-transcription factors in systemic lupus erythematosus patients. Mediat. Inflamm. 2012, 2012, 495934. [Google Scholar] [CrossRef] [PubMed]
- Zubiaur, M.; Izquierdo, M.; Terhorst, C.; Malavasi, F.; Sancho, J. CD38 ligation results in activation of the Raf-1/mitogen-activated protein kinase and the CD3-zeta/zeta-associated protein-70 signaling pathways in Jurkat T lymphocytes. J. Immunol. 1997, 159, 193–205. [Google Scholar] [PubMed]
- Patton, D.T.; Wilson, M.D.; Rowan, W.C.; Soond, D.R.; Okkenhaug, K. The PI3K p110delta regulates expression of CD38 on regulatory T cells. PLoS ONE 2011, 6, e17359. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Muchamuel, T.; Sakaguchi, S.; Andrade, S.; Menon, S.; Howard, M. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/W F1 mice. J. Exp. Med. 1994, 179, 305–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorente, L.; Richaud-Patin, Y.; García-Padilla, C.; Claret, E.; Jakez-Ocampo, J.; Cardiel, M.H.; Alcocer-Varela, J.; Grangeot-Keros, L.; Alarcón-Segovia, D.; Wijdenes, J.; et al. Clinical and biologic effects of anti–interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000, 43, 1790–1800. [Google Scholar] [CrossRef]
- Yin, Z.; Bahtiyar, G.; Zhang, N.; Liu, L.; Zhu, P.; Robert, M.E.; McNiff, J.; Madaio, M.P.; Craft, J. IL-10 regulates murine lupus. J. Immunol. 2002, 169, 2148–2155. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Ishiura, N.; Nakashima, H.; Kuwano, Y.; Okochi, H.; Tamaki, K.; Sato, S.; Tedder, T.F.; Fujimoto, M. Regulatory B cells (B10 Cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J. Immunol. 2010, 184, 4801–4809. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.A.; Norena, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, A.; Miyagaki, T.; DiLillo, D.J.; Matsushita, T.; Horikawa, M.; Kountikov, E.I.; Spolski, R.; Poe, J.C.; Leonard, W.J.; Tedder, T.F. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 2012, 491, 264–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalampokis, I.; Yoshizaki, A.; Tedder, T.F. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res. Ther. 2013, 15 (Suppl. 1), S1. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Bouaziz, J.D.; Haas, K.M.; Poe, J.C.; Fujimoto, M.; Tedder, T.F. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008, 28, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Bouaziz, J.D.; Matsushita, T.; Tsubata, T.; Tedder, T.F. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J. Immunol. 2009, 182, 7459–7472. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.M.; Watanabe, R.; Matsushita, T.; Nakashima, H.; Ishiura, N.; Okochi, H.; Fujimoto, M.; Tedder, T.F. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 Mice. J. Immunol. 2010, 184, 4789–4800. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Dittel, B.N. Mechanisms of regulatory B cell function in autoimmune and inflammatory diseases beyond IL-10. J. Clin. Med. 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Han, S.; Lee, P.Y.; Khaybullin, R.; Shumyak, S.; Lu, L.; Chatha, A.; Afaneh, A.; Zhang, Y.; Xie, C.; et al. Pathogenesis of diffuse alveolar hemorrhage in murine lupus. Arthritis Rheumatol. 2017, 69, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodriguez, S.; Rosal-Vela, A.; Botta, D.; Cumba Garcia, L.M.; Zumaquero, E.; Prados-Maniviesa, V.; Cerezo-Wallis, D.; Lo Buono, N.; Robles-Guirado, J.A.; Guerrero, S.; et al. CD38 promotes pristane-induced chronic inflammation and increases susceptibility to experimental lupus by an apoptosis-driven and TRPM2-dependent mechanism. Sci. Rep. 2018, 8, 3357. [Google Scholar] [CrossRef] [PubMed]
- Hubert, S.; Rissiek, B.; Klages, K.; Huehn, J.; Sparwasser, T.; Haag, F.; Koch-Nolte, F.; Boyer, O.; Seman, M.; Adriouch, S. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2-P2X7 pathway. J. Exp. Med. 2010, 207, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Scheuplein, F.; Rissiek, B.; Driver, J.P.; Chen, Y.G.; Koch-Nolte, F.; Serreze, D.V. A recombinant heavy chain antibody approach blocks ART2 mediated deletion of an iNKT cell population that upon activation inhibits autoimmune diabetes. J. Autoimmun. 2010, 34, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rissiek, B.; Haag, F.; Boyer, O.; Koch-Nolte, F.; Adriouch, S. ADP-ribosylation of P2X7: A matter of life and death for regulatory T cells and natural killer T cells. Curr. Top. Microbiol. Immunol. 2015, 384, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Postigo, J.; Iglesias, M.; Cerezo-Wallis, D.; Rosal-Vela, A.; Garcia-Rodriguez, S.; Zubiaur, M.; Sancho, J.; Merino, R.; Merino, J. Mice Deficient in CD38 Develop an Attenuated Form of Collagen Type II-Induced Arthritis. PLoS ONE 2012, 7, e33534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sag, D.; Krause, P.; Hedrick, C.C.; Kronenberg, M.; Wingender, G. IL-10–producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J. Clin. Investig. 2014, 124, 3725–3740. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Pantoja, M.; Lopez-Herrera, G.; Romero-Ramirez, H.; Santos-Argumedo, L.; Chavez-Rueda, A.K.; Hernandez-Cueto, A.; Flores-Munoz, M.; Rodriguez-Alba, J.C. CD38 protein deficiency induces autoimmune characteristics and its activation enhances IL-10 production by regulatory B cells. Scand. J. Immunol. 2018, 87, e12664. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Q.; Singh, A.K.; Wilson, M.T.; Satoh, M.; Stanic, A.K.; Park, J.J.; Hong, S.; Gadola, S.D.; Mizutani, A.; Kakumanu, S.R.; et al. Immunoregulatory role of CD1d in the hydrocarbon oil-induced model of lupus nephritis. J. Immunol. 2003, 171, 2142–2153. [Google Scholar] [CrossRef] [PubMed]
- DiLillo, D.J.; Matsushita, T.; Tedder, T.F. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann. N. Y. Acad. Sci. 2010, 1183, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, A.; Mizoguchi, E.; Takedatsu, H.; Blumberg, R.S.; Bhan, A.K. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 2002, 16, 219–230. [Google Scholar] [CrossRef]
- Nakano, H.; Yanagita, M.; Gunn, M.D. Cd11c+ B220+ Gr-1+ Cells in Mouse Lymph Nodes and Spleen Display Characteristics of Plasmacytoid Dendritic Cells. J. Exp. Med. 2001, 194, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Alba, J.C.; Moreno-Garcia, M.E.; Sandoval-Montes, C.; Rosales-Garcia, V.H.; Santos-Argumedo, L. CD38 induces differentiation of immature transitional 2 B lymphocytes in the spleen. Blood 2008, 111, 3644–3652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushita, T.; Tedder, T.F. Identifying regulatory B cells (B10 cells) that produce IL-10 in mice. Methods Mol. Biol. 2011, 677, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Blair, P.A.; Isenberg, D.A.; Mauri, C. A Regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity 2016, 44, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Rowland, S.L.; Riggs, J.M.; Gilfillan, S.; Bugatti, M.; Vermi, W.; Kolbeck, R.; Unanue, E.R.; Sanjuan, M.A.; Colonna, M. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J. Exp. Med. 2014, 211, 1977–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cockayne, D.A.; Muchamuel, T.; Grimaldi, J.C.; Muller-Steffner, H.; Randall, T.D.; Lund, F.E.; Murray, R.; Schuber, F.; Howard, M.C. Mice deficient for the ecto-nicotinamide adenine dinucleotide glycohydrolase CD38 exhibit altered humoral immune responses. Blood 1998, 92, 1324–1333. [Google Scholar] [PubMed]
- Ohlrogge, W.; Haag, F.; Lohler, J.; Seman, M.; Littman, D.R.; Killeen, N.; Koch-Nolte, F. Generation and characterization of ecto-ADP-ribosyltransferase ART2.1/ART2.2-deficient mice. Mol. Cell. Biol. 2002, 22, 7535–7542. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Reeves, W.H. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J. Exp. Med. 1994, 180, 2341–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burlock, B.; Richardson, G.; García-Rodríguez, S.; Guerrero, S.; Zubiaur, M.; Sancho, J. The Role of CD38 on the Function of Regulatory B Cells in a Murine Model of Lupus. Int. J. Mol. Sci. 2018, 19, 2906. https://doi.org/10.3390/ijms19102906
Burlock B, Richardson G, García-Rodríguez S, Guerrero S, Zubiaur M, Sancho J. The Role of CD38 on the Function of Regulatory B Cells in a Murine Model of Lupus. International Journal of Molecular Sciences. 2018; 19(10):2906. https://doi.org/10.3390/ijms19102906
Chicago/Turabian StyleBurlock, Brianna, Gabrielle Richardson, Sonia García-Rodríguez, Salvador Guerrero, Mercedes Zubiaur, and Jaime Sancho. 2018. "The Role of CD38 on the Function of Regulatory B Cells in a Murine Model of Lupus" International Journal of Molecular Sciences 19, no. 10: 2906. https://doi.org/10.3390/ijms19102906
APA StyleBurlock, B., Richardson, G., García-Rodríguez, S., Guerrero, S., Zubiaur, M., & Sancho, J. (2018). The Role of CD38 on the Function of Regulatory B Cells in a Murine Model of Lupus. International Journal of Molecular Sciences, 19(10), 2906. https://doi.org/10.3390/ijms19102906