Amphiphilic Polymeric Micelles Based on Deoxycholic Acid and Folic Acid Modified Chitosan for the Delivery of Paclitaxel
Abstract
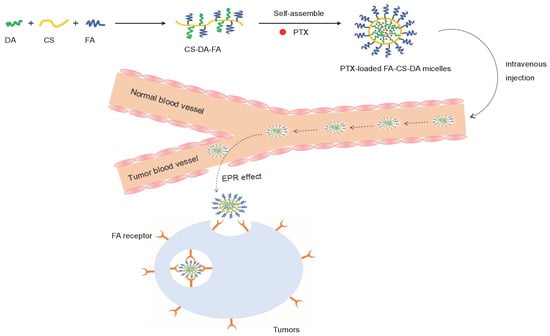
1. Introduction
2. Results and Discussion
2.1. Preparation of FA-CS-DA
2.2. Characterization of FA-CS-DA
2.2.1. Proton Nuclear Magnetic Resonance (1H-NMR) Characterization
2.2.2. Fourier-Transform Infrared (FTIR) Characterization
2.2.3. Critical Micelle Concentration (CMC) of FA-CS-DA
2.3. Preparation of PTX-Loaded FA-CS-DA Micelles
2.4. Characterization of PTX-Loaded FA-CS-DA Micelles
2.4.1. Particle Size and Zeta Potential
2.4.2. Transmission Electron Microscopy (TEM) Observation
2.4.3. X-Ray Diffraction (XRD) Analysis
2.5. In Vitro Cytotoxicity Study
2.6. In Vivo Tumor Growth Inhibition Study
3. Materials and Methods
3.1. Materials
3.2. Animals and Cell Lines
3.3. Synthesis of FA-CS-DA
3.4. Characterization of FA-CS-DA
3.4.1. 1H-NMR Characterization
3.4.2. FTIR Characterization
3.4.3. Measurement of the Degree of Substitution (DS)
3.4.4. Determination of Critical Micelle Concentration (CMC)
3.5. Preparation of PTX-Loaded FA-CS-DA Micelles
3.6. Characterization of PTX-Loaded FA-CS-DA Micelles
3.6.1. Measurement of Particle Size and Zeta Potential
3.6.2. TEM Observation
3.6.3. XRD Analysis
3.7. Determination of Drug Loading and Drug Encapsulation Efficiency
3.8. In Vitro Cytotoxicity Study
3.9. In Vivo Antitumor Activity Study
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sofias, A.M.; Dunne, M.; Storm, G.; Allen, C. The battle of “nano” paclitaxel. Adv. Drug Deliv. Rev. 2017, 122, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Eloy, J.O.; Petrilli, R.; Topan, J.F.; Antonio, H.M.R.; Barcellos, J.P.A.; Chesca, D.L.; Serafini, L.N.; Tiezzi, D.G.; Lee, R.J.; Marchetti, J.M. Co-loaded paclitaxel/rapamycin liposomes: Development, characterization and in vitro and in vivo evaluation for breast cancer therapy. Colloids Surf. B Biointerfaces 2016, 141, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Tatiparti, K.; Sau, S.; Gawde, K.A.; Iyer, A.K. Copper-free ‘click’ chemistry-based synthesis and characterization of carbonic anhydrase-IX anchored albumin-paclitaxel nanoparticles for targeting tumor hypoxia. Int. J. Mol. Sci. 2018, 19, 838. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, X.; Zu, Y.; Zhang, Y. Preparation and characterization of paclitaxel nanosuspension using novel emulsification method by combining high speed homogenizer and high pressure homogenization. Int. J. Pharm. 2015, 490, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Yang, H. Targeted nanosystems: Advances in targeted dendrimers for cancer therapy. Nanomedicine 2016, 12, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Sun, S.; Gong, X.; Li, Q.; Yan, P.; Cui, F. Polymeric micelles based on modified glycol chitosan for paclitaxel delivery: Preparation, characterization and evaluation. Int. J. Mol. Sci. 2018, 19, 1550. [Google Scholar] [CrossRef] [PubMed]
- Cong, Z.; Shi, Y.; Wang, Y.; Wang, Y.; Niu, J.E.; Chen, N.; Xue, H. A novel controlled drug delivery system based on alginate hydrogel/chitosan micelle composites. Int. J. Biol. Macromol. 2018, 107, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, Z.; Wang, F.; Luan, Y. Active-targeting docetaxel-loaded mixed micelles for enhancing antitumor efficacy. J. Mol. Liq. 2018, 264, 172–178. [Google Scholar] [CrossRef]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, S.; Wang, Y.; Wang, X.; Wang, Q.; Chen, M. Advances in self-assembled chitosan nanomaterials for drug delivery. Biotechnol. Adv. 2014, 32, 1301–1316. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Fu, Y.; Liu, Y.; Chen, Q.; Mu, Y.; Zhou, J.; Li, L.; Xu, W.; Yin, T. N-mercapto acetyl-N′-octyl-O, N″-glycol chitosan as an efficiency oral delivery system of paclitaxel. Carbohydr. Polym. 2018, 181, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.S.; Almeida, A.; Prezotti, F.; Cury, B.; Campana-Filho, S.P.; Sarmento, B. Synthesis and characterization of 3,6-O,O′- dimyristoyl chitosan micelles for oral delivery of paclitaxel. Colloids Surf. B Biointerfaces 2017, 152, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.C.; Jiang, Y.; Xie, Y.; Qiu, L.L.; Yang, Q.; Lu, H.Y. Novel amphiphilic folic acid-cholesterol-chitosan micelles for paclitaxel delivery. Oncotarget 2017, 8, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Yin, C. Trimethyl chitosan based conjugates for oral and intravenous delivery of paclitaxel. Acta Biomater. 2017, 53, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.; Nazarpak, M.H.; Solouk, A.; Akbari, S.; Hasani-Sadrabadi, M.M. Magnetic responsive of paclitaxel delivery system based on SPION and palmitoyl chitosan. J. Magn. Magn. Mater. 2017, 421, 316–325. [Google Scholar] [CrossRef]
- Skorik, Y.A.; Golyshev, A.A.; Kritchenkov, A.S.; Gasilova, E.R.; Poshina, D.N.; Sivaram, A.J.; Jayakumar, R. Development of drug delivery systems for taxanes using ionic gelation of carboxyacyl derivatives of chitosan. Carbohydr. Polym. 2017, 162, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Sharma, S.; Khan, I.; Gothwal, A.; Sharma, A.K.; Singh, Y.; Chourasia, M.K.; Kumar, V. Enhanced apoptotic and anticancer potential of paclitaxel loaded biodegradable nanoparticles based on chitosan. Int. J. Biol. Macromol. 2017, 98, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Sun, S.; Li, X.; Piao, H.; Piao, H.; Cui, F.; Fang, L. α-Tocopherol succinate-modified chitosan as a micellar delivery system for paclitaxel: Preparation, characterization and in vitro/in vivo evaluations. Int. J. Pharm. 2012, 423, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Heřmánková, E.; Žák, A.; Poláková, L.; Hobzová, R.; Hromádka, R.; Širc, J. Polymeric bile acid sequestrants: Review of design, in vitro binding activities, and hypocholesterolemic effects. Eur. J. Med. Chem. 2018, 144, 300–317. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F.; Hagey, L.R. Bile acids: Chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell. Mol. Life Sci. 2008, 65, 2461–2483. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Du, H.; Zhai, G. Self-assembled nanoparticles based on chondroitin sulfate-deoxycholic acid conjugates for docetaxel delivery: Effect of degree of substitution of deoxycholic acid. Colloids Surf. B Biointerfaces 2016, 146, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Dhas, N.L.; Ige, P.P.; Kudarha, R.R. Design, optimization and in-vitro study of folic acid conjugated-chitosan functionalized PLGA nanoparticle for delivery of bicalutamide in prostate cancer. Powder Technol. 2015, 283, 234–245. [Google Scholar] [CrossRef]
- Scomparin, A.; Salmaso, S.; Eldar-Boock, A.; Ben-Shushan, D.; Ferber, S.; Tiram, G.; Shmeeda, H.; Landa-Rouben, N.; Leor, J.; Caliceti, P.; et al. A comparative study of folate receptor-targeted doxorubicin delivery systems: Dosing regimens and therapeutic index. J. Control. Release 2015, 208, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Vo, C.L.N.; Kang, T.; Oh, E.; Lee, B.J. New method and characterization of self-assembled gelatin–oleic nanoparticles using a desolvation method via carbodiimide/N-hydroxysuccinimide (EDC/NHS) reaction. Eur. J. Pharm. Biopharm. 2015, 89, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Băran, A.; Stîngă, G.; Anghel, D.-F.; Iovescu, A.; Tudose, M. Comparing the spectral properties of pyrene as free molecule, label and derivative in some colloidal systems. Sens. Actuators B Chem. 2014, 197, 193–199. [Google Scholar] [CrossRef]
- Zhang, C.; Qu, G.; Sun, Y.; Wu, X.; Yao, Z.; Guo, Q.; Ding, Q.; Yuan, S.; Shen, Z.; Ping, Q.; et al. Pharmacokinetics, biodistribution, efficacy and safety of N-octyl-O-sulfate chitosan micelles loaded with paclitaxel. Biomaterials 2008, 29, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zuo, Z.; Du, J.; Wang, Y.; Sun, R.; Cao, Z.; Ye, X.; Wang, J.; Leong, K.W.; Wang, J. Surface charge critically affects tumor penetration and therapeutic efficacy of cancer nanomedicines. Nano Today 2016, 11, 133–144. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.C.; Kang, S.W.; Kwon, I.C.; Kim, Y.H.; Jeong, S.Y. Synthesis and the micellar characteristics of poly(ethylene oxide)–deoxycholic acid conjugates. Langmuir 2000, 16, 4792–4797. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Y.; Zhang, D.; Zhang, Q.; Zheng, D.; Hao, L.; Liu, Y.; Duan, C.; Jia, L.; Liu, G. Folate-mediated targeted and intracellular delivery of paclitaxel using a novel deoxycholic acid-O-carboxymethylated chitosan–folic acid micelles. Int. J. Nanomed. 2012, 7, 325–337. [Google Scholar]
- Yen, H.; Young, Y.; Tsai, T.; Cheng, K.; Chen, X.; Chen, Y.; Chen, C.; Young, J.; Hong, P. Positively charged gold nanoparticles capped with folate quaternary chitosan: Synthesis, cytotoxicity, and uptake by cancer cells. Carbohydr. Polym. 2018, 183, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Krajicek, M.E. Mucoadhesive polymers as platforms for peroral peptide delivery and absorption: Synthesis and evaluation of different chitosan-EDTA conjugates. J. Control. Release 1998, 50, 215–223. [Google Scholar] [CrossRef]
- Liang, N.; Sun, S.; Hong, J.; Tian, J.; Fang, L.; Cui, F. In vivo pharmacokinetics, biodistribution and antitumor effect of paclitaxel-loaded micelles based on α-tocopherol succinate-modified chitosan. Drug Deliv. 2016, 23, 2651–2660. [Google Scholar] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Liang, N.; Wang, D.; Yan, P.; Kawashima, Y.; Cui, F.; Sun, S. Amphiphilic Polymeric Micelles Based on Deoxycholic Acid and Folic Acid Modified Chitosan for the Delivery of Paclitaxel. Int. J. Mol. Sci. 2018, 19, 3132. https://doi.org/10.3390/ijms19103132
Li L, Liang N, Wang D, Yan P, Kawashima Y, Cui F, Sun S. Amphiphilic Polymeric Micelles Based on Deoxycholic Acid and Folic Acid Modified Chitosan for the Delivery of Paclitaxel. International Journal of Molecular Sciences. 2018; 19(10):3132. https://doi.org/10.3390/ijms19103132
Chicago/Turabian StyleLi, Liang, Na Liang, Danfeng Wang, Pengfei Yan, Yoshiaki Kawashima, Fude Cui, and Shaoping Sun. 2018. "Amphiphilic Polymeric Micelles Based on Deoxycholic Acid and Folic Acid Modified Chitosan for the Delivery of Paclitaxel" International Journal of Molecular Sciences 19, no. 10: 3132. https://doi.org/10.3390/ijms19103132
APA StyleLi, L., Liang, N., Wang, D., Yan, P., Kawashima, Y., Cui, F., & Sun, S. (2018). Amphiphilic Polymeric Micelles Based on Deoxycholic Acid and Folic Acid Modified Chitosan for the Delivery of Paclitaxel. International Journal of Molecular Sciences, 19(10), 3132. https://doi.org/10.3390/ijms19103132