Traditional Chinese Medicine as a Potential Source for HSV-1 Therapy by Acting on Virus or the Susceptibility of Host
Abstract
:1. Introduction
1.1. Herpes Simplex Virus Type I (HSV-1) and Related Disease
1.2. Mechanism and Defects of Current Anti-HSV-1 Drugs
2. Natural Products as Potential Resources for New Antiviral Drugs
3. Natural Anti-HSV-1 Products from TCM
3.1. Extracts with Potential Anti-HSV-1 Activities
3.2. Pure Compounds Isolated from TCM with Anti-HSV-1 Activities
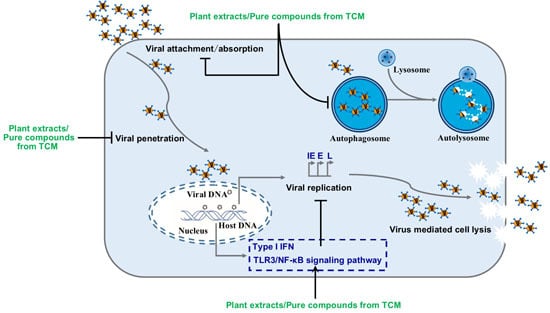
4. The Pharmacodynamic Mechanism of TCM against HSV-1
4.1. TCM Resists HSV-1 by Enhancing Organism Immunity
4.2. TCM Exerts Anti-HSV-1 Effect by Inducing Autophagy
4.3. TCM Exerts Antiviral Effects by Inhibiting HSV-1 Replication or Inactivation of HSV-1
4.4. Natural Anti-HSV-1 Products with Unclear Mechanism
5. Advantages and Limitations of TCM in the Prevention and Treatment of HSV-1 Infection
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HSV-1 | Herpes simplex virus type 1 |
| ACV | Acyclovir |
| TCM | Traditional Chinese Medicine |
| LFE | Lychee flower extract |
| mTOR | Mammalian target of rapamycin |
| p70S6K | p70S6 kinase |
| MHC-I | Major histocompatibility complex I |
| AqMOL | Aqueous extract |
| EMSA | Electrophoretic mobility shift assay |
| ISG | Interferon stimulated genes |
| CPE | Cytopathic effect |
| APS | Astragalus polysaccharide |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin 6 |
| TLR3 | Toll-like receptor 3 |
| NF-κB | Nuclear factor-κb |
| 1246TGG | 1,2,4,6-tetra-O-galloyl-β-d-glucose |
| OB | Ocimum basilicum |
| SI | Selectivity index |
| YCHT | Yin Chen Hao Tang |
| HCWEs | H. cordata water extracts |
References
- Laine, R.F.; Albecka, A.; van de Linde, S.; Rees, E.J.; Crump, C.M.; Kaminski, C.F. Structural analysis of herpes simplex virus by optical super-resolution imaging. Nat. Commun. 2015, 6, 5980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef] [PubMed]
- Vink, E.I.; Smiley, J.R.; Mohr, I. Subversion of host responses to energy insufficiency by Us3 supports herpes simplex virus 1 replication during stress. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Ives, A.M.; Bertke, A.S. Stress hormones epinephrine and corticosterone selectively modulate herpes simplex virus 1 (HSV-1) and HSV-2 productive infections in adult sympathetic, but not sensory, neurons. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Avgousti, D.C.; Weitzman, M.D. Stress flips a chromatin switch to wake up latent virus. Cell Host Microbe 2015, 18, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Cliffe, A.R.; Arbuckle, J.H.; Vogel, J.L.; Geden, M.J.; Rothbart, S.B.; Cusack, C.L.; Strahl, B.D.; Kristie, T.M.; Deshmukh, M. Neuronal stress pathway mediating a histone methyl/phospho switch is required for herpes simplex virus reactivation. Cell Host Microbe 2015, 18, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Messer, H.G.; Jacobs, D.; Dhummakupt, A.; Bloom, D.C. Inhibition of H3K27me3-specific histone demethylases JMJD3 and UTX blocks reactivation of herpes simplex virus 1 in trigeminal ganglion neurons. J. Virol. 2015, 89, 3417–3420. [Google Scholar] [CrossRef] [PubMed]
- Uchakin, P.N.; Parish, D.C.; Dane, F.C.; Uchakina, O.N.; Scheetz, A.P.; Agarwal, N.K.; Smith, B.E. Fatigue in medical residents leads to reactivation of herpes virus latency. Interdiscip. Perspect. Infect. Dis. 2011, 2011, 571340. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Cai, S.L.; Kim, J.; Nanez, A.; Sahin, M.; MacLean, K.H.; Inoki, K.; Guan, K.L.; Shen, J.; Person, M.D.; et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc. Natl. Acad. Sci. USA 2010, 107, 4153–4158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichihashi, M.; Nagai, H.; Matsunaga, K. Sunlight is an important causative factor of recurrent herpes simplex. Cutis 2004, 74, 14–18. [Google Scholar] [PubMed]
- Marques-Silva, L.; Castro, W.H.; Gomez, E.L.C.; Guimaraes, A.L.S.; Silva, M.S.L.; Gomez, R.S. The impact of dental surgery on HSV-1 reactivation in the oral mucosa of seropositive patients. J. Oral Maxillofac. Surg. 2007, 65, 2269–2272. [Google Scholar] [CrossRef] [PubMed]
- Zhua, L.Q.; Thompson, J.; Ma, F.R.; Eudy, J.; Jones, C. Effects of the synthetic corticosteroid dexamethasone on bovine herpesvirus 1 productive infection. Virology 2017, 505, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kook, I.; Jones, C. The serum and glucocorticoid-regulated protein kinases (SGK) stimulate bovine herpesvirus 1 and herpes simplex virus 1 productive infection. Virus Res. 2016, 222, 106–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsia, S.C.; Bedadala, G.R.; Balish, M.D. Effects of thyroid hormone on HSV-1 gene regulation: Implications in the control of viral latency and reactivation. Cell Biosci. 2011, 1. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.M.; Leong, M.L.L.; Kim, B.; Wang, E.; Park, J.; Hemmings, B.A.; Firestone, G.L. Hyperosmotic stress stimulates promoter activity and regulates cellular utilization of the serum- and glucocorticoid-inducible protein kinase (Sgk) by a p38 MAPK-dependent pathway. J. Biol. Chem. 2000, 275, 25262–25272. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xie, P.; Xu, M.M.; Li, P.; Zao, G.N. The influence of stress factors on the reactivation of latent herpes simplex virus type 1 in infected mice. Cell Biochem. Biophys. 2011, 61, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Steel, A.J.; Eslick, G.D. Herpes viruses increase the risk of Alzheimer’s disease: A meta-analysis. J. Alzheimers Dis. 2015, 47, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.A.; Harris, E.A. Herpes simplex virus type 1 and other pathogens are key causative factors in sporadic Alzheimer’s disease. J. Alzheimers Dis. 2015, 48, 319–353. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Lazzarotto, T.; Ianni, M.; Porcellini, E.; Forti, P.; Masliah, E.; Gabrielli, L.; Licastro, F. Herpes virus in Alzheimer’s disease: Relation to progression of the disease. Neurobiol. Aging 2014, 35, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Civitelli, L.; Marcocci, M.E.; Celestino, I.; Piacentini, R.; Garaci, E.; Grassi, C.; De Chiara, G.; Palamara, A.T. Herpes simplex virus type 1 infection in neurons leads to production and nuclear localization of APP intracellular domain (AICD): Implications for Alzheimer’s disease pathogenesis. J. Neurovirol. 2015, 21, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, R.; Li Puma, D.D.; Ripoli, C.; Marcocci, M.E.; De Chiara, G.; Garaci, E.; Palamara, A.T.; Grassi, C. Herpes simplex virus type-1 infection induces synaptic dysfunction in cultured cortical neurons via GSK-3 activation and intraneuronal amyloid-beta protein accumulation. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- He, R.R.; Kurihara, H. Shanghuo syndrome in traditional chinese medicine. World Sci. Technol. 2008, 10, 37–41. [Google Scholar] [CrossRef]
- Zhu, S.R.; Luo, X.; Li, Y.F.; Hiroshi, K.; He, R.R. Emotional stress-induced Shanghuo syndrome increases disease susceptibility. Zhongguo Zhong Yao Za Zhi 2018, 43, 1529–1535. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- De, S.K.; Hart, J.C.; Breuer, J. Herpes simplex virus and varicella zoster virus: Recent advances in therapy. Curr. Opin. Infect. Dis. 2015, 28, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ooi, V.E.; Chan, P.K.; Ang, P.O., Jr. Isolation and characterization of a sulfated polysaccharide from the brown alga Sargassum patens and determination of its anti-herpes activity. Biochem. Cell Biol. 2003, 81, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R. New approaches to the therapy of HSV infections. Herpes 2006, 13, 53–55. [Google Scholar] [PubMed]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.; Masarcikova, R.; Berchova, K. Bioactive natural products with anti-herpes simplex virus properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penesyan, A.; Kjelleberg, S.; Egan, S. Development of novel drugs from marine surface associated microorganisms. Mar. Drugs 2010, 8, 438–459. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Ather, A.; Thompson, K.D.; Gambari, R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antivir. Res. 2005, 67, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.M.; Chiang, S.T.; Chang, Y.Y.; Chen, Y.C.; Yang, D.J.; Chen, Y.Y.; Lin, H.W.; Tseng, J.K. Lychee flower extract inhibits proliferation and viral replication of HSV-1-infected corneal epithelial cells. Mol. Vis. 2016, 22, 129–137. [Google Scholar] [PubMed]
- Lipipun, V.; Kurokawa, M.; Suttisri, R.; Taweechotipatr, P.; Pramyothin, P.; Hattori, M.; Shiraki, K. Efficacy of Thai medicinal plant extracts against herpes simplex virus type 1 infection in vitro and in vivo. Antivir. Res. 2003, 60, 175–180. [Google Scholar] [CrossRef]
- He, Y.C.; Lu, Z.H.; Shi, P.; Hao, J.C.; Zhao, Z.J.; Xie, H.T.; Mao, P.; Chen, S.J. Anti-herpes simplex virus activities of bioactive extracts from Antrodia camphorata mycelia. Antivir. Ther. 2016, 21, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Lin, Y.L.; Liu, C.P.; Tsai, W.J. Herpes simplex virus type 1 propagation in HeLa cells interrupted by Nelumbo nucifera. J. Biomed. Sci. 2005, 12, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zhang, C.H.; Wang, L.J.; Cui, Y.X.; Qi, R.B.; Yang, C.R.; Zhang, Y.J.; Wei, X.Y.; Lu, D.X.; Wang, Y.F. In vitro anti-viral activity of the total alkaloids from Tripterygium hypoglaucum against herpes simplex virus type 1. Virol. Sin. 2010, 25, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.C.; Ng, L.T.; Cheng, P.W.; Chiang, W.; Lin, C.C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, C.; Mandalari, G.; Smeriglio, A.; Trombetta, D.; Pizzo, M.M.; Pennisi, R.; Sciortino, M.T. Almond skin extracts abrogate HSV-1 replication by blocking virus binding to the cell. Viruses 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Lin, L.T.; Huang, H.H.; Yang, C.M.; Lin, C.C. Yin Chen Hao Tang, a Chinese prescription, inhibits both herpes simplex virus type-1 and type-2 infections in vitro. Antivir. Res. 2008, 77, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Nawawi, A.; Nakamura, N.; Meselhy, M.R.; Hattori, M.; Kurokawa, M.; Shiraki, K.; Kashiwaba, N.; Ono, M. In vivo antiviral activity of Stephania cepharantha against herpes simplex virus Type-1. Phytother. Res. 2001, 15, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.C.; Chang, J.S.; Chen, C.C.; Ng, L.T.; Lin, C.C. Anti-Herpes simplex virus activity of Bidens pilosa and Houttuynia cordata. Am. J. Chin. Med. 2003, 31, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.Y.; Ho, B.C.; Lee, S.Y.; Chang, S.Y.; Kao, C.L.; Lee, S.S.; Lee, C.N. Houttuynia cordata targets the beginning stage of herpes simplex virus infection. PLoS ONE 2015, 10, e0115475. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Chang, Y.Y.; Lin, H.W.; Chen, Y.C.; Hsu, S.H.; Lin, J.T. Inhibitory effect of litchi (Litchi chinensis Sonn.) flower on lipopolysaccharide-induced expression of proinflammatory mediators in RAW264.7 cells through NF-kappaB, ERK, and JAK2/STAT3 inactivation. J. Agric. Food Chem. 2014, 62, 3458–3465. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, M.; Wadhwani, A.; Kai, H.; Hidaka, M.; Yoshida, H.; Sugita, C.; Watanabe, W.; Matsuno, K.; Hagiwara, A. Activation of cellular immunity in herpes simplex virus type 1-infected mice by the oral administration of aqueous extract of moringa oleifera lam. leaves. Phytother. Res. 2016, 30, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.C.; Su, C.R.; Ku, Y.C.; Wu, T.S. The constituents and their bioactivities of houttuynia cordata. Chem. Pharm. Bull. 2009, 57, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Gao, H.; Zhu, Q.C.; Wang, Y.Q.; Li, T.; Mu, Z.Q.; Wu, H.L.; Peng, T.; Yao, X.S. Houttuynoids A-E, anti-herpes simplex virus active flavonoids with novel skeletons from houttuynia cordata. Org. Lett. 2012, 14, 1772–1775. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Peng, T.; Gao, H.; Zhu, Q.; Chen, S.; Li, T.; Xiao, G.; Wu, H. Houttuynoid and Preparation Method and Application Thereof. 2013. Available online: https://patents.google.com/patent/CN103304609B/en (accessed on 19 October 2018).
- Chen, S.D.; Li, T.; Gao, H.; Zhu, Q.C.; Lu, C.J.; Wu, H.L.; Peng, T.; Yao, X.S. Anti HSV-1 flavonoid derivatives tethered with houttuynin from Houttuynia cordata. Planta Med. 2013, 79, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Chen, G.D.; Fan, H.X.; Hu, D.; Zhou, Z.Q.; Lan, K.H.; Zhang, H.P.; Maeda, H.; Yao, X.S.; Gao, H. Houttuynoid M, an anti-HSV active houttuynoid from houttuynia cordata featuring a bis-houttuynin chain tethered to a flavonoid core. J. Nat. Prod. 2017, 80, 3010–3013. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, L.B.; Wu, H.L.; Chen, S.D.; Zhu, Q.C.; Gao, H.; Yu, X.T.; Wang, Y.; Su, W.H.; Yao, X.S.; et al. Anti-herpes simplex virus type 1 activity of Houttuynoid A, a flavonoid from Houttuynia cordata Thunb. Antivir. Res. 2017, 144, 273–280. [Google Scholar] [CrossRef] [PubMed]
- He, L.W.; Liu, H.Q.; Chen, Y.Q.; Yang, J.Y.; Wang, T.L.; Li, W. Total synthesis and anti-viral activities of an extract of Radix isatidis. Molecules 2014, 19, 20906–20912. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ikeda, T.; Kaku, M.; Zhu, X.H.; Okawa, M.; Yokomizo, K.; Uyeda, M.; Nohara, T. A new lignan glycoside and phenylethanoid glycosides from Strobilanthes cusia BREMEK. Chem. Pharm. Bull. 2004, 52, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Du, Q.; Liao, P.Y.; Chen, Z.P.; Wang, D.; Yang, C.R.; Kitazato, K.; Wang, Y.F.; Zhang, Y.J. Notoginsenoside ST-4 inhibits virus penetration of herpes simplex virus in vitro. J. Asian Nat. Prod. Res. 2011, 13, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.R.; Luo, J.; Hou, W.; Xiao, H.; Yang, Z.Q. The effect of emodin, an anthraquinone derivative extracted from the roots of Rheum tanguticum, against herpes simplex virus in vitro and in vivo. J. Ethnopharmacol. 2011, 133, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.F.; Pei, Y.; Qu, C.; Lai, Z.C.; Ren, Z.; Yang, K.; Xiong, S.; Zhang, Y.J.; Yang, C.R.; Wang, D.; et al. In vitro anti-herpes simplex virus activity of 1,2,4,6-tetra-O-galloyl-beta-d-glucose from Phyllanthus emblica L. (Euphorbiaceae). Phytother. Res. 2011, 25, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Guo, Y.S.; Wang, C.H.; Li, G.Q.; Xu, J.J.; Chung, H.Y.; Ye, W.C.; Li, Y.L.; Wang, G.C. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chem. 2014, 152, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C.C. Antiviral activity of Plantago major extracts and related compounds in vitro. Antivir. Res. 2002, 55, 53–62. [Google Scholar] [CrossRef]
- Li, H.; Zhou, C.; Pan, Y.; Gao, X.; Wu, X.; Bai, H.; Zhou, L.; Chen, Z.; Zhang, S.; Shi, S.; et al. Evaluation of antiviral activity of compounds isolated from Ranunculus sieboldii and Ranunculus sceleratus. Planta Med. 2005, 71, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Du, H.; Cui, S.; Liu, T.; Yang, G.; Sun, H.; Tao, W.; Jiang, B.; Yu, L.; You, F.E. fischeriana root compound Dpo activates antiviral innate immunity. Front. Cell. Infect. Microbiol. 2017, 7, 456. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.H.; Yin, F.L.; Xin, X.G.; Mao, S.M.; Hu, P.P.; Zhao, C.Z.; Sun, X.N. Astragalus polysaccharide protects astrocytes from being infected by HSV-1 through TLR3/NF-kappa B signaling pathway. Evid. Based Complement. Altern. Med. 2014, 2014, 285356. [Google Scholar] [CrossRef] [PubMed]
- Rezeng, C.; Yuan, D.; Long, J.; Suonan, D.; Yang, F.; Li, W.; Tong, L.; Jiumei, P. Alantolactone exhibited anti-herpes simplex virus 1 (HSV-1) action in vitro. Biosci. Trends 2015, 9, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Zandi, K.; Ramedani, E.; Mohammadi, K.; Tajbakhsh, S.; Deilami, I.; Rastian, Z.; Fouladvand, M.; Yousefi, F.; Farshadpour, F. Evaluation of antiviral activities of curcumin derivatives against HSV-1 in Vero cell line. Nat. Prod. Commun. 2010, 5, 1935–1938. [Google Scholar] [PubMed]
- Zhou, M.; Xu, M.; Ma, X.X.; Zheng, K.; Yang, K.; Yang, C.R.; Wang, Y.F.; Zhang, Y.J. Antiviral triterpenoid saponins from the roots of Ilex asprella. Planta Med. 2012, 78, 1702–1705. [Google Scholar] [CrossRef] [PubMed]
- Sekita, Y.; Murakami, K.; Yumoto, H.; Hirao, K.; Amoh, T.; Fujiwara, N.; Hirota, K.; Fujii, H.; Matsuo, T.; Miyake, Y.; et al. Antibiofilm and anti-inflammatory activities of houttuynia cordata decoction for oral care. Evid. Based Complement. Altern. Med. 2017, 2017, 2850947. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Chae, H.S.; Chin, Y.W.; Kim, J. Alkaloids from aerial parts of Houttuynia cordata and their anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2017, 27, 2807–2811. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Wang, Z.X.; Yang, Z.Y.; Wang, J.J.; Xu, Y.X.; Tan, R.X.; Li, E.G. Houttuynia cordata blocks HSV infection through inhibition of NF-kappa B activation. Antivir. Res. 2011, 92, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, C.Y.; Ho, T.Y. Emodin is a novel alkaline nuclease inhibitor that suppresses herpes simplex virus type 1 yields in cell cultures. Br. J. Pharmacol. 2008, 155, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.S.; Perez-Fons, L.; Estepa, A.; Micol, V. Membrane-related effects underlying the biological activity of the anthraquinones emodin and barbaloin. Biochem. Pharmacol. 2004, 68, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Battistutta, R.; Sarno, S.; De Moliner, E.; Papinutto, E.; Zanotti, G.; Pinna, L.A. The replacement of ATP by the competitive inhibitor emodin induces conformational modifications in the catalytic site of protein kinase CK2. J. Biol. Chem. 2000, 275, 29618–29622. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.; Lee, Y.H.; Lee, C.H.; Lee, S.K. Emodin, an anthraquinone derivative isolated from the rhizomes of Rheum palmatum, selectively inhibits the activity of casein kinase II as a competitive inhibitor. Planta Med. 1999, 65, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Frette, X.C.; Christensen, L.P.; Grevsen, K. Chitosan oligosaccharides promote the content of polyphenols in greek oregano (Origanum vulgare ssp. hirtum). J. Agric. Food Chem. 2012, 60, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.H.; Chan, L.P.; Ding, H.Y.; So, E.C.; Lin, R.J.; Wang, H.M.; Chen, Y.G.; Chou, T.H. Free radical scavenging activity of 4-(3,4-Dihydroxybenzoyloxymethyl)phenyl-O-beta-d-glucopyranoside from origanum vulgare and its protection against oxidative damage. J. Agric. Food Chem. 2012, 60, 7690–7696. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S.; El, S.N.; Karagozlu, N.; Sahin, S. Antioxidant and antimicrobial activities of essential oils obtained from oregano (Origanum vulgare ssp. hirtum) by using different extraction methods. J. Med. Food 2011, 14, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Loo, C.Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef] [PubMed]
- Kutluay, S.B.; Doroghazi, J.; Roemer, M.E.; Triezenberg, S.J. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology 2008, 373, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Andrilenas, K.K.; Ramlall, V.; Kurland, J.; Leung, B.; Harbaugh, A.G.; Siggers, T. DNA-binding landscape of IRF3, IRF5 and IRF7 dimers: Implications for dimer-specific gene regulation. Nucleic Acids Res. 2018, 46, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Konno, H.; Konno, K.; Barber, G.N. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell 2013, 155, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Wang, C.G.; Jiang, Q.F.; Lv, M.Z.; Gao, P.F.; Yu, X.Y.; Mu, P.; Zhang, R.; Bi, S.; Feng, J.M.; et al. NEMO-IKK beta are essential for IRF3 and NF-kappa B activation in the cGAS-STING pathway. J. Immunol. 2017, 199, 3222–3233. [Google Scholar] [CrossRef] [PubMed]
- English, L.; Chemali, M.; Duron, J.; Rondeau, C.; Laplante, A.; Gingras, D.; Alexander, D.; Leib, D.; Norbury, C.; Lippe, R.; et al. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat. Immunol. 2009, 10, 480–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Lee, S.; Jung, J.U. When autophagy meets viruses: A double-edged sword with functions in defense and offense. Semin. Immunopathol. 2010, 32, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ooi, L.S.; Wang, H.; But, P.P.; Ooi, V.E. Antiviral activities of medicinal herbs traditionally used in southern mainland China. Phytother. Res. 2004, 18, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Jie, C.; Luo, Z.; Chen, H.; Wang, M.; Yan, C.; Mao, Z.F.; Xiao, G.K.; Kurihara, H.; Li, Y.F.; He, R.R. Indirubin, a bisindole alkaloid from Isatis indigotica, reduces H1N1 susceptibility in stressed mice by regulating MAVS signaling. Oncotarget 2017, 8, 105615–105629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Jie, C.; Tang, L.P.; Meng, H.; Li, X.B.; Li, Y.B.; Chen, L.X.; Yan, C.; Kurihara, H.; Li, F.; et al. New insights into the effects and mechanism of a classic traditional Chinese medicinal formula on influenza prevention. Phytomedicine 2017, 27, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, Y.F.; Tang, L.P.; Tsoi, B.; Chen, M.; Chen, H.; Chen, X.M.; Tan, R.R.; Kurihara, H.; He, R.R. A new mechanism of vitamin C effects on A/FM/1/47(H1N1) virus-induced pneumonia in restraint-stressed mice. BioMed Res. Int. 2015, 2015, 675149. [Google Scholar] [CrossRef] [PubMed]
- Schachtele, S.J.; Hu, S.X.; Little, M.R.; Lokensgard, J.R. Herpes simplex virus induces neural oxidative damage via microglial cell Toll-like receptor-2. J. Neuroinflamm. 2010, 7. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Su, A.; Fu, Y.; Wang, X.; Lv, X.; Xu, W.; Xu, S.; Wang, H.; Wu, Z. Harmine blocks herpes simplex virus infection through downregulating cellular NF-kappaB and MAPK pathways induced by oxidative stress. Antivir. Res. 2015, 123, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Orvedahl, A.; Alexander, D.; Talloczy, Z.; Sun, Q.; Wei, Y.; Zhang, W.; Burns, D.; Leib, D.A.; Levine, B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 2007, 1, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Pilli, M.; Arko-Mensah, J.; Ponpuak, M.; Roberts, E.; Master, S.; Mandell, M.A.; Dupont, N.; Ornatowski, W.; Jiang, S.; Bradfute, S.B.; et al. TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation. Immunity 2012, 37, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Weidberg, H.; Elazar, Z. TBK1 mediates crosstalk between the innate immune response and autophagy. Sci. Signal. 2011, 4. [Google Scholar] [CrossRef] [PubMed]
- Siracusano, G.; Venuti, A.; Lombardo, D.; Mastino, A.; Esclatine, A.; Sciortino, M.T. Early activation of MyD88-mediated autophagy sustains HSV-1 replication in human monocytic THP-1 cells. Sci. Rep. 2016, 6, 31302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, S.; Xu, X.; Liao, Y.; Wang, Y.; Wang, J.; Feng, M.; Wang, L.; Zhang, Y.; He, Z.; Yang, F.; et al. Attenuated phenotype and immunogenic characteristics of a mutated herpes simplex virus 1 strain in the rhesus macaque. Viruses 2018, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guo, Y.; Fan, S.; Cui, P.; Feng, M.; Wang, L.; Zhang, Y.; Liao, Y.; Zhang, X.; Li, Q. Attenuated phenotypes and analysis of a herpes simplex virus 1 strain with partial deletion of the UL7, UL41 and LAT genes. Virol. Sin. 2017, 32, 404–414. [Google Scholar] [CrossRef] [PubMed]


| Source | Extracts | Target/Mechanism | IC50 (μg/mL) | CC50 (μg/mL) | In Vitro | In Vivo | HSV-1 Strain | MOI | References |
|---|---|---|---|---|---|---|---|---|---|
| Lychee flower | Water and ethanol | Inhibition of mTOR and p70s6k phosphorylation | Not mentioned | Not mentioned | √ | × | Not mentioned | 1 pfu/cell | [32] |
| Moringa oleifera | Ethanol | Not mentioned | 100.0 ± 5.3 | 875 ± 35 | √ | √ | 7401H | 100 pfu/0.2 mL (60 mm dishes) | [33] |
| Ventilago denticulata | Ethanol | Not mentioned | 46.3 ± 1.5 | 838 ± 53 | √ | √ | 7401H | 100 pfu/0.2 mL (60 mm dishes) | [33] |
| Antrodia camphorata mycelia | Crude extract Fraction A Fraction B | Not mentioned | 61.2 ± 5.5 8.2 ± 1.80 120.0 ± 3.5 | 485.0 | √ | × | F | 2 pfu/cell | [34] |
| 197.0 | |||||||||
| 235.0 | |||||||||
| Nelumbo nucifera | NN-B-5 | Interruption of αTIF/C1/Oct-1/GARAT multiproteins/DNA complexes formation | 21.3 ± 1.6 | Not mentioned | √ | × | KOS/TK-HSV-1 | 100 pfu/well | [35] |
| Tripterygium hypoglaucum | Total alkaloids | Not mentioned | 6.5 | 46.6 | √ | × | SM44 | 100 TCID50 | [36] |
| Ocimum basilicum | Water Ethanol | Not mentioned | 90.9 ± 2.6 108.3 ± 2.4 | 1469.3 684.8 | √ | × | KOS | 20 TCID50 | [37] |
| Almond skin | Methanol | Inhibition of viral adsorption and blocking the production of viral particles | Not mentioned | Not mentioned | √ | × | F/VP26GFP-HSV-1 | 1 pfu/cell | [38] |
| Yin Chen Hao Tang (YCHT) | Water | Not mentioned | 142.5 ± 1.7 | 850.7 ± 1.7 | √ | × | KOS | 100 pfu/well | [39] |
| Stephania cepharantha | Methanol | Not mentioned | 18 | Not mentioned | √ | √ | 7401H | 100 pfu/0.2 mL (60 mm dishes) | [40] |
| CHCl3-soluble fraction (alkaloid raction) | 8 | ||||||||
| Houttuynia cordata | Water | Not mentioned | 822.39 | >1000 | √ | × | Not mentioned | Not mentioned | [41] |
| Houttuynia cordata | Water | Inhibition of NF-κB activation and blocking viral binding/penetration/replication | 692 | >100,000 | √ | × | F | 1 pfu/cell | [42] |
| Compounds | Type | Target/Mechanism | IC50 (μg/mL) | CC50 (μg/mL) | In Vitro | In Vivo | HSV-1 Strain | MOI | References |
|---|---|---|---|---|---|---|---|---|---|
| Quercetin (1) | Flavonoid | Inhibition of NF-κB activation and viral entry | 52.9 | >100,000 | √ | × | F | 1 pfu/cell | [42] |
| Isoquercitrin (2) | Inhibition of NF-κB activation | 0.42 | |||||||
| Norcepharadione B (3) | Alkaloid | Not mentioned | 170 μM | Not mentioned | √ | × | KOS | 3 pfu/cell | [45] |
| Houttuynoid A (4) | Flavonoid | Not mentioned | 23.50 ± 1.82 | 166.38 | √ | × | Not mentioned | Not mentioned | [46] |
| Houttuynoid B (5) | 57.71 ± 8.03 | 181.79 | |||||||
| Houttuynoid C (6) | 50.75 ± 11.07 | 531.35 | |||||||
| Houttuynoid D (7) | 59.89 ± 6.63 | 180.87 | |||||||
| Houttuynoid E (8) | 42.03 ±10.22 | 134.92 | |||||||
| Houttuynoid F (9) | Flavonoid | Not mentioned | Not mentioned | Not mentioned | √ | × | Blue | Not mentioned | [47] |
| Houttuynoid G (10) | Flavonoid | Not mentioned | 38.46 ± 9.57 | 113.10 ± 12.16 | √ | × | Blue | 0.5 pfu/cell | [48] |
| Houttuynoid H (11) | 14.10 ± 0.11 | 44.55 ± 4.63 | |||||||
| Houttuynoid I (12) | 62.00 ± 2.06 | 63.06 ± 8.34 | |||||||
| Houttuynoid J (13) | 70.76 ± 2.22 | 100.87 ± 6.14 | |||||||
| Houttuynoid K (14) | Flavonoid | Not mentioned | Not mentioned | Not mentioned | √ | × | Blue | Not mentioned | [47] |
| Houttuynoid L (15) | |||||||||
| Houttuynoid M (16) | Flavonoid | Not mentioned | 17.72 | >200 | √ | √ | Blue/F | 0.5 pfu/cell | [49] |
| Houttuynoid A (4) | 12.42 | Not mentioned | |||||||
| Houttuynoid A (4) | Flavonoid | Not mentioned | 23.50 ± 1.82 | 166.36 ± 9.27 | √ | √ | Blue/F | 0.5 pfu/cell | [50] |
| Source | Compounds | Type | Target/Mechanism | IC50 (μg/mL) | CC50 (μg/mL) | In Vitro | In Vivo | HSV-1 Strain | MOI | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Radix isatidis | 3-(furan-2-yl)-7-hydroxyisoquinolin-1(2H)-one (17) | Aglycone derivative | Not mentioned | 15.3 | 90.9 | √ | × | Not mentioned | 100 TCID50/mL, 20 μL/well | [51] |
| 3-(Furan-2-yl)-7-(((2S,3R,5S,6R)-3,4,5-trihydroxy-6-(hydro-xymethyl)tetrahydro-2H-pyran-2-yl)oxy) isoquinolin-1(2H)-one (18) | Glucoside derivative | Not mentioned | 42.4 | 72.1 | √ | × | Not mentioned | 100 TCID50/mL, 20 μL/well | ||
| 3-(5-(Hydroxymethyl)furan-2-yl)-7-(((2S,3R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)isoquinolin-1(2H)-one (19) | Isoquinoline derivative | Not mentioned | 79.1 | 619.4 | √ | × | Not mentioned | 100 TCID50/mL, 20 μL/well | ||
| Strobilanthes cusia | Lupeol (20) | Triterpenoid | Not mentioned | 11.70 | 49.3 | √ | × | KOS | 100 pfu/cell | [52] |
| Panax notoginseng | notoginsenoside ST-4 (21) | Dammarane-type saponin | HSV-1 penetration and viral protein (vp5) synthesis | 16.47 ± 0.67 | 510.64 ± 4.56 | √ | × | F | 30 pfu/well | [53] |
| Rheum tanguticum | emodin (22) | Anthraquinone derivative | Not mentioned | Not mentioned | Not mentioned | √ | √ | F | 100 TCID50/mL | [54] |
| Phyllanthus emblica | 1,2,4,6-tetra-O-galloyl-β-d-glucose (1246TGG) (23) | Polyphenolic | Not mentioned | 10.77 ± 0.61 | >253.63 | √ | × | Not mentioned | 30 pfu/well (24-well plates) | [55] |
| Origanum vulgare | acacetin-7-O-[6′′′-O-acetyl-β-d-galactopy-ranosyl-(1→2)]-β-d-glucopyranoside (24) | Phenolic compound | Not mentioned | 38.5 | Not mentioned | √ | × | F | 100 TCID50, 100 μL | [56] |
| 2,5-dihydroxybenzoic acid (25) | 32.7 | |||||||||
| Plantago major | chlorogenic acid (26) | Phenolic compound | Not mentioned | 47.6 | 3995 | √ | × | KOS | 0.002–0.025 pfu/cell | [57] |
| caffeic acid (27) | Phenolic compound | 15.3 | 10,293 | |||||||
| baicalein (28) | Flavonoid | 4.7 | 19.5 | |||||||
| vanillic acid (29) | Phenolic compound | 88.1 | 1338 | |||||||
| Ranunculus sceleratus | protocatechuyl aldehyde (30) | Phenolic aldehyde | Not mentioned | 17.34 ± 1.2 | >200 | √ | × | Not mentioned | 100 pfu/well | [58] |
| Stephania cepharantha | FK-3000 (31) | Alkaloid | Not mentioned | 7.8 | Not mentioned | √ | √ | 7401H | 100 pfu, 60 mm dishes | [40] |
| Euphorbia Fischeriana | Dpo (32) | Not mentioned | STING/IRFs/ELF4 dependent way | Not mentioned | Not mentioned | × | √ | Not mentioned | Not mentioned | [59] |
| Astragalus | astragalus polysaccharide (33) | Polysaccharide | TLR3/NF-κB Signaling Pathway | Not mentioned | 120 | √ | × | SM44 | Not mentioned | [60] |
| Inulae Radix (Tu-Mu-Xiang) | alantolactone (34) | Sesquiterpene lactone | Not mentioned | 0.04 | >1 | √ | × | Not mentioned | Not mentioned | [61] |
| Curcuma longa L. | curcumin (35) | Phenolic | Not mentioned | 33.0 | 484.2 | √ | × | KOS | 100 TCID50 | [62] |
| gallium-curcumin (36) | 13.9 | 255.8 | ||||||||
| Cu-curcumin (37) | 23.1 | 326.6 | ||||||||
| Ilex asprella | asprellanoside A (38) | Triterpenoid Saponin | Not mentioned | 140 | Not mentioned | √ | × | F | 40 pfu/well | [63] |
| oblonganoside H (39) | 180 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, X.-H.; Luo, Z.; Liu, L.-F.; Yan, C.; Yan, C.-Y.; Chen, G.-D.; Gao, H.; Duan, W.-J.; Kurihara, H.; et al. Traditional Chinese Medicine as a Potential Source for HSV-1 Therapy by Acting on Virus or the Susceptibility of Host. Int. J. Mol. Sci. 2018, 19, 3266. https://doi.org/10.3390/ijms19103266
Li W, Wang X-H, Luo Z, Liu L-F, Yan C, Yan C-Y, Chen G-D, Gao H, Duan W-J, Kurihara H, et al. Traditional Chinese Medicine as a Potential Source for HSV-1 Therapy by Acting on Virus or the Susceptibility of Host. International Journal of Molecular Sciences. 2018; 19(10):3266. https://doi.org/10.3390/ijms19103266
Chicago/Turabian StyleLi, Wen, Xiao-Hua Wang, Zhuo Luo, Li-Fang Liu, Chang Yan, Chang-Yu Yan, Guo-Dong Chen, Hao Gao, Wen-Jun Duan, Hiroshi Kurihara, and et al. 2018. "Traditional Chinese Medicine as a Potential Source for HSV-1 Therapy by Acting on Virus or the Susceptibility of Host" International Journal of Molecular Sciences 19, no. 10: 3266. https://doi.org/10.3390/ijms19103266
APA StyleLi, W., Wang, X.-H., Luo, Z., Liu, L.-F., Yan, C., Yan, C.-Y., Chen, G.-D., Gao, H., Duan, W.-J., Kurihara, H., Li, Y.-F., & He, R.-R. (2018). Traditional Chinese Medicine as a Potential Source for HSV-1 Therapy by Acting on Virus or the Susceptibility of Host. International Journal of Molecular Sciences, 19(10), 3266. https://doi.org/10.3390/ijms19103266