PEG-Plasma Hydrogels Increase Epithelialization Using a Human Ex Vivo Skin Model
Abstract
1. Introduction
2. Results
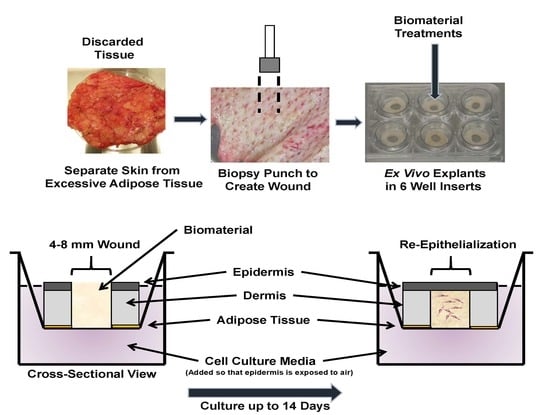
2.1. Initial 4 mm Pilot Study to Determine Feasibility of Ex Vivo Model
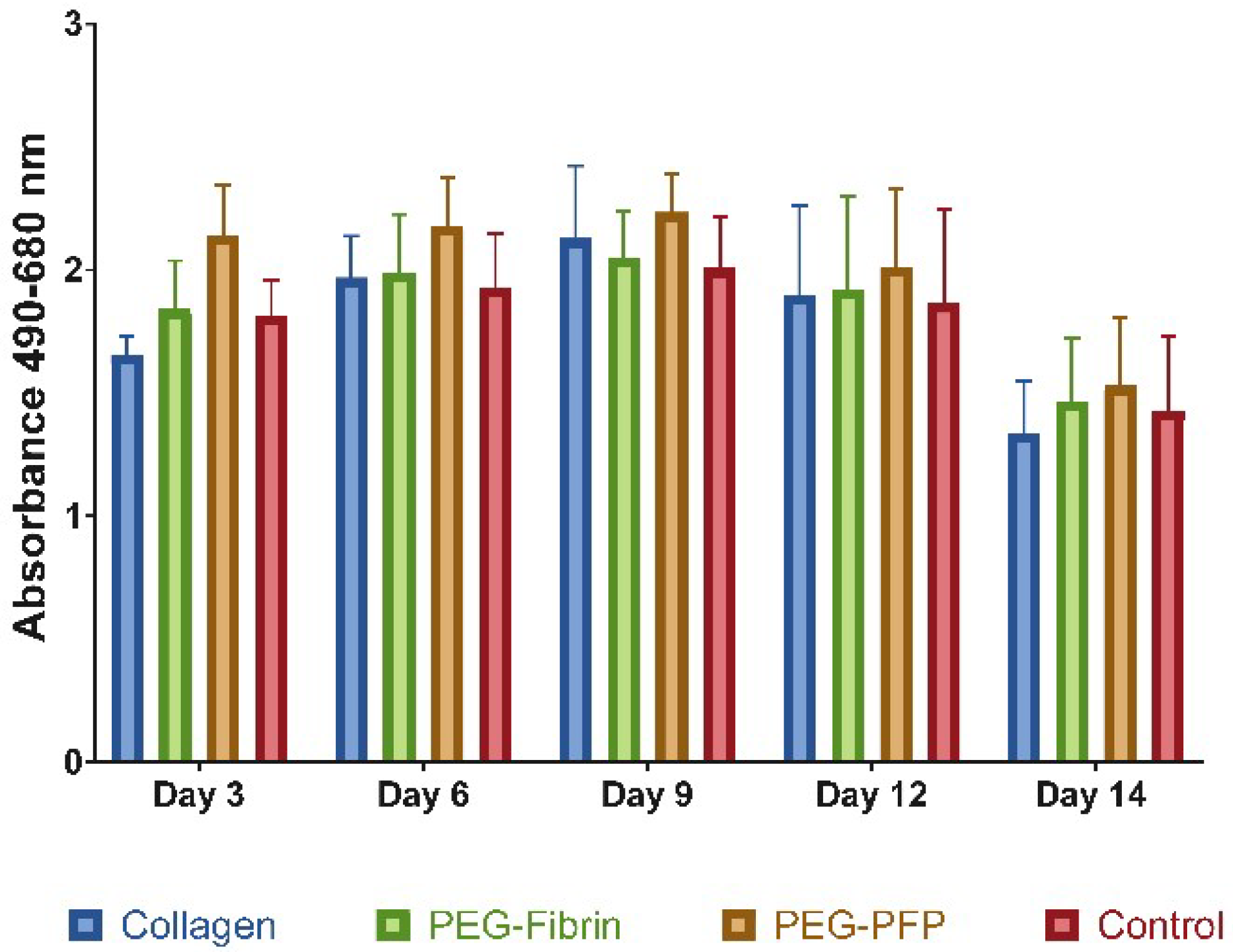
2.2. Hydrogel Treatments do not Increase Cell Death in 8 mm Ex Vivo Explants
2.3. PEG-PFP Hydrogels Increase Wound Closure Rates
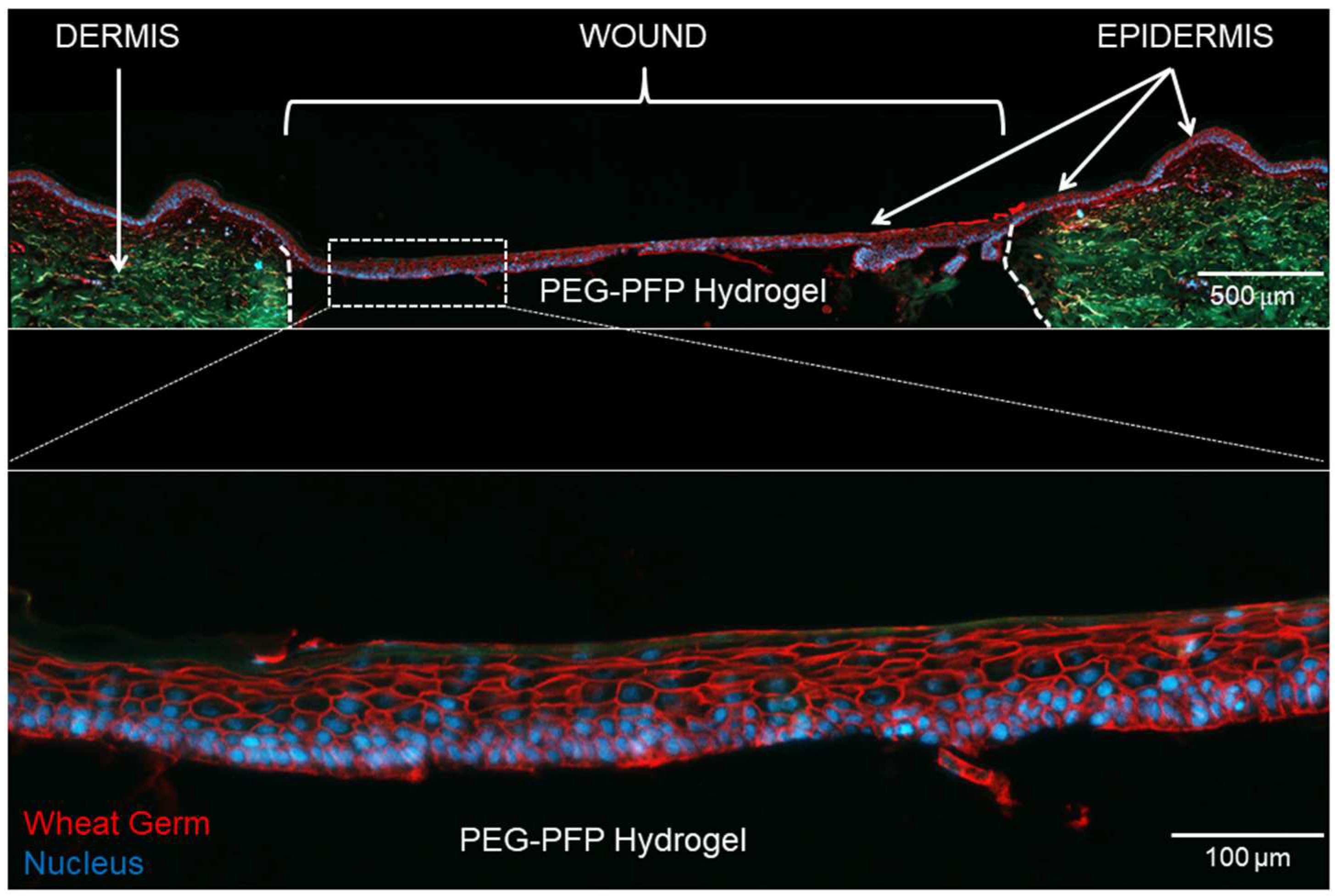
2.4. Epidermal Stratification with Polyethylene Glycol-Platelet Free Plasma (PEG-PFP) Hydrogels
2.5. Cellular Migration and Proliferation with the Hydrogels
2.6. α-SMA Positive Cells Infiltrate into the PEG-PFP Hydrogels
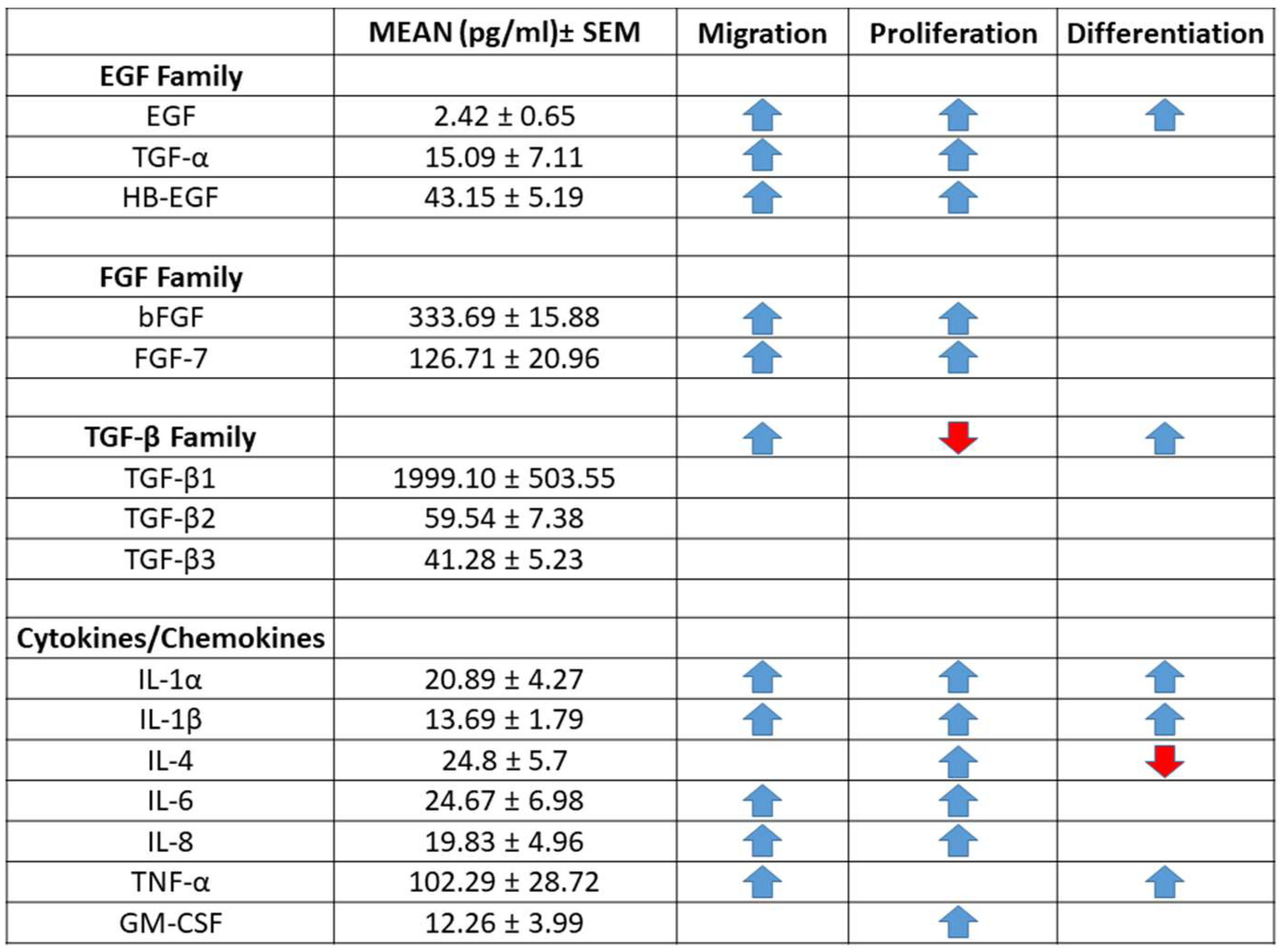
2.7. Human PFP Contains Detectable Levels of Cytokines and Growth Factors
3. Discussion
4. Materials and Methods
4.1. Ex Vivo Model
4.2. Platelet-Free Plasma Preparation
4.3. Quantification of Chemokines in Human PFP
4.4. Hydrogel Formulations
4.4.1. Collagen Hydrogel
4.4.2. PEG-Fibrin Hydrogel
4.4.3. PEG-PFP Hydrogel
4.5. Tissue Viability
4.6. Microscopy Documentation of Wound Healing
4.7. Immunohistochemistry
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PEG | Polyethylene glycol |
| PFP | Platelet-free plasma |
| CK-10 | Cytokeratin 10 |
| α-SMA | Alpha smooth muscle actin |
| WG | Wheat germ |
| FDA | Food and Drug Administration |
| PRP | Platelet-rich plasma |
| ASCs | Adipose derived stem cells |
| HBSS | Hank’s balanced salt solution |
| ABAM | Antibiotic-antimycotic |
| LDH | Lactate dehydrogenase |
| RT | Room temperature |
| H&E | Hematoxylin and eosin |
| PFA | Paraformaldehyde |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick-end labeling |
| TGF | Transforming growth factor |
| EGF | Epidermal growth factor |
| KGF | Keratinocyte growth factor |
| FGF | Fibroblast growth factor |
References
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics; National Ambulatory Medical Care Survey: 2015 State and National Summary Tables. 2015. Available online: https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2015_namcs_web_tables.pdf (accessed on 20 September 2018).
- National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey: 2015 Emergency Department Summary Tables. 2015. Available online: https://www.cdc.gov/nchs/data/nhamcs/web_tables/2015_ed_web_tables (accessed on 20 September 2018).
- U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for Industry Chronic Cutaneous Ulcer and Burn Wounds—Developing Products for Treatment. 2006. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm071324.pdf (accessed on 19 September 2018).
- Li, W.; Zhou, J.; Xu, Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed. Rep. 2015, 3, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- De Wever, B.; Kurdykowski, S.; Descargues, P. Human Skin Models for Research Applications in Pharmacology and Toxicology: Introducing NativeSkin®, the “Missing Link” Bridging Cell Culture and/or Reconstructed Skin Models and Human Clinical Testing. Appl. In Vitro Toxicol. 2015, 1, 26–32. [Google Scholar] [CrossRef]
- Shamir, E.R.; Ewald, A.J. Three-dimensional organotypic culture: Experimental models of mammalian biology and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Salgado, G.; Ng, Y.Z.; Koh, L.F.; Goh, C.S.M.; Common, J.E. Human reconstructed skin xenografts on mice to model skin physiology. Differentiation 2017, 98, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, T.; Bayat, A. Ex vivo evaluation of acellular and cellular collagen-glycosaminoglycan flowable matrices. Biomed. Mater. 2015, 10, 041001. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, T.; Bayat, A. In vitro and ex vivo analysis of hyaluronan supplementation of Integra(R) dermal template on human dermal fibroblasts and keratinocytes. J. Appl. Biomater. Funct. Mater. 2016, 14, e9–e18. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, T.; Yuan, X.F.; Bayat, A. Electrospun silk fibroin fiber diameter influences in vitro dermal fibroblast behavior and promotes healing of ex vivo wound models. J. Tissue Eng. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Lazic, T.; Falanga, V. Bioengineered skin constructs and their use in wound healing. Plast. Reconstr. Surg. 2011, 127, 75s–90s. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, S.C.; Upton, Z.; Bott, K.; Dargaville, T.R. Recent advances in dermal wound healing: Biomedical device approaches. Expert Rev. Med. Device 2010, 7, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Stone Ii, R.; Natesan, S.; Kowalczewski, C.J.; Mangum, L.H.; Clay, N.E.; Clohessy, R.M.; Carlsson, A.H.; Tassin, D.H.; Chan, R.K.; Rizzo, J.A.; et al. Advancements in Regenerative Strategies Through the Continuum of Burn Care. Front. Pharmacol. 2018, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Madaghiele, M.; Demitri, C.; Sannino, A.; Ambrosio, L. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burns Trauma 2014, 2, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Catanzano, O.; D’Esposito, V.; Acierno, S.; Ambrosio, M.R.; De Caro, C.; Avagliano, C.; Russo, P.; Russo, R.; Miro, A.; Ungaro, F.; et al. Alginate-hyaluronan composite hydrogels accelerate wound healing process. Carbohydr. Polym 2015, 131, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Naseri-Nosar, M.; Ziora, Z.M. Wound dressings from naturally-occurring polymers: A review on homopolysaccharide-based composites. Carbohydr. Polym 2018, 189, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M.; Melrose, J. Proteoglycans in Normal and Healing Skin. Adv. Wound Care (New Rochelle) 2015, 4, 152–173. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2018, e1801651. [Google Scholar] [CrossRef]
- Tziveleka, L.A.; Ioannou, E.; Tsiourvas, D.; Berillis, P.; Foufa, E.; Roussis, V. Collagen from the Marine Sponges Axinella cannabina and Suberites carnosus: Isolation and Morphological, Biochemical, and Biophysical Characterization. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Fauzi, M.B.; Lokanathan, Y.; Aminuddin, B.S.; Ruszymah, B.H.I.; Chowdhury, S.R. Ovine tendon collagen: Extraction, characterisation and fabrication of thin films for tissue engineering applications. Mater. Sci. Eng. 2016, 68, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Rittie, L. Type I Collagen Purification from Rat Tail Tendons. Methods Mol. Biol. 2017, 1627, 287–308. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Ramshaw, J.A. Biomedical applications of collagens. J. Biomed. Mater. Res. 2016, 104, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Foster, K. The use of fibrin sealant in burn operations. Surgery 2007, 142, S50–S54. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, D.M.; Roy, D.C.; Becerra, S.C.; Natesan, S.; Christy, R.J. In Situ Delivery of Fibrin-Based Hydrogels Prevents Contraction and Reduces Inflammation. J. Burn Care Res. 2018, 39, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, D.M.; Stone, R., 2nd; Wrice, N.; Laborde, A.; Becerra, S.C.; Natesan, S.; Christy, R.J. Delivery of Allogeneic Adipose Stem Cells in Polyethylene Glycol-Fibrin Hydrogels as an Adjunct to Meshed Autografts After Sharp Debridement of Deep Partial Thickness Burns. Stem Cells Transl. Med. 2018, 7, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.; Zamora, D.O.; Wrice, N.L.; Baer, D.G.; Christy, R.J. Bilayer hydrogel with autologous stem cells derived from debrided human burn skin for improved skin regeneration. J. Burn Care Res. 2013, 34, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.; Zhang, G.; Baer, D.G.; Walters, T.J.; Christy, R.J.; Suggs, L.J. A bilayer construct controls adipose-derived stem cell differentiation into endothelial cells and pericytes without growth factor stimulation. Tissue Eng. Part A 2011, 17, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Pietrzak, W.S.; Blanton, M. Platelet-rich plasma: A review of biology and applications in plastic surgery. Plast. Reconstr. Surg. 2006, 118, 147e–159e. [Google Scholar] [CrossRef] [PubMed]
- Kazakos, K.; Lyras, D.N.; Verettas, D.; Tilkeridis, K.; Tryfonidis, M. The use of autologous PRP gel as an aid in the management of acute trauma wounds. Injury 2009, 40, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Aurora, A.; Wrice, N.; Walters, T.J.; Christy, R.J.; Natesan, S. A PEGylated platelet free plasma hydrogel based composite scaffold enables stable vascularization and targeted cell delivery for volumetric muscle loss. Acta Biomater. 2018, 65, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Moles, C.M.; Bhattacharya, S.S.; LeSaint, M.; Dhamija, Y.; Le, L.D.; King, A.; Kidd, M.; Bouso, M.F.; Shaaban, A.; et al. Comparison of interleukin 10 homologs on dermal wound healing using a novel human skin ex vivo organ culture model. J. Surg. Res. 2014, 190, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Hanel, K.H.; Cornelissen, C.; Luscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care (New Rochelle) 2014, 3, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Raja; Sivamani, K.; Garcia, M.S.; Isseroff, R.R. Wound re-epithelialization: Modulating keratinocyte migration in wound healing. Front. Biosci. 2007, 12, 2849–2868. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.L.; Marshall, C.D.; Longaker, M.T. Minimizing Skin Scarring through Biomaterial Design. J. Funct. Biomater. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Francesko, A.; Petkova, P.; Tzanov, T. Hydrogel Dressings for Advanced Wound Management. Clin. Med. Chem. 2017. [Google Scholar] [CrossRef]
- Fuchs, E.; Raghavan, S. Getting under the skin of epidermal morphogenesis. Nat. Rev. 2002, 3, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.T.; Green, H. Differentiation of the epidermal keratinocyte in cell culture: Formation of the cornified envelope. Cell 1976, 9, 511–521. [Google Scholar] [CrossRef]
- Anacker, D.; Moody, C. Generation of organotypic raft cultures from primary human keratinocytes. J. Vis. Exp. 2012, e3668. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.T.; Rice, R.K.; Lynch, K.A.; Supp, A.P.; Swope, V.B.; Kagan, R.J.; Supp, D.M. Assessment of replication rates of human keratinocytes in engineered skin substitutes grafted to athymic mice. Wound Repair Regen. 2012, 20, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.T.; Kagan, R.J.; Greenhalgh, D.G.; Warner, P.; Yakuboff, K.P.; Palmieri, T.; Warden, G.D. Cultured skin substitutes reduce requirements for harvesting of skin autograft for closure of excised, full-thickness burns. J. Trauma 2006, 60, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.A.; Gibson, A.L.; Schlosser, S.J.; Schurr, M.J.; Allen-Hoffmann, B.L. Chimeric composite skin substitutes for delivery of autologous keratinocytes to promote tissue regeneration. Ann. Surg. 2010, 251, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, A.; Amini-Nik, S.; Jeschke, M.G. Animal models in burn research. Cell. Mol. Life Sci. 2014, 71, 3241–3255. [Google Scholar] [CrossRef] [PubMed]
- Dorsett-Martin, W.A. Rat models of skin wound healing: A review. Wound Repair Regen. 2004, 12, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Steinert, P.M. A model for the hierarchical structure of the human epidermal cornified cell envelope. Cell Death Differ. 1995, 2, 33–40. [Google Scholar] [PubMed]
- Gangatirkar, P.; Paquet-Fifield, S.; Li, A.; Rossi, R.; Kaur, P. Establishment of 3D organotypic cultures using human neonatal epidermal cells. Nat. Protoc. 2007, 2, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.; Thomas-Virnig, C.; Allen-Hoffmann, B.L. Classical human epidermal keratinocyte cell culture. Methods Mol. Biol. 2013, 945, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Helary, C.; Zarka, M.; Giraud-Guille, M.M. Fibroblasts within concentrated collagen hydrogels favour chronic skin wound healing. J. Tissue Eng. Regen. Med. 2012, 6, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, X.; Wang, Z.; Zhang, J.; Suggs, L. A PEGylated fibrin patch for mesenchymal stem cell delivery. Tissue Eng. 2006, 12, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Barthel, L.K.; Raymond, P.A. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J. Histochem. Cytochem. 1990, 38, 1383–1388. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stone, R., II; Wall, J.T.; Natesan, S.; Christy, R.J. PEG-Plasma Hydrogels Increase Epithelialization Using a Human Ex Vivo Skin Model. Int. J. Mol. Sci. 2018, 19, 3156. https://doi.org/10.3390/ijms19103156
Stone R II, Wall JT, Natesan S, Christy RJ. PEG-Plasma Hydrogels Increase Epithelialization Using a Human Ex Vivo Skin Model. International Journal of Molecular Sciences. 2018; 19(10):3156. https://doi.org/10.3390/ijms19103156
Chicago/Turabian StyleStone, Randolph, II, John T. Wall, Shanmugasundaram Natesan, and Robert J. Christy. 2018. "PEG-Plasma Hydrogels Increase Epithelialization Using a Human Ex Vivo Skin Model" International Journal of Molecular Sciences 19, no. 10: 3156. https://doi.org/10.3390/ijms19103156
APA StyleStone, R., II, Wall, J. T., Natesan, S., & Christy, R. J. (2018). PEG-Plasma Hydrogels Increase Epithelialization Using a Human Ex Vivo Skin Model. International Journal of Molecular Sciences, 19(10), 3156. https://doi.org/10.3390/ijms19103156