Cellular Signaling Pathways Activated by Functional Graphene Nanomaterials
Abstract
1. Introduction
2. Functional Graphene Nanomaterials and their Biomedical Applications
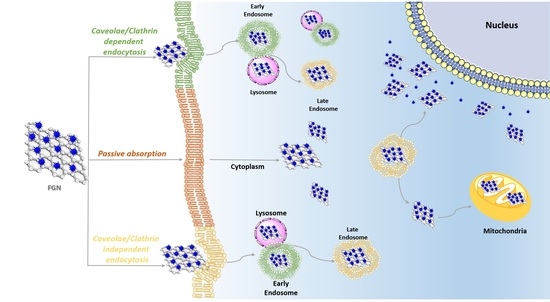
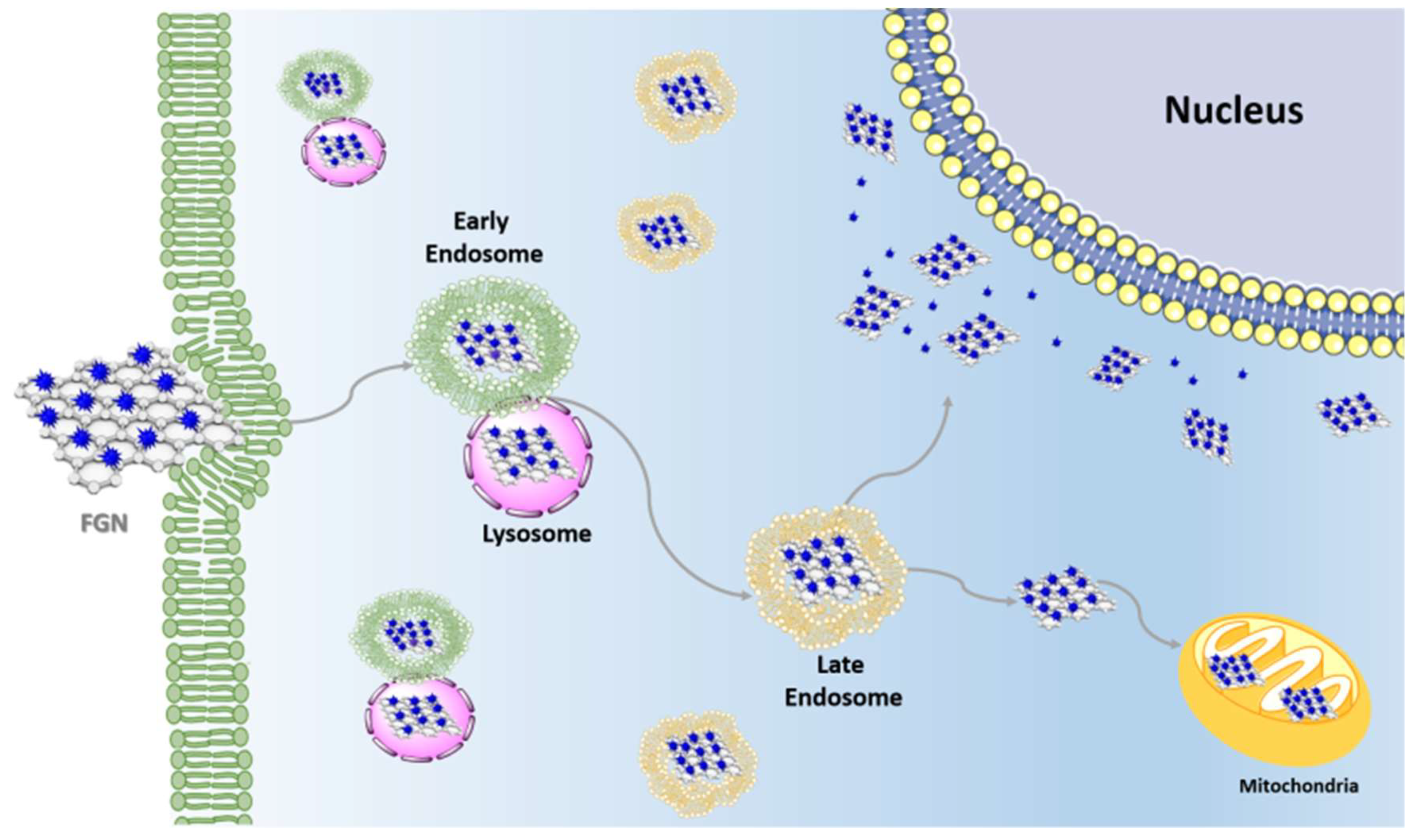
3. Functional Graphene Nanomaterials Cellular Uptake
4. Subcellular Localization of Functional Graphene Nanomaterials
5. Intracellular Molecular Pathways Activated by Functional Graphene Nanomaterials
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AuNPs | Gold nanoparticles |
| DCs | Dendritic cells |
| DNA | Deoxyribonucleic acid |
| Dox | Doxorubicin |
| EGaIn | Eutectic alloy of gallium indium |
| EGFR | Epidermal growth factor receptor |
| EPI | Epirubicin |
| ERK | Extracellular signal–regulated kinases |
| FGN | Functional Graphene Nanomaterials |
| FLG | Few-layer graphene |
| G | Graphene pristine |
| GA | Glycyrrhetinic acid |
| GIAN | Graphene-isolated-Au-nanocrystals |
| GO | Graphene oxide |
| GQDs | Graphene quantum dots |
| G-Red | Reduced graphene oxide |
| GSH | Glutathione |
| HA | Hyaluronic acid |
| MAPK | Mitogen-activated protein kinase |
| MMA | Mitochondria-mediated apoptosis |
| MRI | Magnetic resonance imaging |
| MSCs | Mesenchymal stem cells |
| NIR | Near infrared |
| NO | Nitric oxide |
| NPs | Nanoparticles |
| PARP | Poly ADP ribose polymerase |
| PCI | Photochemical internalization |
| RNA | Ribonucleic acid |
| PDT | Photodynamic therapy |
| PEG | Polyethylene glycol |
| PEG-DSPE | (1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N [amino (polyethylene glycol)] |
| PEI | Polyethyleneimine |
| PS | Photosensitizer |
| PSS | Poly(sodium 4-styrenesulfonate) |
| PTT | Photothermal therapy |
| ROS | Reactive oxygen species |
| SERS | Surface-enhanced Raman scattering |
| Sil | Silibin |
| TPP | Triphenylphosphonium |
| TRITC | Tetramethylrhodamine-isothiocyanate |
| UCNP | Upconversion nanoparticles |
References
- Zhong, Y.; Zhen, Z.; Zhu, H. Graphene: Fundamental research and potential applications. FlatChem 2017, 4, 20–32. [Google Scholar] [CrossRef]
- Tonelli, F.M.P.; Goulart, V.A.M.; Gomes, K.N.; Ladeira, M.S.; Santos, A.K.; Lorençon, E.; Ladeira, L.O.; Resende, R.R. Graphene-based nanomaterials: Biological and medical applications and toxicity. Nanomedicine 2015, 10, 2423–2450. [Google Scholar] [CrossRef] [PubMed]
- Ubani, C.A.; Ibrahim, M.A.; Teridi, M.A.M.; Sopian, K.; Ali, J.; Chaudhary, K.T. Application of graphene in dye and quantum dots sensitized solar cell. Solar Energy 2016, 137, 531–550. [Google Scholar] [CrossRef]
- Thomas, V.J.; Ramaswamy, S. Application of graphene and graphene compounds for environmental remediation. Sci. Adv. Mater. 2016, 8, 477–500. [Google Scholar] [CrossRef]
- Mahdavi, M.; Rahmani, F.; Nouranian, S. Molecular simulation of pH-dependent diffusion, loading, and release of doxorubicin in graphene and graphene oxide drug delivery systems. J. Mater. Chem. B 2016, 4, 7441–7451. [Google Scholar] [CrossRef]
- Malekmohammadi, S.; Hadadzadeh, H.; Farrokhpoura, H.; Amirghofranb, Z. Immobilization of gold nanoparticles on folate-conjugated dendritic mesoporous silica-coated reduced graphene oxide nanosheets: A new nanoplatform for curcumin pH-controlled and targeted delivery. Soft Matter 2018, 14, 2400–2410. [Google Scholar] [CrossRef] [PubMed]
- Dapeng, L.; Wensi, Z.; Xiaoqing, Y.; Zhiqiang, S.; Zhenping, W.; Gang, W. When biomolecule meet graphene: From molecular level interactions to material design and applications. Nanoscale 2016, 47, 19491–19509. [Google Scholar] [CrossRef]
- Silva, M.; Alves, N.M.; Paiva, M.C. Graphene-polymer nanocomposites for biomedical applications. Polym. Adv. Technol. 2018, 29, 687–700. [Google Scholar] [CrossRef]
- Modugno, G.; Ménard-Moyon, C.; Prato, M.; Bianco, A. Carbon nanomaterials combined with metal nanoparticles for theranostic applications. Br. J. Pharmacol. 2015, 172, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Li, S.; Thomas, A.; Kotov, N.A.; Haag, R. Functional Graphene Nanomaterials Based Architectures: Biointeractions, Fabrications, and Emerging Biological Applications. Chem. Rev. 2017, 117, 1826–1914. [Google Scholar] [CrossRef] [PubMed]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef] [PubMed]
- Krishanu, G.; Kishor, S. Biomedical Applications of Graphene Nanomaterials and Beyond. ACS Biomater. Sci. Eng. 2018, 4, 2653–2703. [Google Scholar] [CrossRef]
- Karahan, H.E.; Wang, Y.; Li, W.; Liu, F.; Wang, L.; Sui, X.; Riaza, M.A.; Chen, Y. Antimicrobial graphene materials: The interplay of complex materials characteristics and competing mechanisms. Biomater. Sci. 2018, 6, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wan, J.; Zhang, S.; Zhang, Y.; Lee, S.T.; Liu, Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACSNano 2011, 5, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic acid targeting of CD44 for cancer therapy: From receptor boilogy to nanomedicine. J. Drug Target. 2015, 23, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Bharate, P.; Lai, C.H.; Ziem, B.; Böttcher, C.; Schulz, A.; Beckert, F.; Hatting, B.; Mülhaupt, R.; Seeberger, P.H.; et al. Multivalency at Interfaces: Supramolecular Carbohydrate-Functionalized Graphene Derivatives for Bacterial Capture, Release, and Disinfection. Nano Lett. 2015, 15, 6051–6605. [Google Scholar] [CrossRef] [PubMed]
- Ziem, B.; Thien, H.; Achazi, K.; Yue, C.; Stern, D.; Silberreis, K.; Gholami, M.F.; Beckert, F.; Groger, D.; Mulhaupt, R.; et al. Highly Efficient Multivalent 2D Nanosystems for Inhibition of Orthopoxvirus Particles. Adv. Healthc. Mater 2016, 5, 2922–2930. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Wycisk, V.; Cheng, C.; Chen, W.; Adeli, M.; Haag, R. Functionalized graphene sheets for intracellular controlled release of therapeutic agents. Nanoscale 2017, 9, 18931–18939. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wu, L.; Zhang, J.; Xi, G.; Pang, Y.; Wang, X.; Chen, T. Hydrothermal Reduction of Polyethylenimine and Polyethylene Glycol Dual-Functionalized Nanographene Oxide for High-Efficiency Gene Delivery. ACS Appl. Mater. Interfaces 2016, 8, 31311–31320. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.Z.; Yang, X.Z.; Shi, X.Z.; Tan, X.F.; Peng, R.; Wang, J.; Liu, Z. Polyethylene Glycol and Polyethylenimine Dual-Functionalized Nano-Graphene Oxide for Photothermally Enhanced Gene Delivery. Small 2013, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Neri, G.; Micale, N.; Scala, A.; Fazio, E.; Mazzaglia, A.; Mineo, P.G.; Montesi, M.; Panseri, S.; Tampieri, A.; Grassi, G.; et al. Silibinin-conjugated graphene nanoplatform: Synthesis, characterization and biological evaluation. FlatChem 2017, 1, 134–141. [Google Scholar] [CrossRef]
- Kenry; Lee, W.C.; Loh, K.P.; Lim, C.T. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials 2018, 155, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. JNSC 2018, 8, 123–137. [Google Scholar] [CrossRef]
- Liang, X.; Liang, B.-L.; Panb, Z.; Lang, X.; Zhang, Y.; Wang, G.; Yin, P.; Guo, L. Tuning plasmonic and chemical enhancement for SERS detection on graphene-based Au hybrids. Nanoscale 2015, 7, 20188–20196. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, X.; Jacobson, O.; Lin, L.; Huang, P.; Niu, G.; Ma, Q.; Chen, X. Sequential Drug Release and Enhanced Photothermal and Photoacoustic Effect of Hybrid Reduced Graphene Oxide-Loaded Ultrasmall Gold Nanorod Vesicles for Cancer Therapy. ACSNano 2015, 22, 9199–9209. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.A.; Shimb, M.S.; Heo, C.Y.; Kwon, Y.J. “Combo” nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Biagiotti, G.; Fedeli, S.; Tuci, G.; Luconi, L.; Giambastiani, G.; Brandi, A.; Pisaneschi, F.; Cicchi, S.; Paoli, P. Combined therapies with nanostructured carbon materials: There is room still available at the bottom. J. Mater. Chem. B 2018, 6, 2022–2035. [Google Scholar] [CrossRef]
- Tu, Z.; Achazi, K.; Schulz, A.; Mülhaupt, R.; Thierbach, S.; Rühl, E.; Adeli, M.; Haag, R. Combination of Surface Charge and Size Controls the Cellular Uptake of Functionalized Graphene Sheets. Adv. Funct. Mater. 2017, 27, 1701837–1701848. [Google Scholar] [CrossRef]
- Chatterjee, N.; Eom, H.-J.; Choi, J. A systems toxicology approach to the surface functionality control of Graphene cell interactions. Biomaterials 2014, 35, 1109–1127. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yan, L.-T. Physical principles of graphene cellular interactions: Computational and theoretical accounts. J. Mater. Chem. B 2017, 5, 4290–4306. [Google Scholar] [CrossRef]
- Liao, K.H.; Lin, Y.S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wei, P.; Zhou, Z.; Wei, T. Interactions of graphene with mammalian cells: Molecular mechanisms and biomedical insights. Adv. Drug Deliv. Rev. 2016, 105, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, K.; Luo, Z.; Duan, Y. Preparation and tumor cell model based biobehavioral evaluation of the nanocarrier system using partially reduced graphene oxide functionalized by surfactant. Int. J. Nanomed. 2015, 10, 4605–4620. [Google Scholar] [CrossRef]
- Sinha, A.; Cha, B.G.; Choi, Y.; Nguyen, T.L.; Yoo, P.J.; Jeong, J.H.; Kim, J. Carbohydrate-Functionalized rGO as an Effective Cancer Vaccine for Stimulating Antigen-Specific Cytotoxic T Cells and Inhibiting Tumor Growth. Chem. Mater. 2017, 29, 6883–6892. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Zou, Y.; Lai, X.; Ding, D.; Chen, L.; Zhang, L.; Wu, Y.; Chen, Z.; Tan, W. Elucidating the cellular uptake mechanism of aptamer-functionalized graphene-isolated-Au-nanocrystals with dual-modal imaging. Analyst 2016, 141, 3337–3342. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.M.; Xie, S.; Fang, J.; Lee, S.K.; Sitharaman, B. Nanoparticle-Facilitated Membrane Depolarization-Induced Receptor Activation: Implications on Cellular Uptake and Drug Delivery. ACS Biomater. Sci. Eng. 2016, 2, 2153–2161. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, Y.; Chen, Z.; Hu, Q.; Liu, Y.; Yu, S.; Gao, W.; Dickey, M.D.; Gu, Z. Enhanced Endosomal Escape by Light-Fueled Liquid-Metal Transformer. Nano Lett. 2017, 17, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Bramini, M.; Sacchetti, S.; Armirotti, A.; Rocchi, A.; Vázquez, E.; León Castellanos, V.; Bandiera, T.; Cesca, F.; Benfenati, F. Graphene Oxide Nanosheets Disrupt Lipid Composition, Ca2+ Homeostasis, and Synaptic Transmission in Primary Cortical Neurons. ACS Nano 2016, 10, 7154–7171. [Google Scholar] [CrossRef] [PubMed]
- Mazzaglia, A.; Scala, A.; Sortino, G.; Zagami, R.; Zhu, Y.; Sciortino, M.T.; Pennisi, R.; Pizzo, M.M.; Neri, G.; Grassi, G.; et al. Intracellular trafficking and therapeutic outcome of multiwalledcarbon nanotubes modified with cyclodextrins and polyethylenimine. Coll. Surf. B Biointerfaces 2018, 163, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Choi, J.; Wang, W.; Lee, S.; Nam, T.H.; Choi, W.; Kim, C.J.; Lee, J.K.; Kim, S.H.; Kang, S.S.; et al. Nullifying Tumor Efflux by Prolonged Endolysosome Vesicles: Development of Low Dose Anticancer-Carbon Nanotube Drug. ACS Nano 2013, 22, 8484–8497. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wei, M.; Li, S.; Le, W. Nanomaterial-modulated autophagy: Underlying mechanisms and functional consequences. Nanomedicine 2016, 11, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Xu, B.; Yao, M.; Mao, Z.; Zhang, Y.; Xu, G.; Tang, Q.; Wang, X.; Xia, Y. Graphene oxide quantum dots disrupt autophagic flux by inhibiting lysosome activity in GC-2 and TM4 cell lines. Toxicology 2016, 30, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-C.; Lin, M.-W.; Hsu, M.-N.; Yu-Chen, G.; Chao, Y.-C.; Tuan, H.-Y.; Chiang, C.-S.; Hu, Y.-C. Graphene oxide sensitizes cancer cells to chemotherapeutics by inducing early autophagy events, promoting nuclear trafficking and necrosis. Theranostic 2018, 8, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, Z.; Zheng, Y.; Geng, Y.; Han, C.; Shi, Y.; Sun, H.; Zhang, C.; Chen, Y.; Zhang, L.; et al. Glycyrrhetinic Acid Functionalized Graphene Oxide for Mitochondria Targeting and Cancer Treatment In Vivo. Small 2018, 14, 1703306–1703320. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Liu, J.; Zhao, Z.; Zhao, Y.; Dong, L.; Yang, M.; Zhou, J.; Huo, M. Redox Sensitive Hyaluronic Acid-Decorated Graphene Oxide for Photothermally Controlled Tumor-Cytoplasm- Selective Rapid Drug Delivery. Adv. Funct. Mater. 2017, 27, 1604620–1604632. [Google Scholar] [CrossRef]
- Tabisha, T.A.; Zhang, S.; Winyard, P.G. Developing the next generation of graphene-based platforms for cancer therapeutics: The potential role of reactive oxygen species. Redox Biol. 2018, 15, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhou, F.; Zhang, D.; Chen, Q.; Xing, D. A graphene oxide based smart drug delivery system for tumor mitochondria-targeting photodynamic therapy. Nanoscale 2016, 8, 3530–3538. [Google Scholar] [CrossRef] [PubMed]
- Lammel, T.; Boisseaux, P.; Fernández-Cruz, M.L.; Navas, J.M. Internalization and cytotoxicity of graphene oxide and carboxyl graphene nanoplatelets in the human hepatocellular carcinoma cell line Hep G2. Part. Fibre Toxicol. 2013, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Skovsen, E.; Snyder, J.W.; Lambert, J.D.C.; Ogilby, P.R. Lifetime and Diffusion of Singlet Oxygen in a Cell. J. Phys. Chem. B 2005, 109, 8570–8573. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, L.; Tian, Y.; Guan, X.; Liu, Q.; Li, S.; Qin, X.; Yang, H.; Liu, Y. “Triple-Punch” Anticancer Strategy Mediated by Near-Infrared Photosensitizer/CpG Oligonucleotides Dual-Dressed and Mitochondria-Targeted Nanographene. ACS Appl. Mater. Interfaces 2018, 10, 6942–6955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wen, L.; Huang, R.; Wang, H.; Hu, X.; Xing, D. Mitochondrial specific photodynamic therapy by rare-earth nanoparticles mediated near-infrared graphene quantum dots. Biomaterials 2018, 153, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Wang, F.D.; Tang, Y.; Zhang, X.Z.; Wang, J.Q.; Yang, Y.J. Distribution of graphene oxide and TiO2-graphene oxide composite in A549 cells. Biol. Trace Elem. Res. 2014, 159, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.L.; Wo, Y.; Guo, S.W.; Cui, D.X. Biocompatibility of graphene oxide. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yang, Z.; Duan, G.; Wu, A.; Gu, Z.; Zhang, L.; Chen, C.; Chai, Z.; Ge, C.; Zhou, R. Graphene Oxide Nanosheets Retard Cellular Migration via Disruption of Actin Cytoskeleton. Small 2017, 13, 1602133. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, M.C.; Vila, M.; Feito, M.J.; Linares, J.; Goncalves, G.; Vallet-Regi, M.; Marques, P.A.; Portoles, M.T. The effects of graphene oxide nanosheets localized on F-actin filaments on cell-cycle alterations. Biomaterials 2013, 34, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Chiacchiaretta, M.; Bramini, M.; Rocchi, A.; Armirotti, A.; Giordano, E.; Vázquez, E.; Bandiera, T.; Ferroni, S.; Cesca, F.; Benfenati, F. Graphene Oxide Upregulates the Homeostatic Functions of Primary Astrocytes and Modulates Astrocyte-to-Neuron Communication. Nano Lett. 2018, 18, 5827–5838. [Google Scholar] [CrossRef] [PubMed]
- Mailänder, V.; Landfester, K. Interaction of Nanoparticles with Cells. Biomacromolecules 2009, 10, 2379–2400. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle delivery of cancer drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Robinson, J.T.; Sun, X.M.; Dai, H.J. PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, J.; Yang, G.; Wanga, C.; Zhu, J.J. Direct electrochemistry and electrocatalysis of hemoglobin based on poly (diallyldimethylammonium chloride). functionalized graphene sheets/room temperature ionic liquid composite film. Electrochem. Commun. 2010, 12, 402–405. [Google Scholar] [CrossRef]
- Guo, C.X.; Zheng, X.T.; Lu, Z.S.; Lou, X.W.; Li, C.M. Biointerface by Cell Growth on Layered Graphene-Artificial Peroxidase-Protein Nanostructure for in Situ Quantitative Molecular Detection. Adv. Mater. 2010, 22, 5164–5167. [Google Scholar] [CrossRef] [PubMed]
- Zan, X.; Fang, Z.; Wu, J.; Xiao, F.; Huo, F.; Duan, H. Free-standing Graphene Paper Decorated with 2d-Assembly of Au@Pt Nanoparticles as Flexible Biosensors to Monitor Live Cell Secretion of Nitric Oxide. Biosens, Bioelectron. 2013, 49, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Wang, X.-Y.; Xu, J.-Q.; Xiao, C.; Liu, Y.-H.; Zhang, X.-W.; Liu, J.-T.; Huang, W.-H. Functionalized Graphene-Based Biomimetic Microsensor Interfacing with Living Cells to Sensitively Monitor Nitric Oxide Release. Chem. Sci. 2015, 6, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, L.; Li, Z.; Lin, Y.; Li, J. In Situ Simultaneous Monitoring of Atp and Gtp Using a Graphene Oxide Nanosheet-Based Sensing Platform in Living Cells. Nat. Protoc. 2014, 9, 1944–1955. [Google Scholar] [CrossRef] [PubMed]
- He, X.-P.; Zhu, B.-W.; Zang, Y.; Li, J.; Chen, G.-R.; Tian, H.; Long, Y.-T. Dynamic Tracking of Pathogenic Receptor Expression of Live Cells Using Pyrenyl Glycoanthraquinone-Decorated Graphene Electrodes. Chem. Sci. 2015, 6, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhu, B.-W.; Qu, S.; He, X.-P.; Chen, G.-R. “Clicked” Galactosyl Anthraquinone on Graphene Electrodes for the Label-Free Impedance Detection of Live Cancer Cells. Dyes Pigm. 2015, 121, 312–315. [Google Scholar] [CrossRef]
- Yang, X.Y.; Niu, G.L.; Cao, X.F.; Wen, Y.K.; Xiang, R.; Duan, H.Q.; Chen, Y.S.J. The preparation of functionalized graphene oxide for targeted intracellular delivery of siRNA. Mater. Chem. 2012, 22, 6649–6654. [Google Scholar] [CrossRef]
- Depan, D.; Shah, J.; Misra, R.D.K. Controlled release of drug from folate-decorated and graphene mediated drug delivery system: Synthesis, loading efficiency, and drug release response. Mater. Sci. Eng. C 2011, 31, 1305–1312. [Google Scholar] [CrossRef]
- Qin, X.C.; Guo, Z.Y.; Liu, Z.M.; Zhang, W.; Wan, M.M.; Yang, B.W. Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. J. Photochem. Photobiol. B Biol. 2013, 120, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, W.; Xu, Z.; Fan, W.; Peng, W.; Luo, S. Comparative toxicity of pristine graphene oxide and its carboxylimidazole or polyethylene glycol functionalized products to Daphnia magna: A two generation study. Environ. Pollut. 2018, 237, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhao, K.; Li, W.; Yang, N.; Liu, Y.; Chen, C.; Wei, T. The interactions between pristine graphene and macrophages and the production of cytokines/chemokines via TLR- and NF-κB-related signaling pathways. Biomaterials 2012, 33, 6933–6942. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Dhifaf, A.; Pescatori, J.M.; Manetti, R.; Fozza, C.; Sgarrella, F.; Bedognetti, D.; Bianco, A.; Kostarelos, K.; Delogu, L.G. Molecular and Genomic Impact of Large and Small Lateral Dimension Graphene Oxide Sheets on Human Immune Cells from Healthy Donors. Adv. Healthc. Mater. 2016, 21, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Russier, J.; León, V.; Orecchioni, M.; Hirata, E.; Virdis, P.; Fozza, C.; Sgarrella, F.; Cuniberti, G.; Prato, M.; Vázquez, E.; et al. Few-Layer Graphene Kills Selectively Tumor Cells from Myelomonocytic Leukemia Patients. Angew. Chem. 2017, 129, 3060–3065. [Google Scholar] [CrossRef]
- Orecchioni, M.; Bedognetti, D.; Newman, L.; Fuoco, C.; Spada, F.; Hendrickx, W.; Marincola, F.M.; Sgarrella, F.; Rodrigues, A.F.; Ménard-Moyon, C.; et al. Single-cell mass cytometry and transcriptome profiling reveal the impact of graphene on human immune cells. Nat. Commun. 2017, 24, 1109. [Google Scholar] [CrossRef] [PubMed]
- Tabish, T.A.; Pranjol, M.Z.I.; Hayat, H.; Rahat, A.A.M.; Abdullah, T.M.; Whatmore, J.L.; Zhang, S.W. In vitro toxic effects of reduced graphene oxide nanosheets on lung cancer cells. Nanotechnology 2018, 28, 504001. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Liu, J.; Wu, J.; Yin, Q.; Liang, H.; Chen, A.; Shao, L. Graphene oxide and reduced graphene oxide induced neural pheochromocytoma-derived PC12 cell lines apoptosis and cell cycle alterations via the ERK signaling pathways. Int. J. Nanomed. 2017, 2, 5501–5510. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, L.; Ying, L.; Yujian, F.; Taotao, W.; Le Guyader, L.; Ge, G.; Liu, R.-S.; Chang, Y.-Z.; Chen, C. The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways. Biomaterials 2012, 33, 402–411. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piperno, A.; Scala, A.; Mazzaglia, A.; Neri, G.; Pennisi, R.; Sciortino, M.T.; Grassi, G. Cellular Signaling Pathways Activated by Functional Graphene Nanomaterials. Int. J. Mol. Sci. 2018, 19, 3365. https://doi.org/10.3390/ijms19113365
Piperno A, Scala A, Mazzaglia A, Neri G, Pennisi R, Sciortino MT, Grassi G. Cellular Signaling Pathways Activated by Functional Graphene Nanomaterials. International Journal of Molecular Sciences. 2018; 19(11):3365. https://doi.org/10.3390/ijms19113365
Chicago/Turabian StylePiperno, Anna, Angela Scala, Antonino Mazzaglia, Giulia Neri, Rosamaria Pennisi, Maria Teresa Sciortino, and Giovanni Grassi. 2018. "Cellular Signaling Pathways Activated by Functional Graphene Nanomaterials" International Journal of Molecular Sciences 19, no. 11: 3365. https://doi.org/10.3390/ijms19113365
APA StylePiperno, A., Scala, A., Mazzaglia, A., Neri, G., Pennisi, R., Sciortino, M. T., & Grassi, G. (2018). Cellular Signaling Pathways Activated by Functional Graphene Nanomaterials. International Journal of Molecular Sciences, 19(11), 3365. https://doi.org/10.3390/ijms19113365