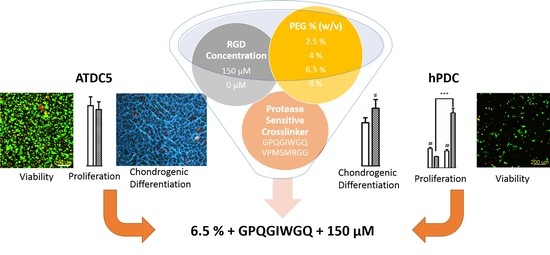
In Vitro Screening of Molecularly Engineered Polyethylene Glycol Hydrogels for Cartilage Tissue Engineering using Periosteum-Derived and ATDC5 Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Screening Using hPDCs Encapsulated in PEG Hydrogels
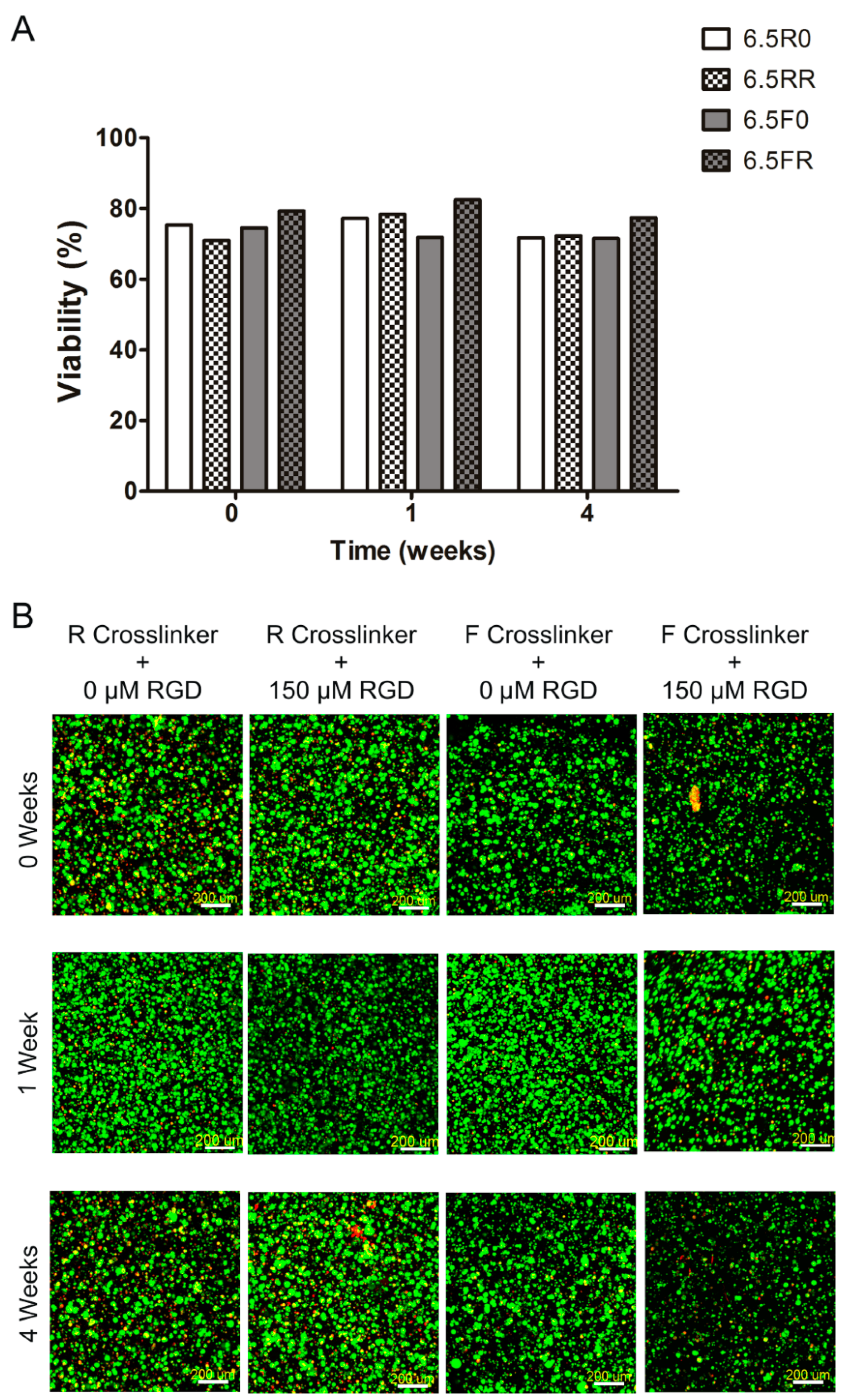
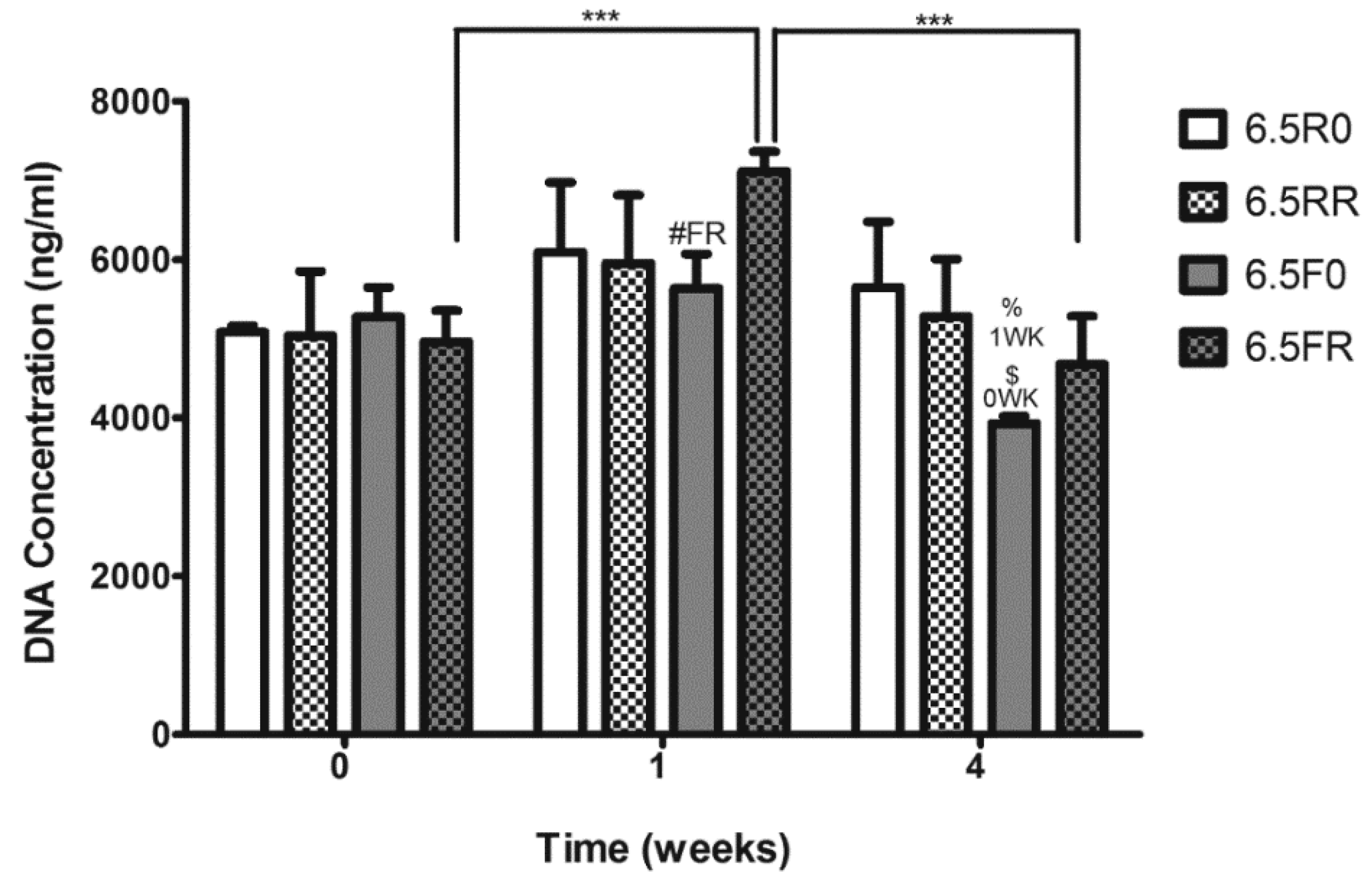
2.1.1. Hydrogel Composition Affects the Encapsulated hPDCs’ Viability and Proliferation when Cultured in Growth Medium
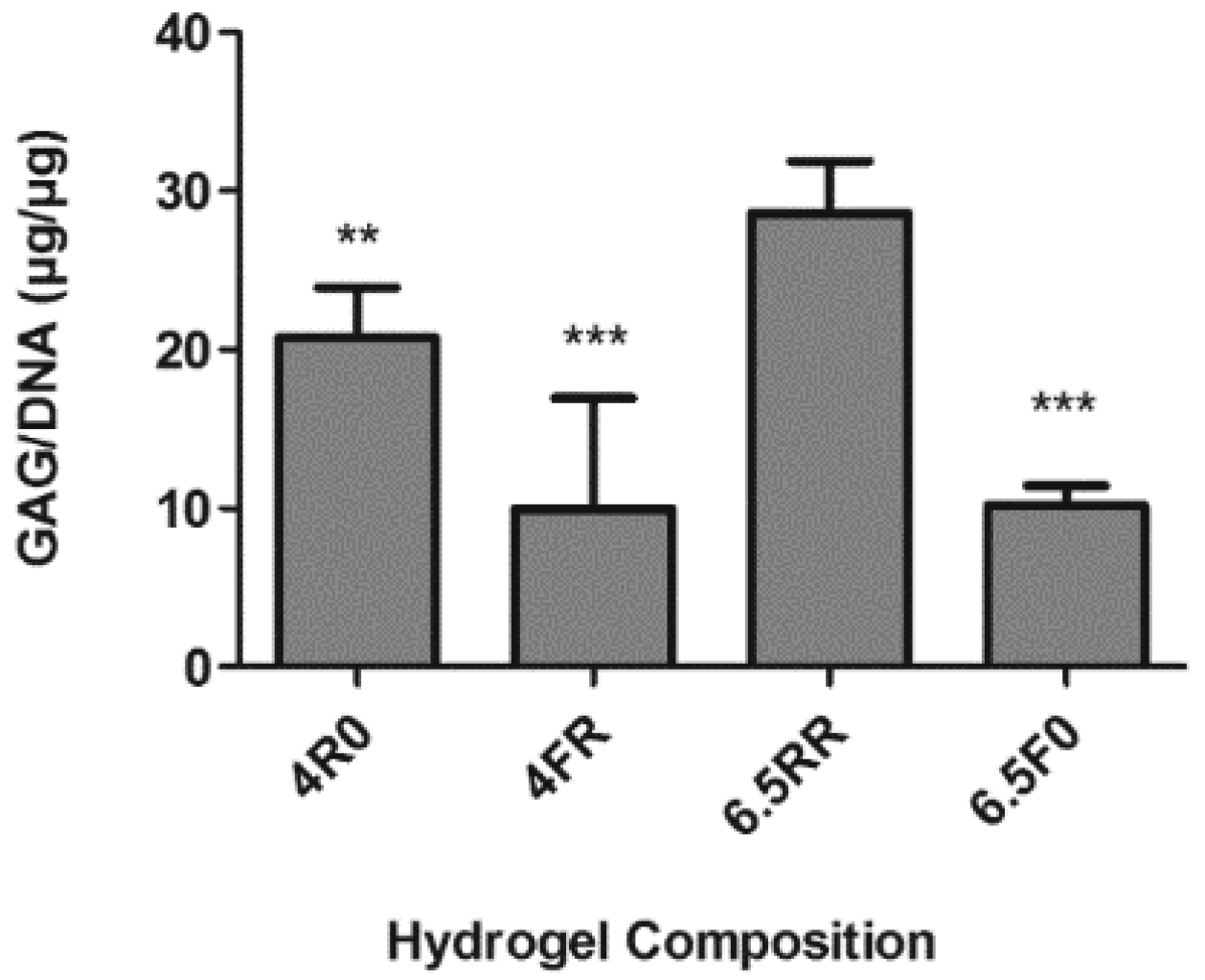
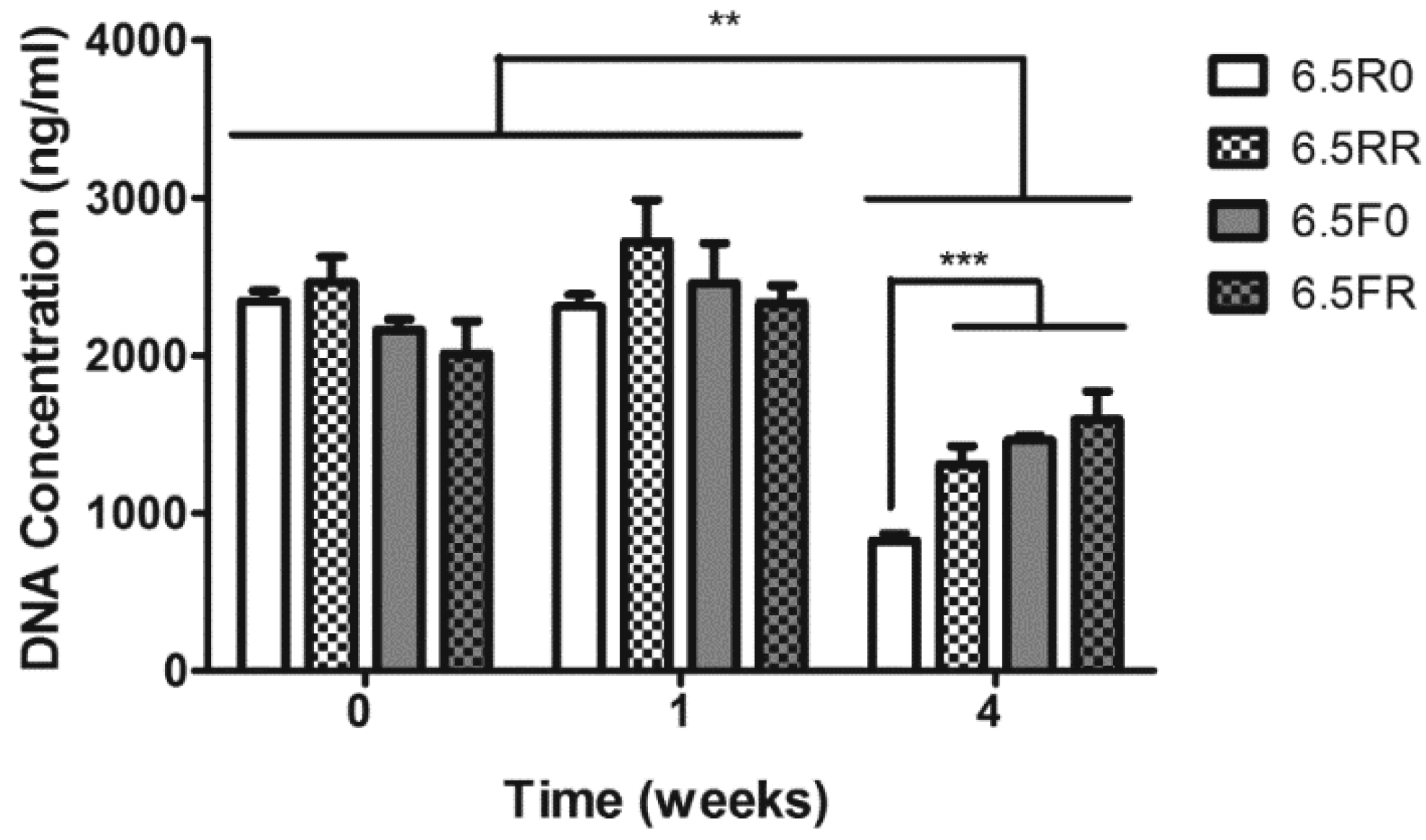
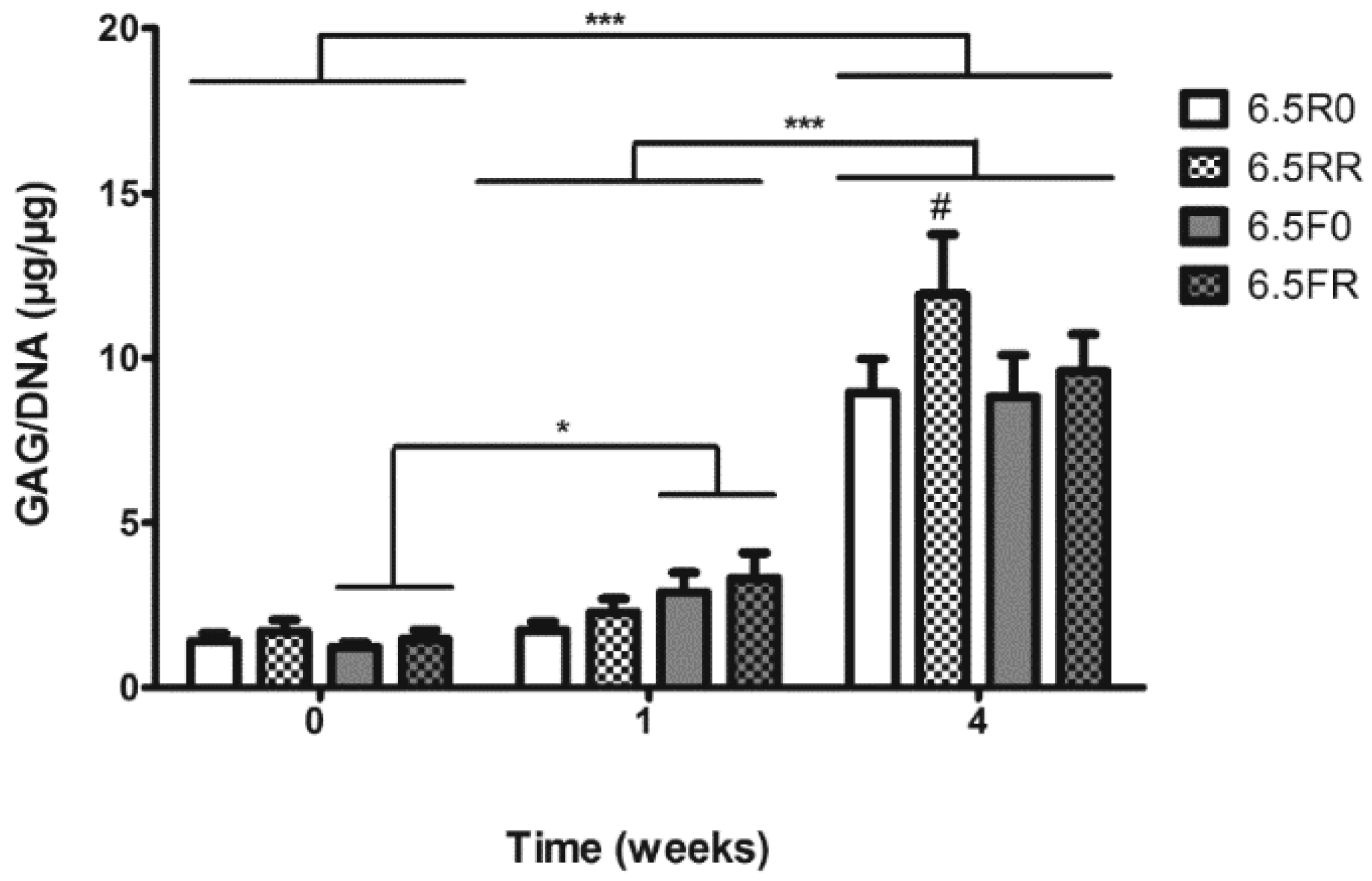
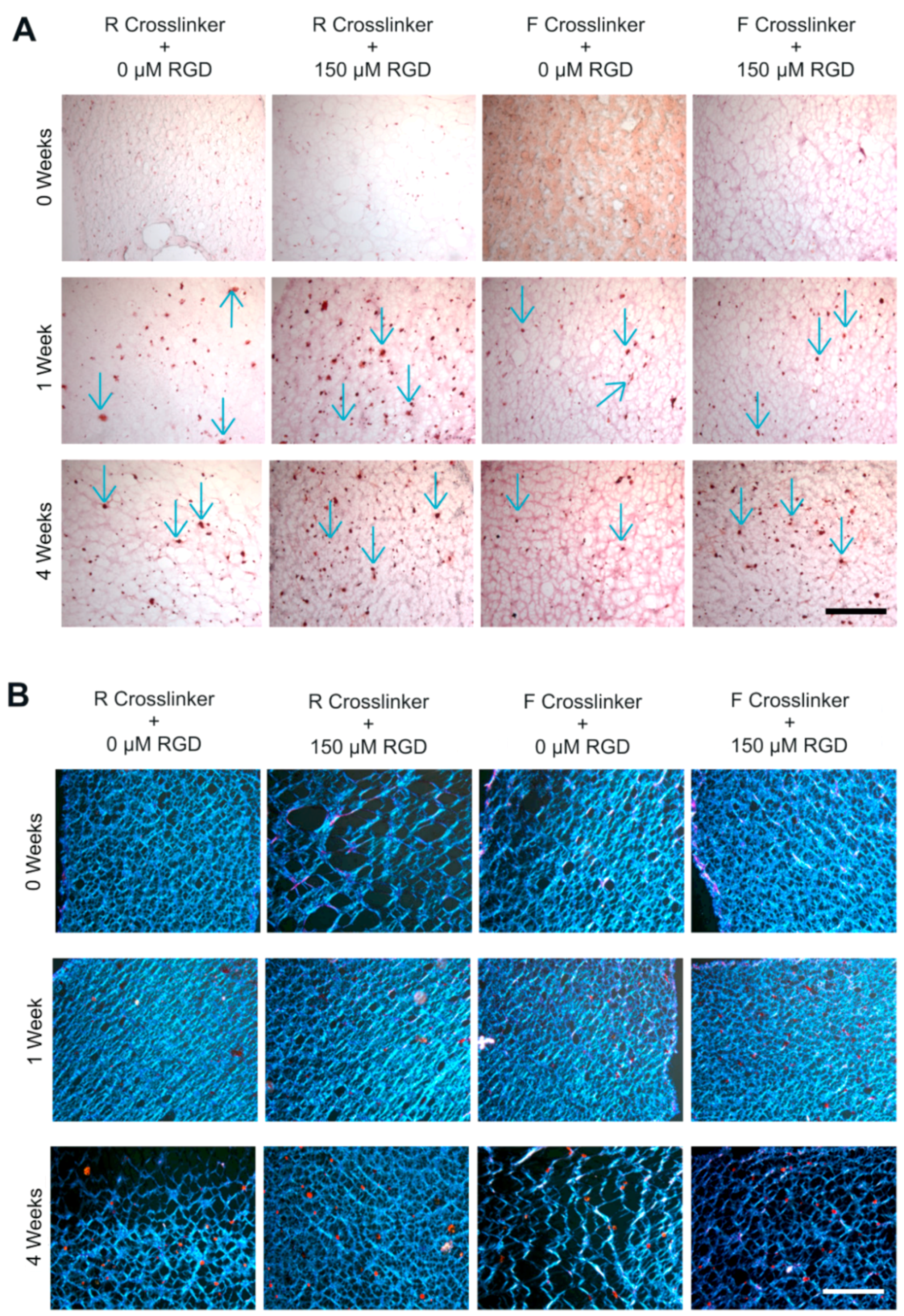
2.1.2. GAG Production of hPDCs Encapsulated in PEG-VS Hydrogels Increases over Time when Cultured in Chondrogenic Differentiation Medium
2.2. Screening Using ATDC5 Cells Encapsulated in PEG Hydrogels and Cultured in Chondrogenic Differentiation Medium
2.2.1. Extent of ATDC5 Viability and Proliferation when Encapsulated within PEG Hydrogels and Cultured in Chondrogenic Differentiation Medium
2.2.2. GAG and Collagen Production by ATDC5 Cells Encapsulated in Protease Degradable PEG-VS Hydrogels
3. Materials and Methods
3.1. hPDC Cell Culture
3.2. ATDC5 Cell Culture
3.3. Hydrogel Preparation and Cell Encapsulation
3.4. Culture Conditions
3.5. Cell Viability Assay
3.6. PicoGreen/Quant-iT DNA Quantification
3.7. Design of Experiments Analysis
3.8. DMMB GAG Assay
3.9. Histology
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACI | Autologous chondrocyte implantation |
| CS | Chondroitin sulfate |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMMB | Dimethylmethylene blue |
| DoE | Design of experiments |
| DTT | Dithiothreitol |
| ECM | Extracellular matrix |
| ETH-1 | Ethidium homodimer-1 |
| FBS | Fetal bovine serum |
| GAG | Glycosaminoglycan |
| GM | Growth medium |
| hPDC | Human periosteum-derived cell |
| Ihh | Indian hedgehog |
| ITS+ | Insulin transferrin selenium + |
| kcat | Catalytic rate constant |
| KM | Michaelis constant |
| MM | Maintenance medium |
| MMP | Matrix metalloproteinase |
| MSC | Mesenchymal stem cell |
| Mw | Molecular weight |
| OCT | Optimal cutting temperature |
| PAMPS | Poly(2-acrylamido-2-methyl-1-propanesulfonic acid) |
| PBE | Phosphate buffered EDTA |
| PBS | Phosphate buffered saline |
| PDMAAm | Poly(dimethylacrylamide) |
| PEG | Polyethylene glycol |
| PEG-DM | Polyethylene glyocol di-methacrylate |
| PEG-VS | Vinyl sulfone end-functionalized polyethylene glycol |
| pKa | Dissociation constant |
| PTH | Parathyroid hormone |
| PTHrP | Parathyroid hormone receptor protein |
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Leonidakis, K.A.; Soultan, A.H.; Piluso, S.; Kudva, A.K.; Van den Broeck, L.; Meiga, T.O.; Gibney, R.; Patterson. Biomimetic materials. In Concise Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; Mishra, M.K., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 189–213. [Google Scholar]
- Moreira Teixeira, L.S.; Patterson, J.; Luyten, F.P. Skeletal tissue regeneration: Where can hydrogels play a role? Int. Orthop. 2014, 38, 1861–1876. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Mankin, H.J. Articular cartilage: Tissue design and chondrocyte-matrix interactions. Instr. Course Lect. 1998, 47, 477–486. [Google Scholar] [PubMed]
- Patterson, J.; Martino, M.M.; Hubbell, J.A. Biomimetic materials in tissue engineering. Mater. Today 2010, 13, 14–22. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Lauer-Fields, J.L.; Schmoekel, H.G.; Metters, A.T.; Weber, F.E.; Fields, G.B.; Hubbell, J.A. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc. Natl. Acad. Sci. USA 2003, 100, 5413–5418. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Durand, K.L.; Anseth, K.S. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. J. Biomed. Mater. Res. Part A 2003, 67, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Chowdhury, T.T.; Lee, D.A.; Bader, D.L.; Anseth, K.S. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann. Biomed. Eng. 2004, 32, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Anseth, K.S. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J. Biomed. Mater. Res. 2002, 59, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Bender, R.J.; Durand, K.L.; Anseth, K.S. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: Engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnol. Bioeng. 2004, 86, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Tian, Q.; Hedrick, J.L.; Po Hui, J.H.; Ee, P.L.; Yang, Y.Y. Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage. Biomaterials 2010, 31, 7298–7307. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Hubbell, J.A. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules 2003, 4, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Elbert, D.L.; Pratt, A.B.; Lutolf, M.P.; Halstenberg, S.; Hubbell, J.A. Protein delivery from materials formed by self-selective conjugate addition reactions. J. Control. Release 2001, 76, 11–25. [Google Scholar] [CrossRef]
- Canal, T.; Peppas, N.A. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J. Biomed. Mater. Res. 1989, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Metters, A.; Hubbell, J. Network formation and degradation behavior of hydrogels formed by Michael-type addition reactions. Biomacromolecules 2005, 6, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Raeber, G.P.; Lutolf, M.P.; Hubbell, J.A. Molecularly engineered PEG hydrogels: A novel model system for proteolytically mediated cell migration. Biophys. J. 2005, 89, 1374–1388. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.; Hubbell, J.A. SPARC-derived protease substrates to enhance the plasmin sensitivity of molecularly engineered PEG hydrogels. Biomaterials 2011, 32, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.; Hubbell, J.A. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials 2010, 31, 7836–7845. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.J.; van Gastel, N.; Carmeliet, G.; Luyten, F.P. Uncovering the periosteum for skeletal regeneration: the stem cell that lies beneath. Bone 2015, 70, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C.; Zhang, X.; Knothe Tate, M.L. Current insights on the regenerative potential of the periosteum: molecular, cellular, and endogenous engineering approaches. J. Orthop. Res. 2012, 30, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, O.; Katsube, Y.; Hirose, M.; Ohgushi, H.; Ito, H. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif. Tissue Int. 2008, 82, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum 2005, 52, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Bolander, J.; Ji, W.; Geris, L.; Bloemen, V.; Chai, Y.C.; Schrooten, J.; Luyten, F.P. The combined mechanism of bone morphogenetic protein- and calcium phosphate-induced skeletal tissue formation by human periosteum derived cells. Eur. Cell Mater. 2016, 31, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.F.; Tam, W.L.; Chai, Y.C.; Geris, L.; Luyten, F.P.; Roberts, S.J. Combinatorial analysis of growth factors reveals the contribution of bone morphogenetic proteins to chondrogenic differentiation of human periosteal cells. Tissue Eng. Part C Methods 2016, 22, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Kudva, A.K.; Luyten, F.P.; Patterson, J. RGD-functionalized polyethylene glycol hydrogels support proliferation and in vitro chondrogenesis of human periosteum-derived cells. J. Biomed. Mater. Res. A 2018, 106, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.; Vasiliadis, H.S.; Brittberg, M.; Lindahl, A. Autologous chondrocyte implantation: A long-term follow-up. Am. J. Sports Med. 2010, 38, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Dufour, J.M. Cell lines: Valuable tools or useless artifacts. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, T.; Miwa, Y.; Kimata, K.; Ikawa, Y. A chondrogenic cell line derived from a differentiating culture of AT805 teratocarcinoma cells. Cell Differ. Dev. 1990, 30, 109–116. [Google Scholar] [CrossRef]
- Shukunami, C.; Ishizeki, K.; Atsumi, T.; Ohta, Y.; Suzuki, F.; Hiraki, Y. Cellular hypertrophy and calcification of embryonal carcinoma-derived chondrogenic cell line ATDC5 in vitro. J. Bone Miner. Res. 1997, 12, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Shukunami, C.; Shigeno, C.; Atsumi, T.; Ishizeki, K.; Suzuki, F.; Hiraki, Y. Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: Differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J. Cell Biol. 1996, 133, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Shigeno, C.; Hiraki, Y.; Shukunami, C.; Kohno, H.; Akagi, M.; Konishi, J.; Nakamura, T. Cloning of a mouse smoothened cDNA and expression patterns of hedgehog signalling molecules during chondrogenesis and cartilage differentiation in clonal mouse EC cells, ATDC5. Biochem. Biophys. Res. Commun. 1997, 235, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Tare, R.S.; Howard, D.; Pound, J.C.; Roach, H.I.; Oreffo, R.O. Tissue engineering strategies for cartilage generation—Micromass and three dimensional cultures using human chondrocytes and a continuous cell line. Biochem. Biophys. Res. Commun. 2005, 333, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, D.; Roeffaers, M.; Kerckhofs, G.; Roberts, S.J.; Hofkens, J.; Van de Putte, T.; Van Oosterwyck, H.; Schrooten, J. Fluorescent oxygen sensitive microbead incorporation for measuring oxygen tension in cell aggregates. Biomaterials 2013, 34, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.E.; Roberts, S.J.; Schrooten, J.; Luyten, F.P. A semi-autonomous model of endochondral ossification for developmental tissue engineering. Tissue Eng. Part A 2012, 18, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Yasuda, K.; Ohmiya, Y.; Honma, K.; Chen, Y.M.; Gong, J.P. In vitro differentiation of chondrogenic ATDC5 cells is enhanced by culturing on synthetic hydrogels with various charge densities. Acta Biomater. 2010, 6, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Cao, X.; Li, Y.; Zeng, L.; Yuan, B.; Chen, X. An injectable hyaluronic acid/PEG hydrogel for cartilage tissue engineering formed by integrating enzymatic crosslinking and Diels–Alder “click chemistry”. Polym. Chem. 2014, 5, 1082–1090. [Google Scholar] [CrossRef]
- Kudva, A.K.; Luyten, F.P.; Patterson, J. Initiating human articular chondrocyte re-differentiation in a 3D system after 2D expansion. J. Mater. Sci. Mater. Med. 2017, 28, 156. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E.; Reed, J.C. Anchorage dependence, integrins, and apoptosis. Cell 1994, 77, 477–478. [Google Scholar] [CrossRef]
- Kraehenbuehl, T.P.; Zammaretti, P.; Van der Vlies, A.J.; Schoenmakers, R.G.; Lutolf, M.P.; Jaconi, M.E.; Hubbell, J.A. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: Systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials 2008, 29, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Zustiak, S.P.; Durbal, R.; Leach, J.B. Influence of cell-adhesive peptide ligands on poly(ethylene glycol) hydrogel physical, mechanical and transport properties. Acta Biomater. 2010, 6, 3404–3414. [Google Scholar] [CrossRef] [PubMed]
- Vigen, M.; Ceccarelli, J.; Putnam, A.J. Protease-sensitive PEG hydrogels regulate vascularization in vitro and in vivo. Macromol. Biosci. 2014, 14, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeck, L.; Piluso, S.; Soultan, A.H.; De Volder, M.; Patterson, J. Cytocompatible carbon nanotube reinforced polyethylene glycol composite hydrogels for tissue engineering. 2018; under revision. [Google Scholar]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Sangaj, N.; Razafiarison, T.; Zhang, C.; Varghese, S. Influence of physical properties of biomaterials on cellular behavior. Pharm. Res. 2011, 28, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Munoz-Pinto, D.; Qu, X.; Hou, Y.; Grunlan, M.A.; Hahn, M.S. Influence of hydrogel mechanical properties and mesh size on vocal fold fibroblast extracellular matrix production and phenotype. Acta Biomater. 2008, 4, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Papantoniou, I.; Sonnaert, M.; Lambrechts, T.; Aerts, J.-M.; Geris, L.; Luyten, F.; Schrooten, J. Analysis of gene expression signatures for osteogenic 3D perfusion-bioreactor cell cultures based on a multifactorial DoE approach. Processes 2014, 2, 639–657. [Google Scholar] [CrossRef]
- Maia, F.R.; Lourenco, A.H.; Granja, P.L.; Goncalves, R.M.; Barrias, C.C. Effect of cell density on mesenchymal stem cells aggregation in RGD-alginate 3D matrices under osteoinductive conditions. Macromol. Biosci. 2014, 14, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Kudva, A.K.; Dikina, A.D.; Luyten, F.P.; Alsberg, E.; Patterson, J. Gelatin microspheres releasing transforming growth factor drive in vitro chondrogenesis of human periosteum derived cells in micromass culture. 2018; submitted. [Google Scholar]
- Elisseeff, J. Injectable cartilage tissue engineering. Expert Opin. Biol. Ther. 2004, 4, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Moreira Teixeira, L.S.; Dijkstra, P.J.; Karperien, M.; van Blitterswijk, C.A.; Zhong, Z.Y.; Feijen, J. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Teixeira, L.S.; Dijkstra, P.J.; van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Enzymatically-crosslinked injectable hydrogels based on biomimetic dextran-hyaluronic acid conjugates for cartilage tissue engineering. Biomaterials 2010, 31, 3103–3113. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Anseth, K.S.; Lee, D.A.; Bader, D.L. Crosslinking density influences the morphology of chondrocytes photoencapsulated in PEG hydrogels during the application of compressive strain. J. Orthop. Res. 2004, 22, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Hyllested, J.L.; Veje, K.; Ostergaard, K. Histochemical studies of the extracellular matrix of human articular cartilage—A review. Osteoarthritis Cartilage 2002, 10, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Eyckmans, J.; Roberts, S.J.; Schrooten, J.; Luyten, F.P. A clinically relevant model of osteoinduction: A process requiring calcium phosphate and BMP/Wnt signalling. J. Cell. Mol. Med. 2010, 14, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Cheng, A.W.; Alexander, P.G.; Beck, A.M.; Tuan, R.S. Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution. Tissue Eng. Part A 2014, 20, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
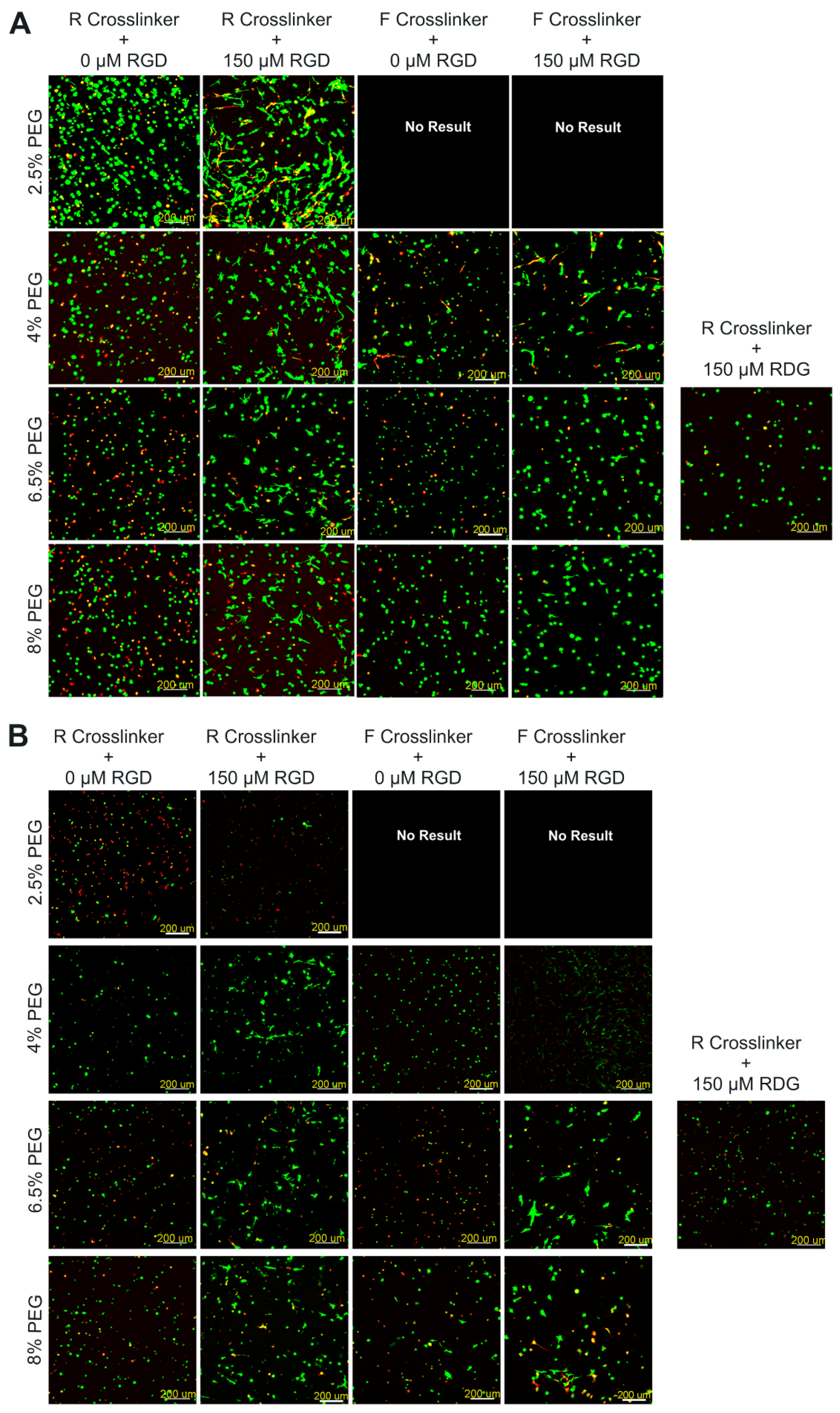
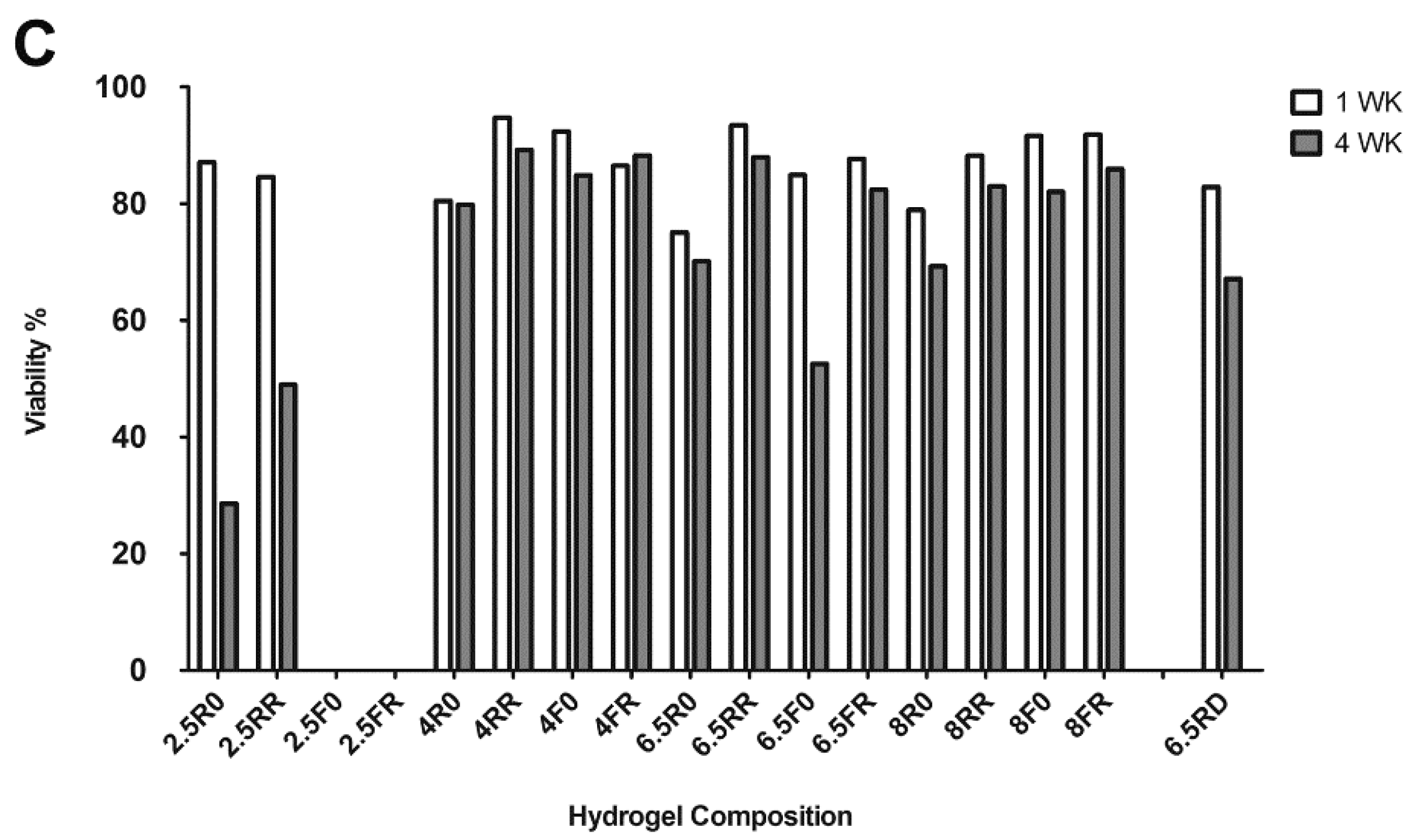


| Nomenclature | % (w/v) PEG | Cross-linker | RGD (µM) |
|---|---|---|---|
| 2.5R0 | 2.5 | Regular | 0 |
| 2.5RR | 2.5 | Regular | 150 |
| 2.5F0 | 2.5 | Fast | 0 |
| 2.5FR | 2.5 | Fast | 150 |
| 4R0 | 4 | Regular | 0 |
| 4RR | 4 | Regular | 150 |
| 4F0 | 4 | Fast | 0 |
| 4FR | 4 | Fast | 150 |
| 6.5R0 | 6.5 | Regular | 0 |
| 6.5RR | 6.5 | Regular | 150 |
| 6.5F0 | 6.5 | Fast | 0 |
| 6.5FR | 6.5 | Fast | 150 |
| 8R0 | 8 | Regular | 0 |
| 8RR | 8 | Regular | 150 |
| 8F0 | 8 | Fast | 0 |
| 8FR | 8 | Fast | 150 |
| 6.5RD | 6.5 | Regular | 150 (scrambled RDG) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudva, A.K.; Luyten, F.P.; Patterson, J. In Vitro Screening of Molecularly Engineered Polyethylene Glycol Hydrogels for Cartilage Tissue Engineering using Periosteum-Derived and ATDC5 Cells. Int. J. Mol. Sci. 2018, 19, 3341. https://doi.org/10.3390/ijms19113341
Kudva AK, Luyten FP, Patterson J. In Vitro Screening of Molecularly Engineered Polyethylene Glycol Hydrogels for Cartilage Tissue Engineering using Periosteum-Derived and ATDC5 Cells. International Journal of Molecular Sciences. 2018; 19(11):3341. https://doi.org/10.3390/ijms19113341
Chicago/Turabian StyleKudva, Abhijith K., Frank P. Luyten, and Jennifer Patterson. 2018. "In Vitro Screening of Molecularly Engineered Polyethylene Glycol Hydrogels for Cartilage Tissue Engineering using Periosteum-Derived and ATDC5 Cells" International Journal of Molecular Sciences 19, no. 11: 3341. https://doi.org/10.3390/ijms19113341
APA StyleKudva, A. K., Luyten, F. P., & Patterson, J. (2018). In Vitro Screening of Molecularly Engineered Polyethylene Glycol Hydrogels for Cartilage Tissue Engineering using Periosteum-Derived and ATDC5 Cells. International Journal of Molecular Sciences, 19(11), 3341. https://doi.org/10.3390/ijms19113341