Copper(II) Bis(diethyldithiocarbamate) Induces the Expression of Syndecan-4, a Transmembrane Heparan Sulfate Proteoglycan, via p38 MAPK Activation in Vascular Endothelial Cells
Abstract
:1. Introduction
2. Results
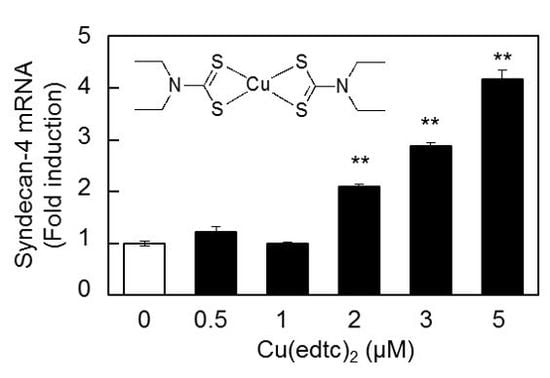
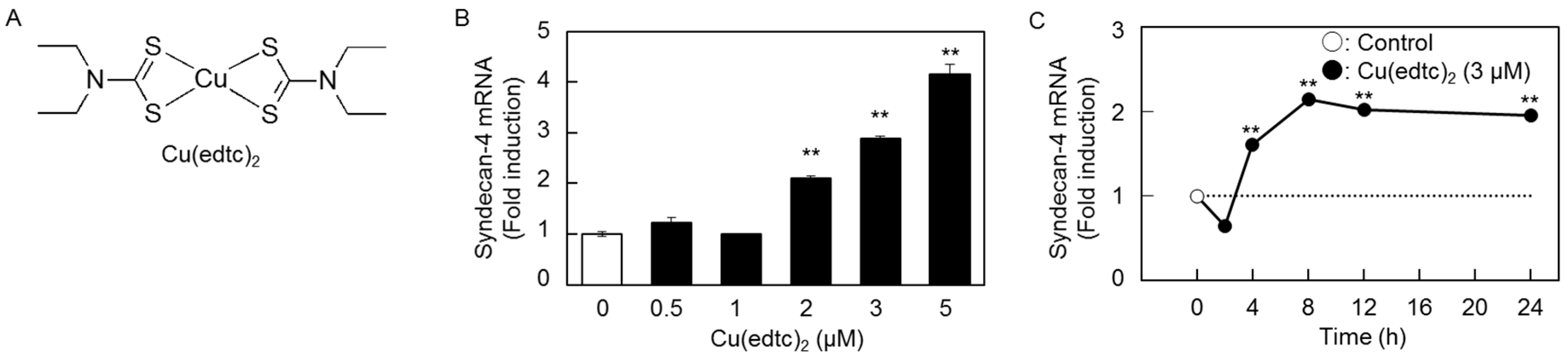
2.1. Cu(edtc)2 Induces Syndecan-4 Expression in Vascular Endothelial Cells
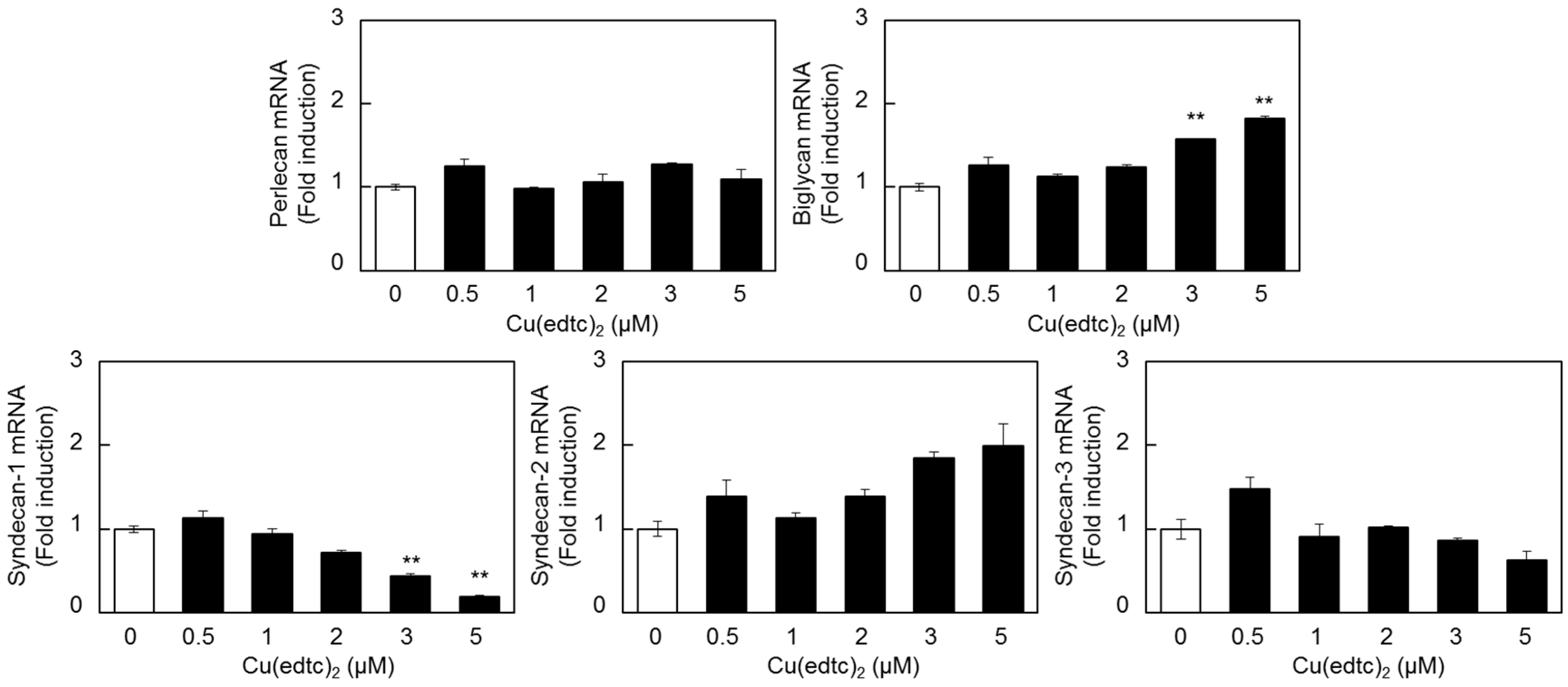
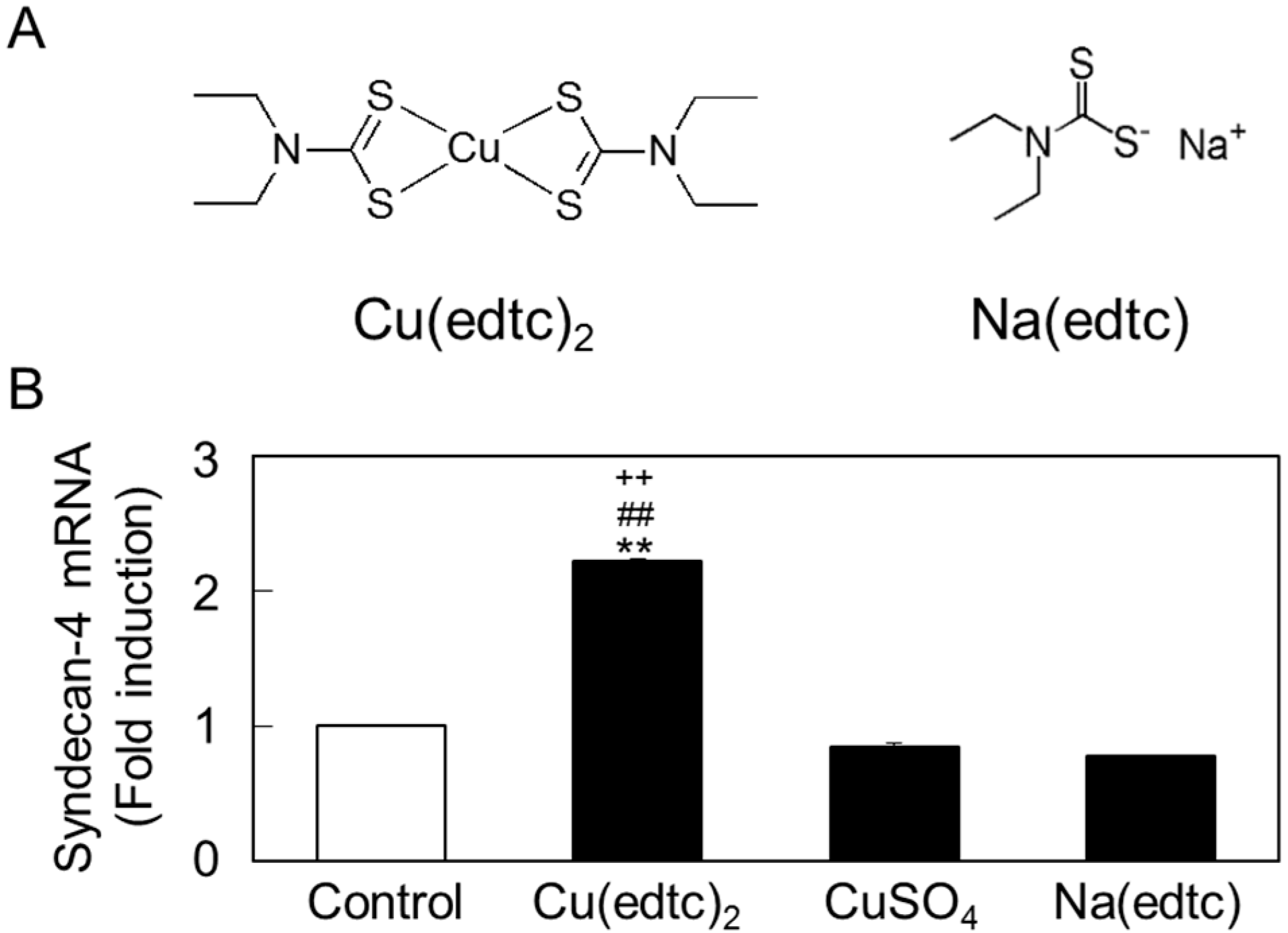
2.2. Characterization of Upregulation of Syndecan-4 mRNA Expression by Cu(edtc)2
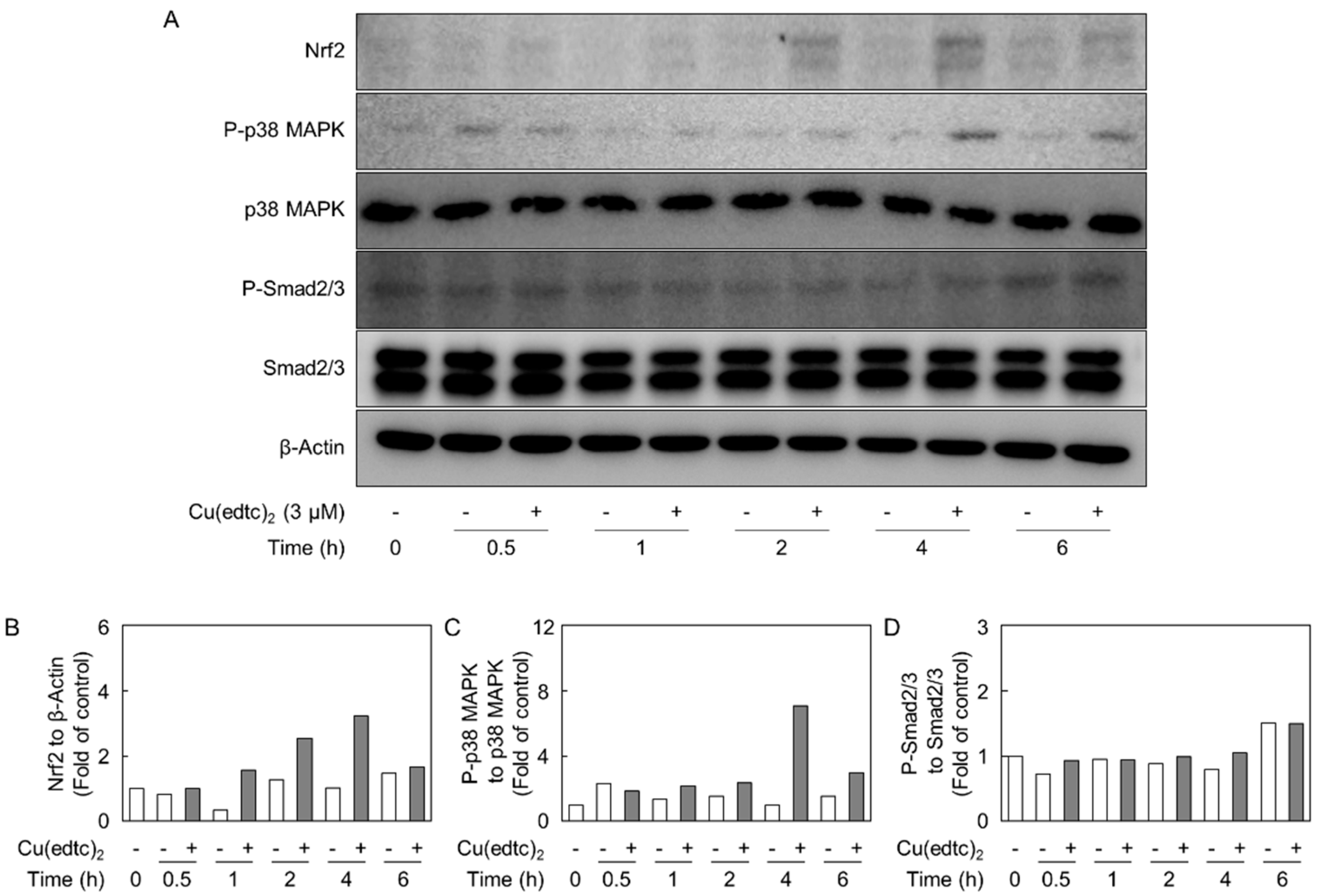
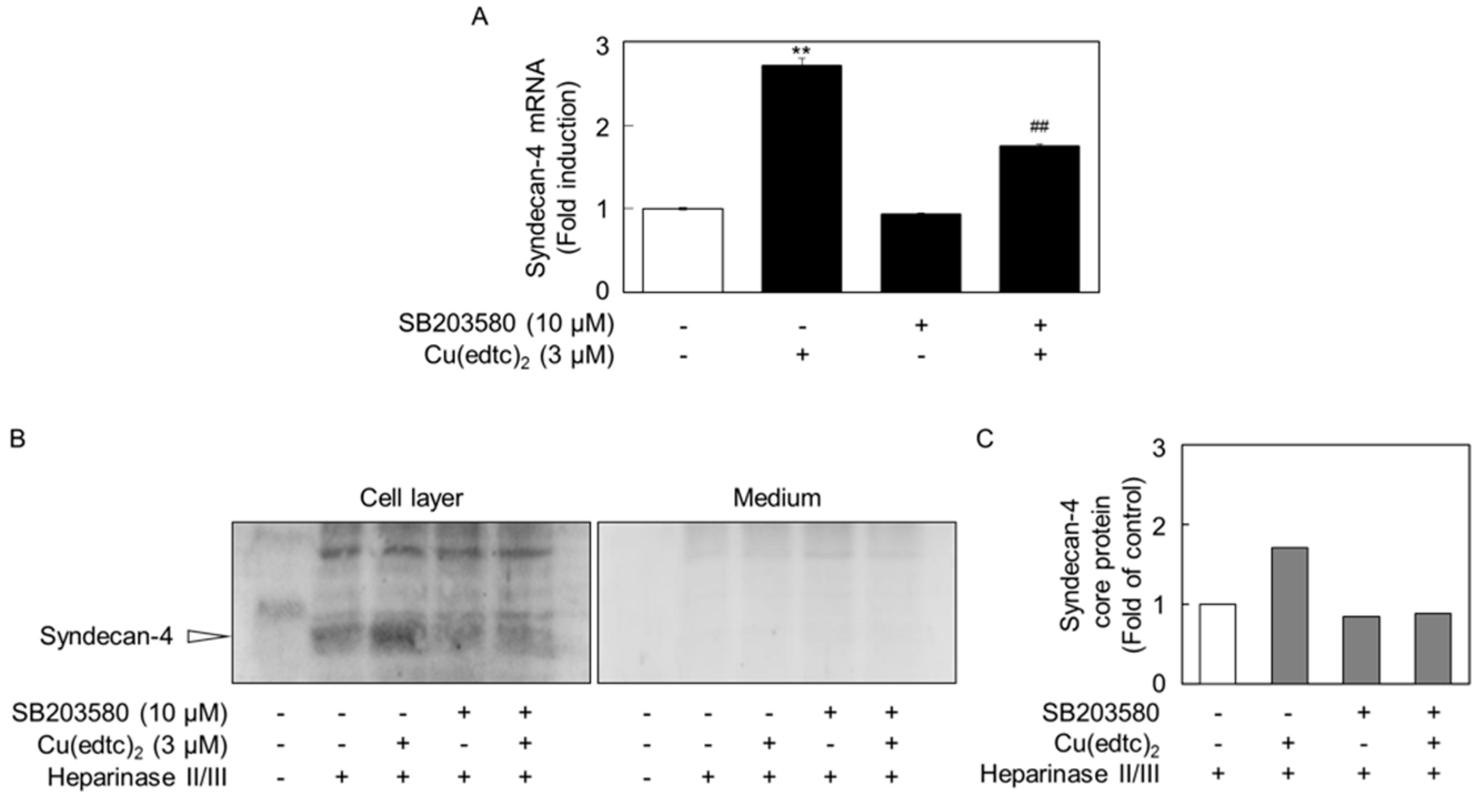
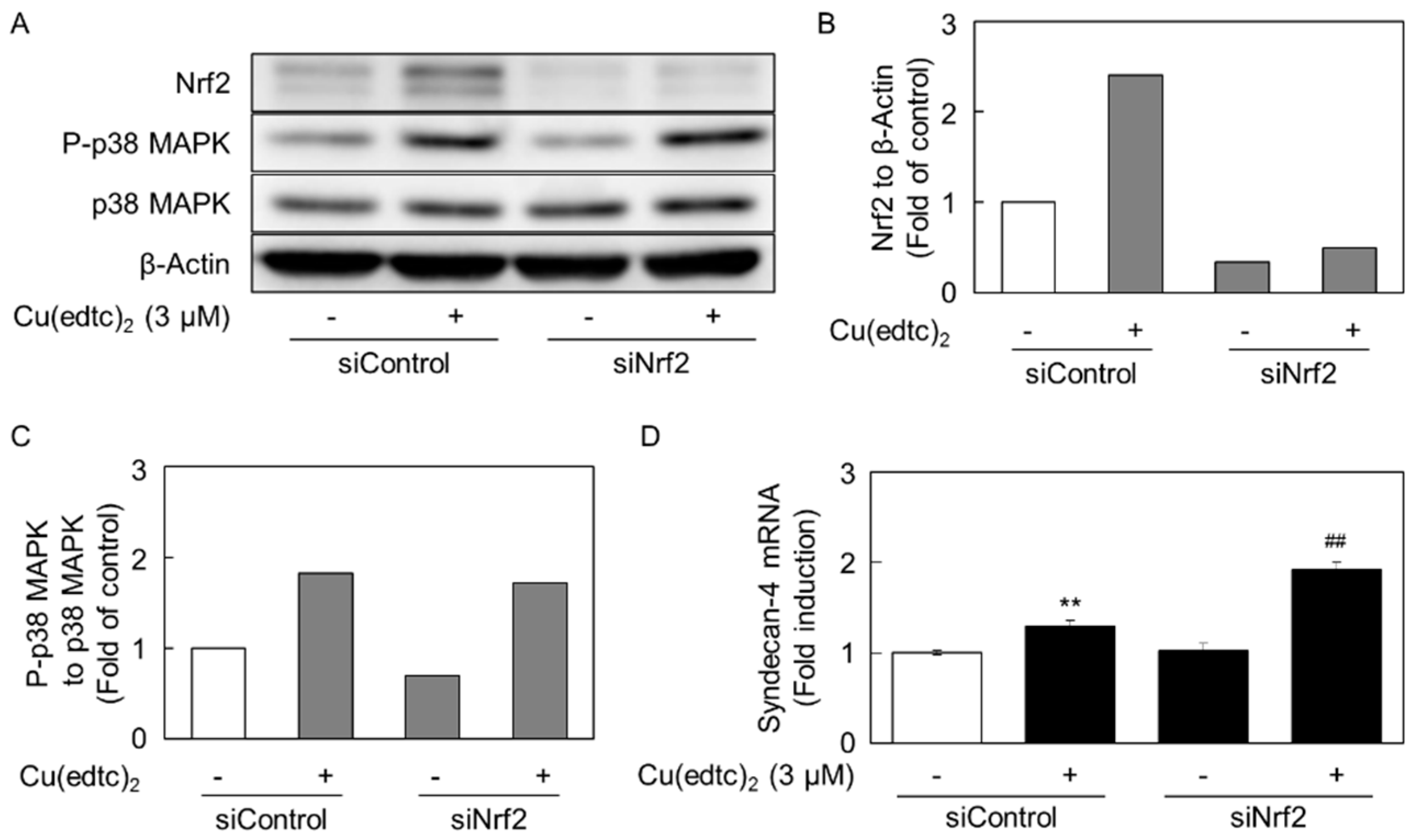
2.3. Cu(edtc)2 Induces Endothelial Syndecan-4 Expression via p38 MAPK Activation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis
4.3. Cell Culture and Treatments
4.4. siRNA Transfection
4.5. Real-Time RT-PCR
4.6. Proteoglycan Core Protein Extraction and Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of interest
Abbreviations
| Cu(edtc)2 | Copper(II) bis(diethyldithiocarbamate) |
| Fe(edtc)3 | Iron(III) tris(diethyldithiocarbamate) |
| Ni(edtc)2 | Nickel(II) bis(diethyldithiocarbamate) |
| Na(edtc) | Sodium diethyldithiocarbamate |
| Zn(edtc)2 | Zinc(II) bis(diethyldithiocarbamate) |
References
- Ruoslahti, E. Structure and biology of proteoglycans. Annu. Rev. Cell Biol. 1988, 4, 229–255. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, I.; Multhaupt, H.A.B.; Couchman, J.R. Proteoglycans, ion channels and cell-matrix adhesion. Biochem. J. 2017, 474, 1965–1979. [Google Scholar] [CrossRef] [PubMed]
- De Agostini, A.I.; Watkins, S.C.; Slayter, H.S.; Youssoufian, H.; Rosenberg, R.D. Localization of anticoagulantly active heparan sulfate proteoglycans in vascular endothelium: Antithrombin binding on cultured endothelial cells and perfused rat aorta. J. Cell Biol. 1990, 111, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T. Targeted gene disruption of natural anticoagulant proteins in mice. Int. J. Hematol. 2002, 76 (Suppl. S2), 36–39. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Lin, C.F.; Li, C.F.; Sun, D.P.; Wang, L.Y.; Hsing, C.H. Anesthetic propofol overdose causes vascular hyperpermeability by reducing endothelial glycocalyx and ATP production. Int. J. Mol. Sci. 2015, 16, 12092–12107. [Google Scholar] [CrossRef] [PubMed]
- Camejo, G. The interaction of lipids and lipoproteins with the intercellular matrix of arterial tissue: Its possible role in atherogenesis. Adv. Lipid Res. 1982, 19, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, M. Basement membrane proteins: Structure, assembly, and cellular interactions. Crit. Rev. Biochem. Mol. Biol. 1992, 27, 93–127. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, C.; Deng, X.; Fujiwara, Y.; Kaji, T. Proteoglycans predominantly synthesized by human brain microvascular endothelial cells in culture are perlecan and biglycan. J. Health Sci. 2005, 51, 576–583. [Google Scholar] [CrossRef]
- Saku, T.; Furthmayr, H. Characterization of the major heparan sulfate proteoglycan secreted by bovine aortic endothelial cells in culture. Homology to the large molecular weight molecule of basement membranes. J. Biol. Chem. 1989, 264, 3514–3523. [Google Scholar] [PubMed]
- Kojima, T.; Shworak, N.W.; Rosenberg, R.D. Molecular cloning and expression of two distinct cDNA-encoding heparan sulfate proteoglycan core proteins from a rat endothelial cell line. J. Biol. Chem. 1992, 267, 4870–4877. [Google Scholar] [PubMed]
- Järveläinen, H.T.; Kinsella, M.G.; Wight, T.N.; Sandell, L.J. Differential expression of small chondroitin/dermatan sulfate proteoglycans, PG-I/biglycan and PG-II/decorin, by vascular smooth muscle and endothelial cells in culture. J. Biol. Chem. 1991, 266, 23274–23281. [Google Scholar] [PubMed]
- Shworak, N.W.; Kojima, T.; Rosenberg, R.D. Isolation and characterization of ryudocan and syndecan heparan sulfate proteoglycans, core proteins, and cDNAs from a rat endothelial cell line. Haemostasis 1993, 23 (Suppl. S1), 161–176. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, N.; Mulligan-Kehoe, M.J.; Corti, F.; Simon, D.D.; Ross, T.D.; Rhodes, J.M.; Wang, T.Z.; Mejean, C.O.; Simons, M.; Humphrey, J.; et al. Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 17308–17313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiguro, K.; Kadomatsu, K.; Kojima, T.; Muramatsu, H.; Iwase, M.; Yoshikai, Y.; Yanada, M.; Yamamoto, K.; Matsushita, T.; Nishimura, M.; et al. Syndecan-4 deficiency leads to high mortality of lipopolysaccharide-injected mice. J. Biol. Chem. 2001, 276, 47483–47488. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Ikesue, M.; Danzaki, K.; Morimoto, J.; Sato, M.; Tanaka, S.; Kojima, T.; Tsutsui, H.; Uede, T. Syndecan-4 prevents cardiac rupture and dysfunction after myocardial infarction. Circ. Res. 2011, 108, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Fujie, T.; Hara, T.; Kaji, T. Toxicology of organic-inorganic hybrid molecules: Bio-organometallics and its toxicology. J. Toxicol. Sci. 2016, 41, SP81–SP88. [Google Scholar] [CrossRef] [PubMed]
- Fujie, T.; Murakami, M.; Yoshida, E.; Tachinami, T.; Shinkai, Y.; Fujiwara, Y.; Yamamoto, C.; Kumagai, Y.; Naka, H.; Kaji, T. Copper diethyldithiocarbamate as an activator of Nrf2 in cultured vascular endothelial cells. J. Biol. Inorg. Chem. 2016, 21, 263–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujie, T.; Segawa, Y.; Yoshida, E.; Kimura, T.; Fujiwara, Y.; Yamamoto, C.; Satoh, M.; Naka, H.; Kaji, T. Induction of metallothionein isoforms by copper diethyldithiocarbamate in cultured vascular endothelial cells. J. Toxicol. Sci. 2016, 41, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujie, T.; Okino, S.; Yoshida, E.; Yamamoto, C.; Naka, H.; Kaji, T. Copper diethyldithiocarbamate as an inhibitor of tissue plasminogen activator synthesis in cultured human coronary endothelial cells. J. Toxicol. Sci. 2017, 42, 553–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, T.; Yoshida, E.; Fujiwara, Y.; Yamamoto, C.; Kaji, T. Transforming growth factor-beta1 modulates the expression of syndecan-4 in cultured vascular endothelial cells in a biphasic manner. J. Cell. Biochem. 2017, 118, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E.; Kurita, M.; Eto, K.; Kumagai, Y.; Kaji, T. Methylmercury promotes prostacyclin release from cultured human brain microvascular endothelial cells via induction of cyclooxygenase-2 through activation of the EGFR-p38 MAPK pathway by inhibiting protein tyrosine phosphatase 1B activity. Toxicology 2017, 392, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, T.; Kojima, T.; Matsuzaki, H.; Nakamura, T.; Yoshida, E.; Fujiwara, Y.; Yamamoto, C.; Saito, S.; Kaji, T. Induction of syndecan-4 by organic-inorganic hybrid molecules with a 1,10-phenanthroline structure in cultured vascular endothelial cells. Int. J. Mol. Sci. 2017, 18, 352. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Yoshida, E.; Shinkai, Y.; Yamamoto, C.; Fujiwara, Y.; Kumagai, Y.; Kaji, T. Biglycan intensifies ALK5-Smad2/3 signaling by TGF-beta1 and downregulates syndecan-4 in cultured vascular endothelial cells. J. Cell. Biochem. 2017, 118, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Rahmani, M.; Conrad, D.; Subler, M.; Dent, P.; Grant, S. The proteasome inhibitor bortezomib interacts synergistically with histone deacetylase inhibitors to induce apoptosis in Bcr/Abl+ cells sensitive and resistant to STI571. Blood 2003, 102, 3765–3774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, T.; Imaizumi, T.; Matsumiya, T.; Tamo, W.; Hatakeyama, M.; Yoshida, H.; Munakata, H.; Fukuda, I.; Satoh, K. Effect of MG132, a proteasome inhibitor, on the expression of growth related oncogene protein-alpha in human umbilical vein endothelial cells. Cytokine 2003, 24, 67–73. [Google Scholar] [CrossRef]
- Yamamoto, N.; Sawada, H.; Izumi, Y.; Kume, T.; Katsuki, H.; Shimohama, S.; Akaike, A. Proteasome inhibition induces glutathione synthesis and protects cells from oxidative stress: Relevance to parkinson disease. J. Biol. Chem. 2007, 282, 4364–4372. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.F.; Bian, Q.; Jiang, J.K.; Thomas, C.J.; Taylor, A.; Pereira, P.; Shang, F. Proteasome inactivation promotes p38 mitogen-activated protein kinase-dependent phosphatidylinositol 3-kinase activation and increases interleukin-8 production in retinal pigment epithelial cells. Mol. Biol. Cell 2009, 20, 3690–3699. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Y.; Small, G.W.; Orlowski, R.Z. Proteasome inhibitors induce a p38 mitogen-activated protein kinase (MAPK)-dependent anti-apoptotic program involving MAPK phosphatase-1 and Akt in models of breast cancer. Breast Cancer Res. Treat. 2006, 100, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Honma, Y.; Shimizu, S.; Takehara, T.; Harada, M. Sorafenib enhances proteasome inhibitor-induced cell death via inactivation of Akt and stress-activated protein kinases. J. Gastroenterol. 2014, 49, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, E.; Suzuki, A.; Murata, M.; Ando, Y.; Kato, I.; Takagi, Y.; Takagi, A.; Murate, T.; Saito, H.; Kojima, T. Molecular mechanisms of syndecan-4 upregulation by TNF-alpha in the endothelium-like EAhy926 cells. J. Biochem. 2013, 154, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, H.; Li, X.; Pan, H.; Li, Z.; Wang, J.; Zheng, Z. TNF-alpha and TGF-beta1 regulate syndecan-4 expression in nucleus pulposus cells: Role of the mitogen-activated protein kinase and NF-kappaB pathways. Connect. Tissue Res. 2015, 56, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Lovborg, H.; Oberg, F.; Rickardson, L.; Gullbo, J.; Nygren, P.; Larsson, R. Inhibition of proteasome activity, nuclear factor-kappaB translocation and cell survival by the antialcoholism drug disulfiram. Int. J. Cancer 2006, 118, 1577–1580. [Google Scholar] [CrossRef] [PubMed]
- Traenckner, E.B.; Wilk, S.; Baeuerle, P.A. A proteasome inhibitor prevents activation of NF-kappa B and stabilizes a newly phosphorylated form of I kappa B-alpha that is still bound to NF-kappa B. EMBO J. 1994, 13, 5433–5441. [Google Scholar] [CrossRef] [PubMed]
- Jaouen, G. Bioorganometallics; Wiley-VCH: Weinheim, Germany, 2006; ISBN 9783527309900. [Google Scholar]
- Hendrickson, A.R.; Martin, R.L.; Rohde, N.M. Dithiocarbamates of copper (I), copper (II), and copper (III). An electrochemical study. Inorg. Chem. 1976, 15, 2115–2119. [Google Scholar] [CrossRef]


| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Perlecan | ATGGCAGCGATGAAGCGGAC | TTGTGGACACGCAGCGGAAC |
| Syndecan-1 | CAGTCAGGAGACAGCATCAG | CCGACAGACATTCCATACC |
| Syndecan-2 | CCAGATGAAGAGGACACAAACG | CCAATAACTCCGCCAGCAA |
| Syndecan-3 | CAAGCAGGCGAGCGTC | GGTGGCAGAGATGAAGTGG |
| Syndecan-4 | TTGCCGTCTTCCTCGTGC | AGGCGTAGAACTCATTGGTGG |
| Biglycan | GCTGCCACTGCCATCTGAG | CGAGGACCAAGGCGTAG |
| GAPDH | AACACCCTCAAGATTGTCAGCAA | ACAGTCTTCTGGGTGGCAGTGA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, T.; Tatsuishi, H.; Banno, T.; Fujie, T.; Yamamoto, C.; Naka, H.; Kaji, T. Copper(II) Bis(diethyldithiocarbamate) Induces the Expression of Syndecan-4, a Transmembrane Heparan Sulfate Proteoglycan, via p38 MAPK Activation in Vascular Endothelial Cells. Int. J. Mol. Sci. 2018, 19, 3302. https://doi.org/10.3390/ijms19113302
Hara T, Tatsuishi H, Banno T, Fujie T, Yamamoto C, Naka H, Kaji T. Copper(II) Bis(diethyldithiocarbamate) Induces the Expression of Syndecan-4, a Transmembrane Heparan Sulfate Proteoglycan, via p38 MAPK Activation in Vascular Endothelial Cells. International Journal of Molecular Sciences. 2018; 19(11):3302. https://doi.org/10.3390/ijms19113302
Chicago/Turabian StyleHara, Takato, Hiroko Tatsuishi, Tomomi Banno, Tomoya Fujie, Chika Yamamoto, Hiroshi Naka, and Toshiyuki Kaji. 2018. "Copper(II) Bis(diethyldithiocarbamate) Induces the Expression of Syndecan-4, a Transmembrane Heparan Sulfate Proteoglycan, via p38 MAPK Activation in Vascular Endothelial Cells" International Journal of Molecular Sciences 19, no. 11: 3302. https://doi.org/10.3390/ijms19113302
APA StyleHara, T., Tatsuishi, H., Banno, T., Fujie, T., Yamamoto, C., Naka, H., & Kaji, T. (2018). Copper(II) Bis(diethyldithiocarbamate) Induces the Expression of Syndecan-4, a Transmembrane Heparan Sulfate Proteoglycan, via p38 MAPK Activation in Vascular Endothelial Cells. International Journal of Molecular Sciences, 19(11), 3302. https://doi.org/10.3390/ijms19113302