The Potential of Intrinsically Magnetic Mesenchymal Stem Cells for Tissue Engineering
Abstract
1. Introduction
2. Extrinsic Magnetization
3. Intrinsic Magnetization
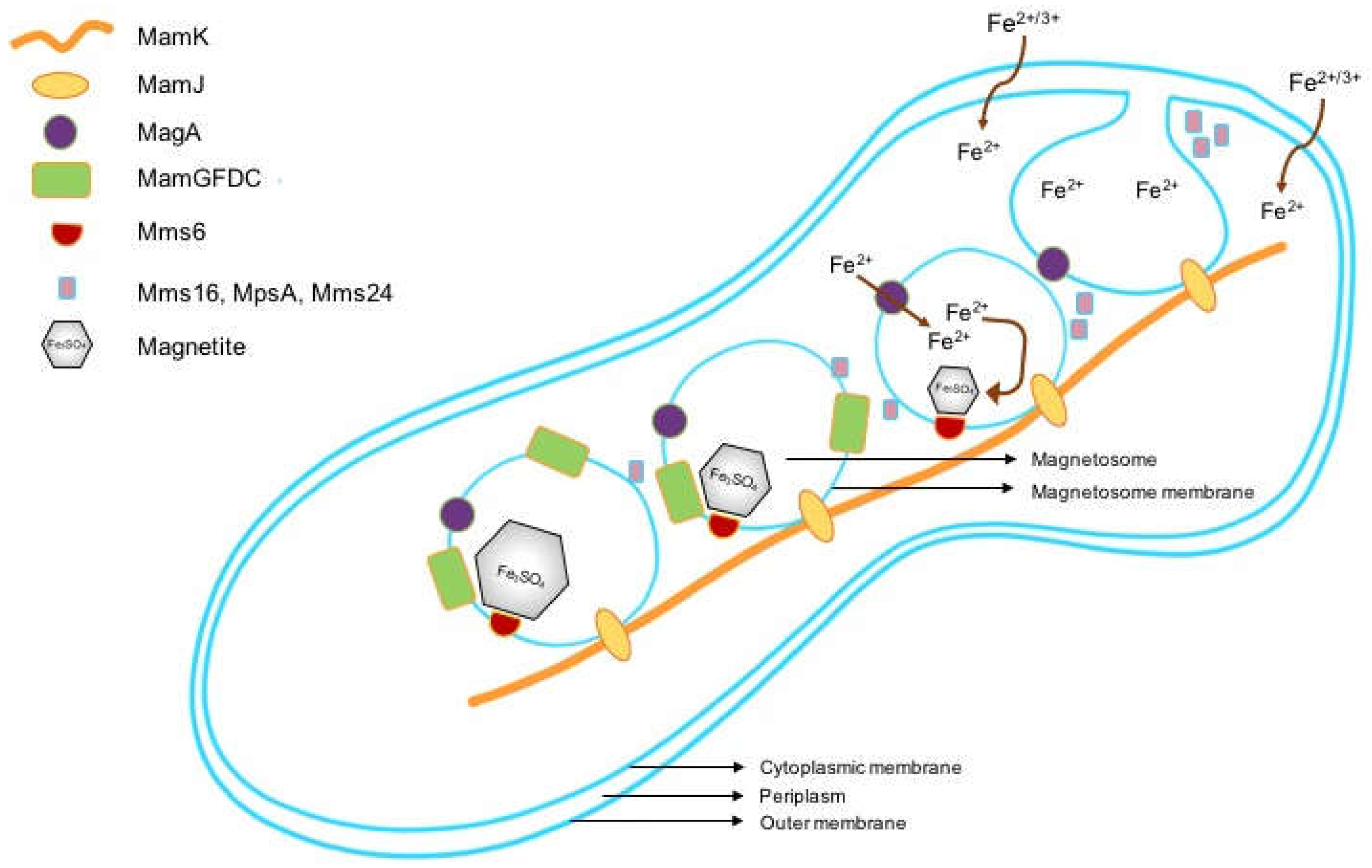
3.1. Magnetotactic Bacteria and Magnetosomes
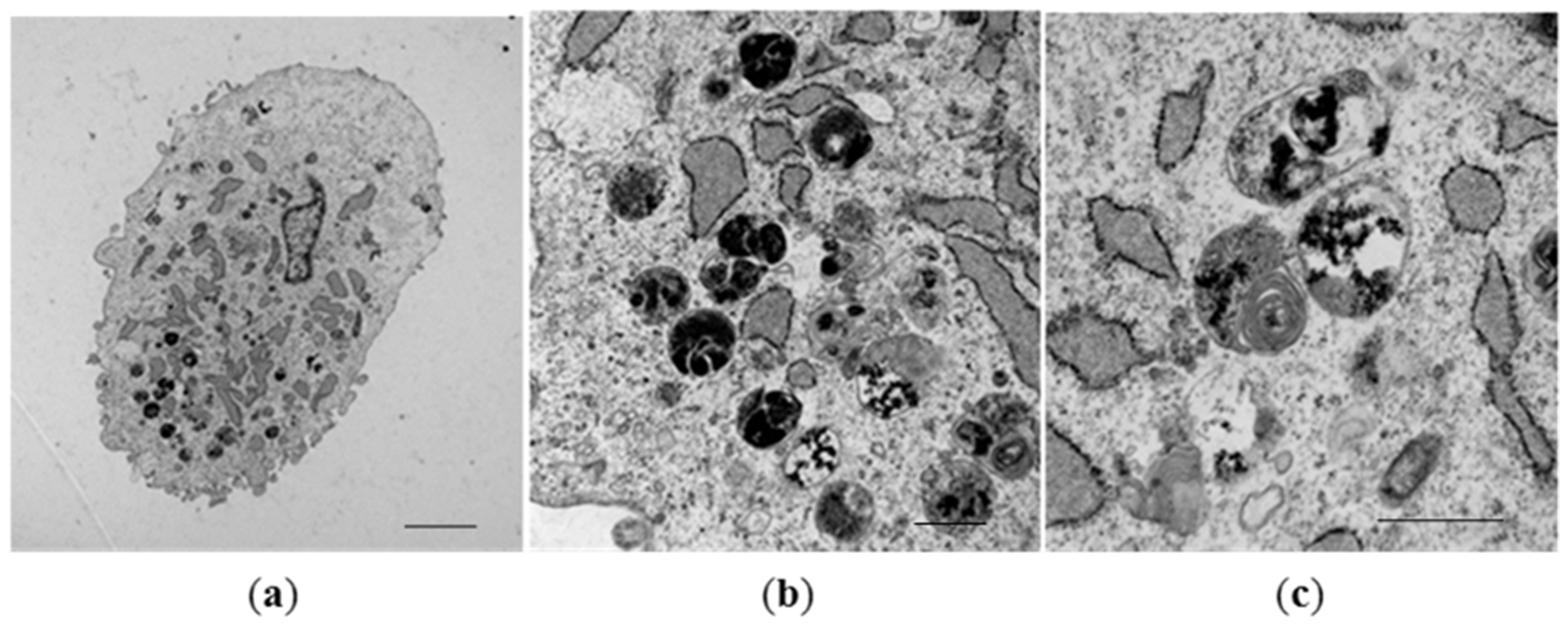
3.2. Formation of Magnetosome-Like Nanoparticles in Mammalian Cells
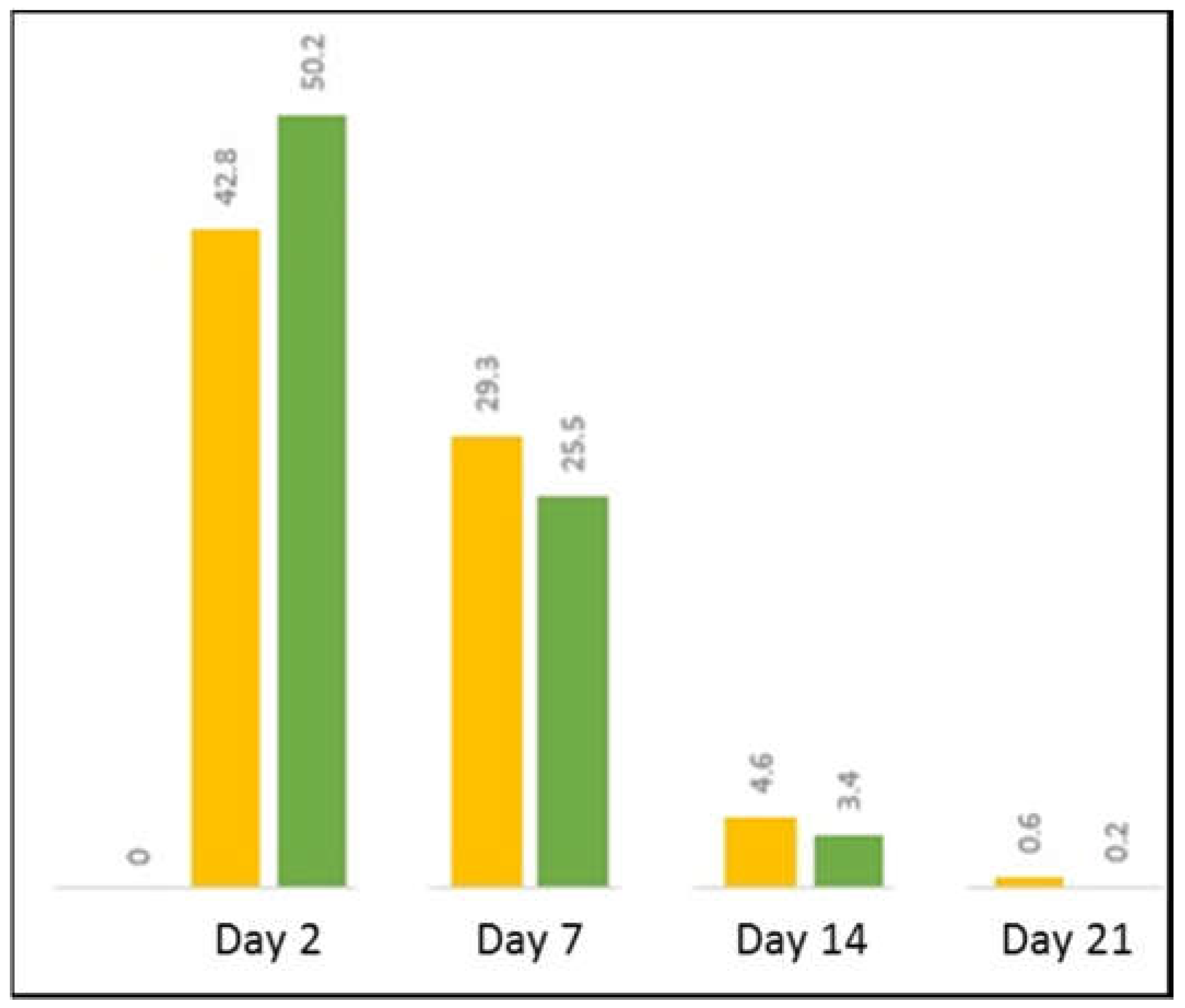
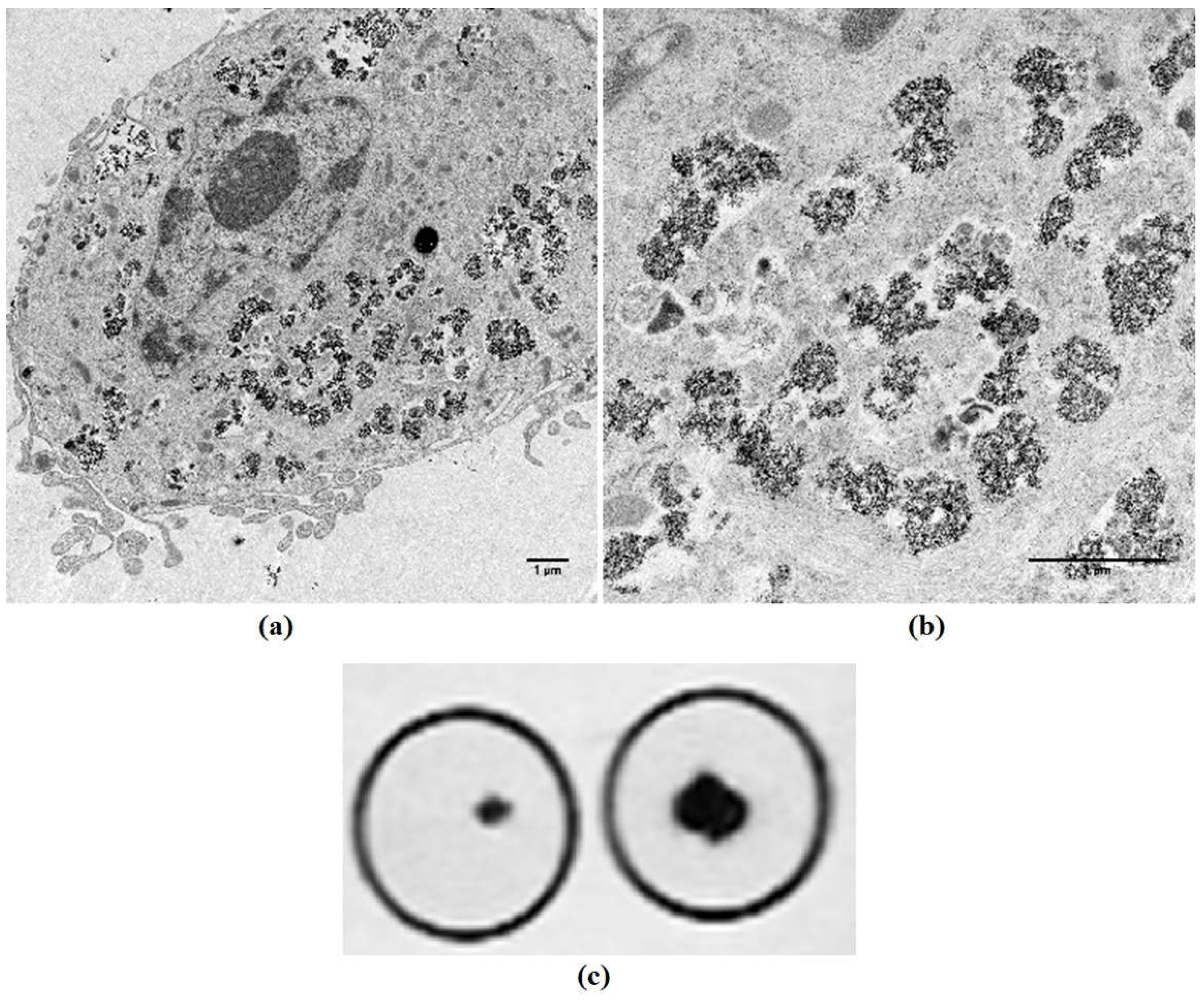
3.3. Biosynthesis of Magnetic Nanoparticles by Human Mesenchymal Stem Cells
4. Tracking, Localization, and Retention of Magnetic MSCs
4.1. MR Imaging
4.2. Magnetic Targeting
5. Magnetic MSCs and Hyperthermia
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gao, Z.; Zhang, L.; Hu, J.; Sun, Y. Mesenchymal stem cells: A potential targeted-delivery vehicle for anti-cancer drug, loaded nanoparticles. Nanomedicine 2013, 9, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Gazeau, F.; Wilhelm, C. Magnetic labeling, imaging and manipulation of endothelial progenitor cells using iron oxide nanoparticles. Future Med. Chem. 2010, 2, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cuschieri, A. Tumour cell labelling by magnetic nanoparticles with determination of intracellular iron content and spatial distribution of the intracellular iron. Int. J. Mol. Sci. 2013, 14, 9111–9125. [Google Scholar] [CrossRef] [PubMed]
- Cores, J.; Caranasos, T.G.; Cheng, K. Magnetically Targeted Stem Cell Delivery for Regenerative Medicine. J. Funct. Biomater. 2015, 6, 526–546. [Google Scholar] [CrossRef] [PubMed]
- Panseri, S.; Montesi, M.; Iafisco, M.; Adamiano, A.; Ghetti, M.; Cenacchi, G.; Tampieri, A. Magnetic Labelling of Mesenchymal Stem Cells with Iron-Doped Hydroxyapatite Nanoparticles as Tool for Cell Therapy. J. Biomed. Nanotechnol. 2016, 12, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Jasmin, G.T.D.S.; de Souza, G.T.; Louzada, R.A.; Rosado-de-Castro, P.H.; Mendez-Otero, R.; Campos de Carvalho, A.C. Tracking stem cells with superparamagnetic iron oxide nanoparticles: Perspectives and considerations. Int. J. Nanomed. 2017, 12, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wang, L.; Che, Y.; Li, Z.; Kong, D. Preparation and evaluation of magnetic nanoparticles for cell labeling. J. Nanosci. Nanotechnol. 2011, 11, 3749–3756. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Zhang, Y.L.; Qian, S.P.; Yu, X.B.; Xie, H.Y.; Zhou, L.; Zheng, S.S. Assessment of biological characteristics of mesenchymal stem cells labeled with superparamagnetic iron oxide particles in vitro. Mol. Med. Rep. 2012, 5, 317–320. [Google Scholar] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.M.; Hsiao, J.K.; Chen, Y.C.; Chien, L.Y.; Yao, M.; Chen, Y.K.; Ko, B.S.; Hsu, S.C.; Tai, L.A.; Cheng, H.Y.; et al. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials 2009, 30, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Hsiao, J.K.; Liu, H.M.; Lai, I.Y.; Yao, M.; Hsu, S.C.; Ko, B.S.; Chen, Y.C.; Yang, C.S.; Huang, D.M. The inhibitory effect of superparamagnetic iron oxide nanoparticle (Ferucarbotran) on osteogenic differentiation and its signaling mechanism in human mesenchymal stem cells. Toxicol. Appl. Pharmacol. 2010, 245, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Henning, T.D.; Sutton, E.J.; Kim, A.; Golovko, D.; Horvai, A.; Ackerman, L.; Sennino, B.; McDonald, D.; Lotz, J.; Daldrup-Link, H.E. The influence of ferucarbotran on the chondrogenesis of human mesenchymal stem cells. Contrast Media Mol. Imaging 2009, 4, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, B.; Cao, M.; Sun, J.; Wu, H.; Zhao, P.; Xing, J.; Yang, Y.; Zhang, X.; Ji, M.; et al. Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials 2016, 86, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, H.; Zarivach, R. Structure prediction of magnetosome-associated proteins. Front. Microbiol. 2014, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Bazylinski, D.A.; Frankel, R.B. Magnetosome formation in prokaryotes. Nat. Rev. Microbiol. 2004, 2, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, R.P. Magnetotactic bacteria. Annu. Rev. Microbiol. 1982, 36, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Barber-Zucker, S.; Keren-Khadmy, N.; Zarivach, R. From invagination to navigation: The story of magnetosome-associated proteins in magnetotactic bacteria. Protein Sci. 2016, 25, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Gorby, Y.A.; Beveridge, T.J.; Blakemore, R.P. Characterization of the bacterial magnetosome membrane. J. Bacteriol. 1988, 170, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Rong, C.; Zhang, C.; Zhang, Y.; Qi, L.; Yang, J.; Guan, G.; Li, Y.; Li, J. FeoB2 Functions in magnetosome formation and oxidative stress protection in Magnetospirillum gryphiswaldense strain MSR-1. J. Bacteriol. 2012, 194, 3972–3976. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E.; Ngo, A.T.; Lefèvre, C.; Lisiecki, I.; Wu, L.F.; Pileni, M.P. Difference between the Magnetic Properties of the Magnetotactic Bacteria and Those of the Extracted Magnetosomes: Influence of the Distance between the Chains of Magnetosomes. J. Phys. Chem. C 2008, 112, 12304–12309. [Google Scholar] [CrossRef]
- Komeili, A. Molecular mechanisms of compartmentalization and biomineralization in magnetotactic bacteria. FEMS Microbiol. Rev. 2012, 36, 232–255. [Google Scholar] [CrossRef] [PubMed]
- Jogler, C.; Schuler, D. Genomics, genetics, and cell biology of magnetosome formation. Annu. Rev. Microbiol. 2009, 63, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Murat, D.; Quinlan, A.; Vali, H.; Komeili, A. Comprehensive genetic dissection of the magnetosome gene island reveals the step-wise assembly of a prokaryotic organelle. Proc. Natl. Acad. Sci. USA 2010, 107, 5593–5598. [Google Scholar] [CrossRef] [PubMed]
- Lohsse, A.; Ullrich, S.; Katzmann, E.; Borg, S.; Wanner, G.; Richter, M.; Voigt, B.; Schweder, T.; Schuler, D. Functional analysis of the magnetosome island in Magnetospirillum gryphiswaldense: The mamAB operon is sufficient for magnetite biomineralization. PLoS ONE 2011, 6, e25561. [Google Scholar] [CrossRef] [PubMed]
- Uebe, R.; Schuler, D. Magnetosome biogenesis in magnetotactic bacteria. Nat. Rev. Microbiol. 2016, 14, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Okamura, Y.; Takeyama, H.; Matsunaga, T. Dynamic analysis of a genomic island in Magnetospirillum sp. strain AMB-1 reveals how magnetosome synthesis developed. FEBS Lett. 2006, 580, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Zeytuni, N.; Ozyamak, E.; Ben-Harush, K.; Davidov, G.; Levin, M.; Gat, Y.; Moyal, T.; Brik, A.; Komeili, A.; Zarivach, R. Self-recognition mechanism of MamA, a magnetosome-associated TPR-containing protein, promotes complex assembly. Proc. Natl. Acad. Sci. USA 2011, 108, E480–E487. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, A.; Murat, D.; Vali, H.; Komeili, A. The HtrA/DegP family protease MamE is a bifunctional protein with roles in magnetosome protein localization and magnetite biomineralization. Mol. Microbiol. 2011, 80, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Mazuyama, E.; Arakaki, A.; Matsunaga, T. MMS6 protein regulates crystal morphology during nano-sized magnetite biomineralization in vivo. J. Biol. Chem. 2011, 286, 6386–6392. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, C.; Burgess, J.G.; Sode, K.; Matsunaga, T. An iron-regulated gene, magA, encoding an iron transport protein of Magnetospirillum sp. strain AMB-1. J. Biol. Chem. 1995, 270, 28392–28396. [Google Scholar] [PubMed]
- Uebe, R.; Henn, V.; Schuler, D. The MagA protein of Magnetospirilla is not involved in bacterial magnetite biomineralization. J. Bacteriol. 2012, 194, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Barber-Zucker, S.; Uebe, R.; Davidov, G.; Navon, Y.; Sherf, D.; Chill, J.H.; Kass, I.; Bitton, R.; Schuler, D.; Zarivach, R. Disease-Homologous Mutation in the Cation Diffusion Facilitator Protein MamM Causes Single-Domain Structural Loss and Signifies Its Importance. Sci. Rep. 2016, 6, 31933. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, A.; Webb, J.; Matsunaga, T. A novel protein tightly bound to bacterial magnetic particles in Magnetospirillum magneticum strain AMB-1. J. Biol. Chem. 2003, 278, 8745–8750. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, A.; Yamagishi, A.; Fukuyo, A.; Tanaka, M.; Matsunaga, T. Co-ordinated functions of Mms proteins define the surface structure of cubo-octahedral magnetite crystals in magnetotactic bacteria. Mol. Microbiol. 2014, 93, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, A.; Tanaka, M.; Lenders, J.J.M.; Thiesbrummel, J.; Sommerdijk, N.A.J.M.; Matsunaga, T.; Arakaki, A. Control of magnetite nanocrystal morphology in magnetotactic bacteria by regulation of mms7 gene expression. Sci. Rep. 2016, 6, 29785. [Google Scholar] [CrossRef] [PubMed]
- Lohsse, A.; Borg, S.; Raschdorf, O.; Kolinko, I.; Tompa, E.; Posfai, M.; Faivre, D.; Baumgartner, J.; Schuler, D. Genetic dissection of the mamAB and mms6 operons reveals a gene set essential for magnetosome biogenesis in Magnetospirillum gryphiswaldense. J. Bacteriol. 2014, 196, 2658–2669. [Google Scholar] [CrossRef] [PubMed]
- Staniland, S.S.; Rawlings, A.E. Crystallizing the function of the magnetosome membrane mineralization protein Mms6. Biochem. Soc. Trans. 2016, 44, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Grunberg, K.; Muller, E.C.; Otto, A.; Reszka, R.; Linder, D.; Kube, M.; Reinhardt, R.; Schuler, D. Biochemical and Proteomic Analysis of the Magnetosome Membrane in Magnetospirillum gryphiswaldense. Appl. Environ. Microbiol. 2004, 70, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Shibata, K.; Hosokawa, M.; Hatakeyama, K.; Arakaki, A.; Gomyo, H.; Mogi, T.; Taguchi, T.; Wake, H.; Tanaami, T.; et al. Characterization of magnetic nanoparticles modified with thiol functionalized PAMAM dendron for DNA recovery. J. Colloid Interface Sci. 2012, 377, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.M.; Rawlings, A.E.; Galloway, J.M.; Staniland, S.S. Using a biomimetic membrane surface experiment to investigate the activity of the magnetite biomineralisation protein Mms6. RSC Adv. 2016, 6, 7356–7363. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, A.; Narumiya, K.; Tanaka, M.; Matsunaga, T.; Arakaki, A. Core Amino Acid Residues in the Morphology-Regulating Protein, Mms6, for Intracellular Magnetite Biomineralization. Sci. Rep. 2016, 6, 35670. [Google Scholar] [CrossRef] [PubMed]
- Murat, D.; Falahati, V.; Bertinetti, L.; Csencsits, R.; Kornig, A.; Downing, K.; Faivre, D.; Komeili, A. The magnetosome membrane protein, MmsF, is a major regulator of magnetite biomineralization in Magnetospirillum magneticum AMB-1. Mol. Microbiol. 2012, 85, 684–699. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.E.; Bramble, J.P.; Walker, R.; Bain, J.; Galloway, J.M.; Staniland, S.S. Self-assembled MmsF proteinosomes control magnetite nanoparticle formation in vitro. Proc. Natl. Acad. Sci. USA 2014, 111, 16094–16099. [Google Scholar] [CrossRef] [PubMed]
- Zurkiya, O.; Chan, A.W.; Hu, X. MagA is sufficient for producing magnetic nanoparticles in mammalian cells, making it an MRI reporter. Magn. Reson. Med. 2008, 59, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Goldhawk, D.E.; Lemaire, C.; McCreary, C.R.; McGirr, R.; Dhanvantari, S.; Thompson, R.T.; Figueredo, R.; Koropatnick, J.; Foster, P.; Prato, F.S. Magnetic resonance imaging of cells overexpressing MagA, an endogenous contrast agent for live cell imaging. Mol. Imaging 2009, 8, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Robledo, B.N.; Harris, S.S.; Hu, X.P. A bacterial gene, mms6, as a new reporter gene for magnetic resonance imaging of mammalian cells. Mol. Imaging 2014, 13, 1–12. [Google Scholar]
- Elfick, A.; Rischitor, G.; Mouras, R.; Azfer, A.; Lungaro, L.; Uhlarz, M.; Herrmannsdorfer, T.; Lucocq, J.; Gamal, W.; Bagnaninchi, P.; et al. Biosynthesis of magnetic nanoparticles by human mesenchymal stem cells following transfection with the magnetotactic bacterial gene mms6. Sci. Rep. 2017, 7, 39755. [Google Scholar] [CrossRef] [PubMed]
- Frankel, R.B.; Blakemore, R.P. Magnetite and magnetotaxis in microorganisms. Adv. Exp. Med. Biol. 1988, 238, 321–330. [Google Scholar] [PubMed]
- Ankrum, J.; Karp, J.M. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol. Med. 2010, 16, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Chemaly, E.R.; Yoneyama, R.; Frangioni, J.V.; Hajjar, R.J. Tracking stem cells in the cardiovascular system. Trends Cardiovasc. Med. 2005, 15, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Chavakis, E.; Urbich, C.; Dimmeler, S. Homing and engraftment of progenitor cells: A prerequisite for cell therapy. J. Mol. Cell. Cardiol. 2008, 45, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, B.M.; Kassem, M. Human mesenchymal stem cells: From basic biology to clinical applications. Gene Ther. 2008, 15, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Andreas, K.; Georgieva, R.; Ladwig, M.; Mueller, S.; Notter, M.; Sittinger, M.; Ringe, J. Highly efficient magnetic stem cell labeling with citrate-coated superparamagnetic iron oxide nanoparticles for MRI tracking. Biomaterials 2012, 33, 4515–4525. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Jin, X.; Dai, G.; Liu, J.; Chen, J.; Yang, L. In vitro targeted magnetic delivery and tracking of superparamagnetic iron oxide particles labeled stem cells for articular cartilage defect repair. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Goldhawk, D.E.; Gelman, N.; Thompson, R.T.; Prato, F.S. Forming Magnetosome-Like Nanoparticles in Mammalian Cells for Molecular MRI. In Design and Applications of Nanoparticles in Biomedical Imaging; Springer: Cham, Switzerland, 2017; pp. 187–203. [Google Scholar]
- Farrell, E.; Wielopolski, P.; Pavljasevic, P.; van Tiel, S.; Jahr, H.; Verhaar, J.; Weinans, H.; Krestin, G.; O’Brien, F.J.; van Osch, G.; et al. Effects of iron oxide incorporation for long term cell tracking on MSC differentiation in vitro and in vivo. Biochem. Biophys. Res. Commun. 2008, 369, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shen, Y.; Sun, A.; Huang, G.; Zhu, H.; Huang, B.; Xu, J.; Song, Y.; Pei, N.; Ma, J.; et al. Magnetic targeting enhances retrograde cell retention in a rat model of myocardial infarction. Stem Cell Res. Ther. 2013, 4, 149. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Liu, X.; Huang, Z.; Pei, N.; Xu, J.; Li, Z.; Wang, Y.; Qian, J.; Ge, J. Comparison of Magnetic Intensities for Mesenchymal Stem Cell Targeting Therapy on Ischemic Myocardial Repair: High Magnetic Intensity Improves Cell Retention but Has No Additional Functional Benefit. Cell Transplant. 2015, 24, 1981–1997. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.H.; Cruz, F.F.; Morales, M.M.; Weiss, D.J.; Rocco, P.R. Magnetic targeting as a strategy to enhance therapeutic effects of mesenchymal stromal cells. Stem Cell Res. Ther. 2017, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Panseri, S.; Russo, A.; Giavaresi, G.; Sartori, M.; Veronesi, F.; Fini, M.; Salter, D.M.; Ortolani, A.; Strazzari, A.; Visani, A.; et al. Innovative magnetic scaffolds for orthopedic tissue engineering. J. Biomed. Mater. Res. A 2012, 100, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Kamei, G.; Kobayashi, T.; Ohkawa, S.; Kongcharoensombat, W.; Adachi, N.; Takazawa, K.; Shibuya, H.; Deie, M.; Hattori, K.; Goldberg, J.L.; et al. Articular cartilage repair with magnetic mesenchymal stem cells. Am. J. Sports Med. 2013, 41, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Ochi, M.; Adachi, N.; Ishikawa, M.; Yanada, S.; Levin, L.S.; Kamei, G.; Kobayashi, T. The safety and efficacy of magnetic targeting using autologous mesenchymal stem cells for cartilage repair. Knee Surg. Sports Traumatol. Arthrosc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, V.; Adamiano, A.; Ausanio, G.; Lanotte, L.; Aquilanti, G.; Coey, J.M.D.; Lantieri, M.; Spina, G.; Fittipaldi, M.; Margaris, G.; et al. Fe-Doping-Induced Magnetism in Nano-Hydroxyapatites. Inorg. Chem. 2017, 56, 4447–4459. [Google Scholar] [CrossRef] [PubMed]
- Yanai, A.; Hafeli, U.O.; Metcalfe, A.L.; Soema, P.; Addo, L.; Gregory-Evans, C.Y.; Po, K.; Shan, X.; Moritz, O.L.; Gregory-Evans, K. Focused magnetic stem cell targeting to the retina using superparamagnetic iron oxide nanoparticles. Cell Transplant. 2012, 21, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Kyrtatos, P.G.; Lehtolainen, P.; Junemann-Ramirez, M.; Garcia-Prieto, A.; Price, A.N.; Martin, J.F.; Gadian, D.G.; Pankhurst, Q.A.; Lythgoe, M.F. Magnetic tagging increases delivery of circulating progenitors in vascular injury. JACC Cardiovasc. Interv. 2009, 2, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Nakamae, T.; Adachi, N.; Kobayashi, T.; Nagata, Y.; Nakasa, T.; Tanaka, N.; Ochi, M. The effect of an external magnetic force on cell adhesion and proliferation of magnetically labeled mesenchymal stem cells. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2010, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Maredziak, M.; Marycz, K.; Lewandowski, D.; Siudzinska, A.; Smieszek, A. Static magnetic field enhances synthesis and secretion of membrane-derived microvesicles (MVs) rich in VEGF and BMP-2 in equine adipose-derived stromal cells (EqASCs)-a new approach in veterinary regenerative medicine. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Maredziak, M.; Marycz, K.; Smieszek, A.; Lewandowski, D.; Toker, N.Y. The influence of static magnetic fields on canine and equine mesenchymal stem cells derived from adipose tissue. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Maredziak, M.; Tomaszewski, K.; Polinceusz, P.; Lewandowski, D.; Marycz, K. Static magnetic field enhances the viability and proliferation rate of adipose tissue-derived mesenchymal stem cells potentially through activation of the phosphoinositide 3-kinase/Akt (PI3K/Akt) pathway. Electromagn. Biol. Med. 2017, 36, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Lepock, J.R. Cellular effects of hyperthermia: Relevance to the minimum dose for thermal damage. Int. J. Hyperther. 2003, 19, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Coss, R.; Linnemans, W. The effects of hyperthermia on the cytoskeleton: A review. Int. J. Hyperther. 1996, 12, 173–196. [Google Scholar] [CrossRef]
- Oei, A.L.; Vriend, L.E.; Crezee, J.; Franken, N.A.; Krawczyk, P.M. Effects of hyperthermia on DNA repair pathways: One treatment to inhibit them all. Radiat. Oncol. 2015, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Yagawa, Y.; Tanigawa, K.; Kobayashi, Y.; Yamamoto, M. Cancer immunity and therapy using hyperthermia with immunotherapy, radiotherapy, chemotherapy, and surgery. J. Cancer Metast. Treat. 2017, 3, 219. [Google Scholar] [CrossRef]
- Hervault, A.; Thanh, N.T.K. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale 2014, 6, 11553–11573. [Google Scholar] [CrossRef] [PubMed]
- Mallory, M.; Gogineni, E.; Jones, G.C.; Greer, L.; Simone II, C.B. Therapeutic hyperthermia: The old, the new, and the upcoming. Crit. Rev. Oncol. Hematol. 2016, 97, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Habash, R.W.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal therapy, part 2: Hyperthermia techniques. Crit. Rev. Biomed. Eng. 2006, 34, 491–542. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.d.; Oliveira, T.R.; Mamani, J.B.; Malheiros, S.M.F.; Pavon, L.F.; Sibov, T.T.; Amaro Junior, E.; Gamarra, L.F. Magnetohyperthermia for treatment of gliomas: Experimental and clinical studies. Einstein 2010, 8, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Abreu, S.C.; Lopes-Pacheco, M.; da Silva, A.L.; Xisto, D.G.; de Oliveira, T.B.; Kitoko, J.Z.; De Castro, L.L.; Amorim, N.R.; Martins, V.; Gonçalves-de-Albuquerque, C.F. Eicosapentaenoic acid enhances the effects of mesenchymal stromal cell therapy in experimental allergic asthma. Front. Immunol. 2018, 9, 1147. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Carrey, J.; Mehdaoui, B.; Respaud, M. Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: Application to magnetic hyperthermia optimization. J. Appl. Phys. 2011, 109, 083921. [Google Scholar] [CrossRef]
- Cheraghipour, E.; Javadpour, S. Cationic albumin-conjugated magnetite nanoparticles, novel candidate for hyperthermia cancer therapy. Int. J. Hyperther. 2013, 29, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Kolosnjaj-Tabi, J.; Wilhelm, C. Magnetic nanoparticles in cancer therapy: How can thermal approaches help? Nanomedicine 2017, 12, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Nandwana, V.; De, M.; Chu, S.; Jaiswal, M.; Rotz, M.; Meade, T.J.; Dravid, V.P. Theranostic magnetic nanostructures (MNS) for cancer. In Nanotechnology-Based Precision Tools for the Detection and Treatment of Cancer; Springer: Cham, Switzerland, 2015; pp. 51–83. [Google Scholar]
- Alphandery, E.; Faure, S.; Seksek, O.; Guyot, F.; Chebbi, I. Chains of magnetosomes extracted from AMB-1 magnetotactic bacteria for application in alternative magnetic field cancer therapy. ACS Nano 2011, 5, 6279–6296. [Google Scholar] [CrossRef] [PubMed]
- Le Fèvre, R.; Durand-Dubief, M.; Chebbi, I.; Mandawala, C.; Lagroix, F.; Valet, J.-P.; Idbaih, A.; Adam, C.; Delattre, J.-Y.; Schmitt, C. Enhanced antitumor efficacy of biocompatible magnetosomes for the magnetic hyperthermia treatment of glioblastoma. Theranostics 2017, 7, 4618. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia—A promising tumour therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef] [PubMed]
- Basel, M.T.; Balivada, S.; Wang, H.; Shrestha, T.B.; Seo, G.M.; Pyle, M.; Abayaweera, G.; Dani, R.; Koper, O.B.; Tamura, M. Cell-delivered magnetic nanoparticles caused hyperthermia-mediated increased survival in a murine pancreatic cancer model. Int. J. Nanomed. 2012, 7, 297. [Google Scholar] [CrossRef] [PubMed]
- Serakinci, N.; Fahrioglu, U.; Christensen, R. Mesenchymal stem cells, cancer challenges and new directions. Eur. J. Cancer 2014, 50, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Reagan, M.R.; Kaplan, D.L. Concise review: Mesenchymal stem cell tumor-homing: Detection methods in disease model systems. Stem Cells 2011, 29, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Kalber, T.L.; Ordidge, K.L.; Southern, P.; Loebinger, M.R.; Kyrtatos, P.G.; Pankhurst, Q.A.; Lythgoe, M.F.; Janes, S.M. Hyperthermia treatment of tumors by mesenchymal stem cell-delivered superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2016, 11, 1973. [Google Scholar] [CrossRef] [PubMed]



| Differentiation Antigen | Non-Transfected MSCs | Non-Transfected MSCs with FeQ | mms6-Transfected MSCs with FeQ |
|---|---|---|---|
| CD34 | 3.1 ± 0.1 | 2.9 ± 0.3 | 3.2 ± 0.2 |
| CD73 | 99.9 ± 0.1 | 99.8 ± 0.1 | 99.8 ± 0.1 |
| CD90 | 99.9 ± 0.1 | 99.6 ± 0.1 | 98.1 ± 0.1 |
| CD105 | 98.8 ± 0.1 | 98.7 ± 0.1 | 98.3 ± 0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerans, F.F.A.; Lungaro, L.; Azfer, A.; Salter, D.M. The Potential of Intrinsically Magnetic Mesenchymal Stem Cells for Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 3159. https://doi.org/10.3390/ijms19103159
Kerans FFA, Lungaro L, Azfer A, Salter DM. The Potential of Intrinsically Magnetic Mesenchymal Stem Cells for Tissue Engineering. International Journal of Molecular Sciences. 2018; 19(10):3159. https://doi.org/10.3390/ijms19103159
Chicago/Turabian StyleKerans, Fransiscus F. A., Lisa Lungaro, Asim Azfer, and Donald M. Salter. 2018. "The Potential of Intrinsically Magnetic Mesenchymal Stem Cells for Tissue Engineering" International Journal of Molecular Sciences 19, no. 10: 3159. https://doi.org/10.3390/ijms19103159
APA StyleKerans, F. F. A., Lungaro, L., Azfer, A., & Salter, D. M. (2018). The Potential of Intrinsically Magnetic Mesenchymal Stem Cells for Tissue Engineering. International Journal of Molecular Sciences, 19(10), 3159. https://doi.org/10.3390/ijms19103159




