1. Introduction
Polyelectrolyte complexes (PECs) are made from two or more macromolecules carrying opposite electrical charges, which can be ionized and electrically complexed with each other in a polar solution [
1]. The polymeric acids (polyanions) or bases (polycations) respond to environmental pH value for their ionization in the pKa interval [
2]. Therefore, in the field of pharmaceutics, PECs have been developed for decades to encapsulate drugs in oral-controlled or sustained-release system, in response to the environmental pH of the gastrointestinal tract (GI tract) [
3]. Besides, PECs are usually formed in polar solvents, such as water or ethanol, to improve the solubility of hydrophobic drugs in pharmaceutics [
4]. Among all developed polymeric materials, natural polymers attract much attention for application in drug delivery system because of their excellent biocompatibility and biodegradability [
5]. The commonly used polyanions are polysaccharides, whose side chains are appended with carboxylic acids, such as pectin, alginates, and acacia gum [
6]. Proteins, such as zein and gelatin, and modified polysaccharides, such as chitosan, are widely utilized as natural polycationic materials [
7,
8,
9]. In addition, natural polymers as PECs have been applied in drug delivery systems to improve the water solubility or for controlled release designs in many studies [
1]. For example, D. Wurster et al. have prepared natural polymers containing cationic type A gelatin and anionic acacia gum to generate PECs for encapsulating hydrophobic agent vitamin A palmitate. The mixing ratios and fabricating methods have been studied and optimized. The PECs could efficiently improve the water solubility of vitamin A palmitate [
10]. B. Stokke et al. have built and optimized PECs with natural polymers containing cationic chitosan (pKa 6.5–6.6) and anionic alginates (pKa 3.4–3.7) at the pKa intervals, and they measured the average diameters upon environmental pH changes from pH 3.0 to 8.0. Moreover, they have found that particle sizes would significantly change at above pH 7.0 for the zeta potential alteration (above the pKa of chitosan), that would be potential in controlled releasing pharmaceutical designs [
8].
Curcumin (diferuloylmethane) is a phenolic component extracted from the rhizome of turmeric [
11]. As a bioactive compound, it has proven its medical efficacy in several tumors [
12,
13,
14], including gastric [
15] and colorectal cancers [
16,
17]. However, its low water solubility and bioavailability limit its clinical uses via oral administration [
18,
19]. Besides, curcumin in a buffer decomposes in a pH-dependent manner, and this degradation accelerates at neutral-basic conditions in preservation, strongly diminishing its pharmacological activity [
20]. Studies have suggested that curcumin could be well formulated to improve its solubility and bioavailability via a reliable controlled- or sustained-release system [
21]. PEC is one of several promising approaches and several attempts to generate PECs for curcumin delivery have been conducted [
22,
23]. K. Hu et al. have fabricated nano-sized zein-pectin PEC for curcumin delivery [
7]. P. Sarika et al. have prepared a cationized gelatin and sodium alginate PEC to improve the water solubility of curcumin, allowing sustained-release of curcumin and enhancing its cytotoxicity in vitro [
24]. A. Anitha et al. have prepared curcumin-loaded dextran sulfate-chitosan PECs to increase the water solubility of curcumin, and the curcumin content also exhibited cytotoxicity toward cancer cells [
25]. Although the principle of PEC formation and studies have proved that PECs would be an applicable formulation for curcumin, it is still difficult to optimize their manufacturing process, including determination of constituent ratios and pH for aggregation [
26]. These manufacturing factors strongly affect the particle size, morphology, loading efficiency, and drug-releasing behaviors of PEC systems [
27]. Thus, it is essential to develop a PEC system with an optimized manufacturing process.
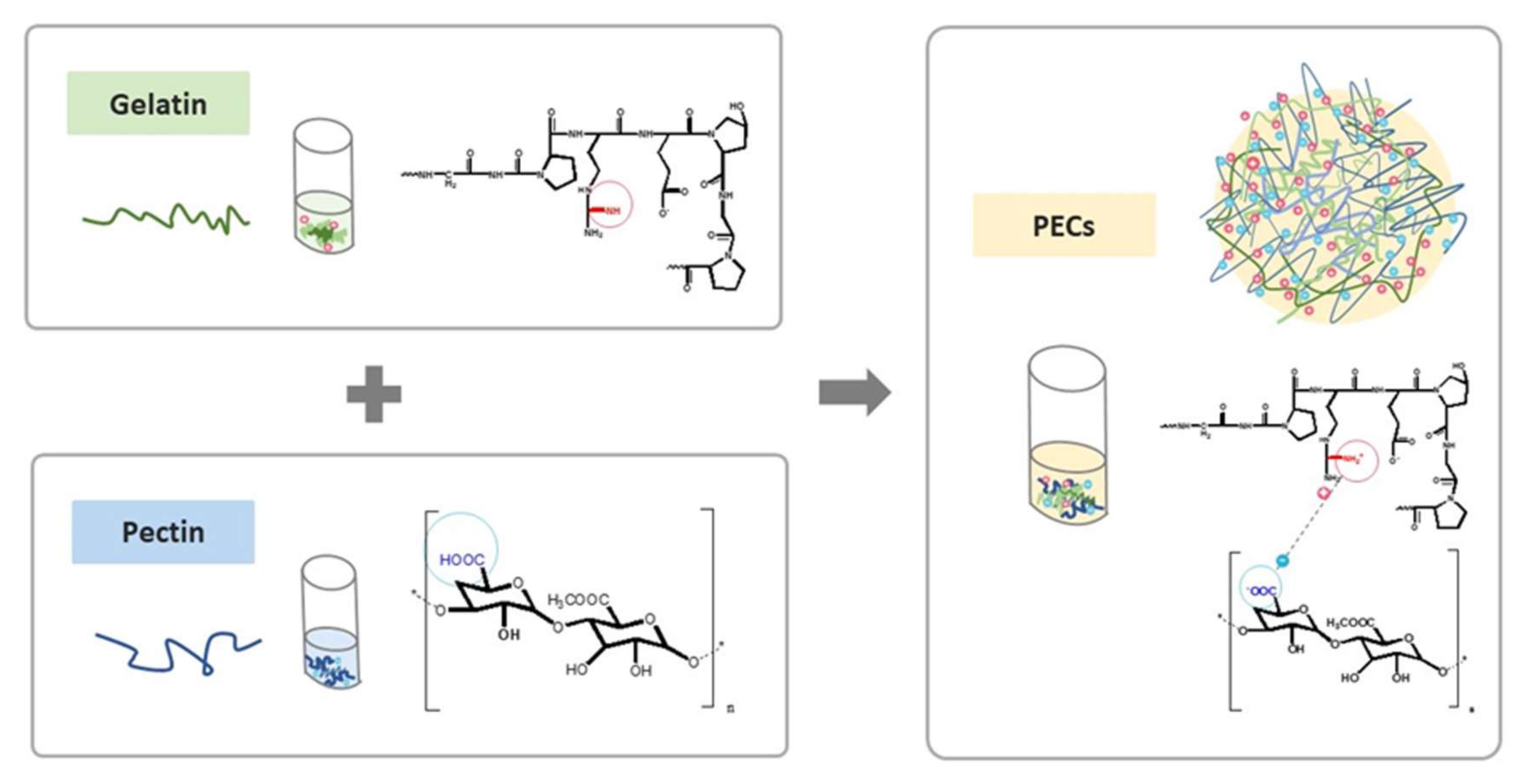
In this study, we proposed a novel PEC composed of natural polymers, and its fabrication was optimized based on its performance in mimetic GI environment. We assembled the natural polycation type B gelatin and the polyanion pectin into a PEC system at an appropriate pH in a polar solvent system, as shown in
Scheme 1. Several studies have previously reported pectin-gelatin PECs, yet type A gelatin was mostly chosen for use in their systems [
26,
28]. Type B gelatin, to our best knowledge, was first introduced in PEC for curcumin delivery. Type B gelatin is derived from collagen after lime hydrolyzation, and its isoelectric point (pI) was 4.8–5.5. The cationized type B gelatin would yield below pH 4.8, such as at pH 4.0. Apart from the electrical properties of type B gelatin, the pI of type A gelatin is approximately 9.0; thus, it is positively charged under this broadline [
29]. Importantly, the pKa of polysaccharides pectin is 3.5 [
30]. This pectin has been utilized to fabricate PEC because it could be electrically neutralized to force payload release in a stomach environment, which has a pH of 2.0 [
31]. Besides, recent studies have pointed out the potential of pectin in colon cancer treatment because its backbone could only be cleaved by colon microorganisms [
32]. Herein, PECs composed of pectin and type B gelatin were prepared at pH 4.0, which could reduce the decomposition of curcumin during manufacture or preservation. The curcumin-releasing profiles of the PECs were further investigated in the mimetic GI environmental pH value and transit periods (pH 2.0 for 2 h to simulate conditions in the stomach, and pH 8.0 for 4 h to mimic pH conditions in the small intestine) [
33]. The remaining curcumin-loaded PECs after pretreatment with mimetic GI tract conditions would be internalized into the colon carcinoma. The biosafety and cytotoxic effect of curcumin-loaded PECs to human normal cells and colon cancer were examined in this study to evaluate the potentials of PECs as a curcumin formulation for future clinical anticancer treatment.
3. Discussion
Curcumin is a potent anti-cancer compound, although its clinical application is limited owing to its hydrophobicity, poor oral bioavailability, and unstable chemical structure. To overcome these challenges, we aimed to design an appropriate curcumin oral formulation for colon cancer treatment. PECs are one of the feasible approaches for achieving this formulation.
PEC is composed of two or more oppositely charged polymers in polar solvents. PEC could not only improve the water solubility of the encapsulated agents but also protect its payload from the environment. Besides, the electrical charge of PEC would change with the environments, thus potentiating oral-controlled or sustained-release drug delivery system using suitable constituents of PEC to enhance oral bioavailability of drugs. In this study, anionic pectin and cationic type B gelatin used to form PEC for curcumin formulation. Pectin-gelatin complexes have been developed [
34]; however, there is a distinction between them and our pectin-type B gelatin PECs. First, our pectin-type B gelatin PECs were nano-sized (264.0 ± 3.1 nm), whereas the PECs reported by other research was micro-sized; hence, they are also called complex coacervates. This means that the aggregation that occurred during their manufacture would be different and new, requiring further exploitation. Besides, previous studies mostly reported type A gelatin as the polycation that interacts with polyanionic pectin. The pI of type A gelatin was 9.0, indicating that at pH 7.0, it is positively charged and capable to self-assemble with pectin (pKa 3.0) to form PECs. As pH increased over 9.0, type A gelatin is electrically neutralized. However, the highest pH in the small intestine is approximately 8.0, far lower than the pI of type A gelatin; thus, the drug-releasing site would be mostly only the upper GI tract, such as the stomach. However, D. Madhavi et al. have suggested sustained-release of curcumin via oral administration, which could increase 10-fold its bioavailability compared to that of unformulated curcumin [
35]. The drug-releasing efficacy and relative bioavailability of pectin-type A gelatin PEC system would be considered. In this study, type B gelatin was first employed in the PEC system. The pI of type B gelatin was 4.8–5.5 until the pH decreased to 4.0, whereas pectin, whose pKa is 3.0, would be self-aggregated with type B gelatin. At pH 2.0, such as in the stomach, the pectin-type B gelatin complex would swell, as shown in
Figure 2, because of the electrically neutralized pectin. The positively charged zeta potential is shown in
Figure 3. As the pH increased to 8.0, type B gelatin was electrically neutralized and the zeta potential of the PECs was negatively charged, breaking the electrical balance of the PEC (
Figure 3). Increased particle sizes and PDI were therefore observed (
Figure 2). The accumulative drug-releasing profile of curcumin-loaded pectin-type B gelatin PECs also revealed their continuous drug-releasing behavior, as shown in
Figure 5. Curcumin was not only abruptly released at pH 2.0 but also released sustainably at pH 8.0, which has not been commonly discussed in other studies. The releasing behavior of our PECs differed from that of pectin-type A gelatin PECs. I. Joseph et al. revealed that micro-sized pectin-type A gelatin PECs release their payloads following sustained release at pH 2.0 [
36], whereas our PECs rapidly released curcumin at pH 2.0 within 1 h. This finding also evidenced the differences in drug-releasing profiles with different components of the PECs.
Micro-sized PECs (complex coacervates) could bear higher drug contents and loading efficacy after optimization, compared to our PECs. M. Saravanan et al. have prepared pectin-gelatin complex coacervation with particle size of 94 μm and high drug content and loading efficiency, which are 57% and 76%, respectively [
26]. Pectin-gelatin complex coacervates were also fabricated by D. Silva el at., with drug content and loading efficiency of 56.7% and 89.5%, respectively [
28]. In this study, the drug content and loading efficiency of pectin-type B gelatin PEC were also discussed. The optimum drug content and loading efficiency were 40% and 53%, respectively, whereas the particle size after encapsulation was 214.67 ± 3.43 nm with narrow particular distribution (PDI was 0.17), as presented in
Figure 4. The low drug contents and loading efficiency were due to the small cavity of nano-sized PECs. The curcumin-loaded nano-sized PECs prepared by P. Sarika et al. also had low drug content and loading efficiency, compared to those of complex coacervates [
24]. The particle size of our curcumin-loaded PECs was slightly smaller than that of bare PEC. The reasonable explanation would be the additional ethanol in the manufacture of these loaded PECs. The ethanol dissolved the curcumin, instead of dispersing it before loading [
37]. Thus, the curcumin would not rapidly aggregate inside the core of PECs during manufacture, resulting in similar particle sizes of PEC after curcumin encapsulation.
The drug-releasing profiles of PECs in mimetic GI tract conditions were also investigated in our study, as shown in
Figure 5. Curcumin-loaded PECs exhibited abrupt release of curcumin at pH 2.0 and the releasing curve was close to the first-order releasing profiles. At pH 8.0, the curcumin-loaded PECs exhibited similar curcumin releasing profiles with zero-order releasing mechanism. I. Joseph et al. have suggested that the constituents of PECs considerably influence their drug-releasing profile [
36]. The drug-releasing profile of our PECs corresponded to the increase in particle size of the bare PECs containing the same ratios of components, as presented in
Figure 2a. Particle sizes were observed to abruptly increase as the pH was adjusted to 2.0, which could be attributed to rapid drug-releasing profile at pH 2.0; the particle size became gradually larger at pH 8.0, indicating the sustained-release behavior of the curcumin-loaded PECs. Moreover, the curcumin-loaded PECs preserved almost 40% of payloads within the PECs, which could directly treat colon cancer cells in the GI tract, and the released curcumin would enter the blood via the GI tract to achieve anti-cancer effect. The remaining curcumin-loaded PECs after passing through the mimetic GI tract conditions have been identified that they could be internalized into the colon cancerous cells and further perform the intracellular drug releasing, shown in
Figure 6.
The cytotoxicity of the curcumin-loaded PECs to HCT116 human cancer cells was accessed by MTT assay, as presented in
Figure 7. In this study, we utilized 1% of DMSO to prepare curcumin solution, which could eliminate the cytotoxicity of DMSO itself [
37]. The cell viabilities shown in
Figure 7 have indicated that curcumin-loaded PECs exhibited stronger toxicity to HCT116 human colon cancer cells than free curcumin after treatment with mimetic GI tract conditions. The cytotoxicity might be attributed to the anti-cancer activity of curcumin. Y. Wang et al. have shown that active curcumin is degraded in a pH-dependent manner; thus, the alkaline (pH 8.0) treatment might reduce the anti-cancer activity of curcumin [
20]. Curcumin-loaded PECs allowed sustained release of curcumin during digestion to maintain curcumin concentration in the blood. Besides, the encapsulated curcumin was protected from external conditions; therefore, the curcumin-loaded PECs could directly carry the bioactive curcumin to cancerous lesions in the colon and efficiently destroyed carcinomas, as evidenced by the results of the internalizing experiments (
Figure 6). The cell viability results (
Figure 7) indicated that the curcumin-loaded PECs exhibited more significant cytotoxicity to HCT116 cells than free curcumin. The results of cell cycle distribution test (
Figure 8) further explained the reason. The cell cycle test results showed that curcumin-treated with mimetic GI tract conditions no longer caused any cell cycle arrest, whereas curcumin-loaded PECs triggered the G2/M phase arrest in HCT116 cells, supporting the results of the studies conducted by A. Jaiswal et al. [
38] and J. Lu et al. [
39]. Our results have greatly supported that our PECs efficiently preserved the therapeutic effect of curcumin as a payload until it reached carcinomas in the colon.
Curcumin-loaded PECs possessed sustained-release properties and could transport active curcumin into tumorous lesions in the colon after treatment with mimetic GI tract conditions. The results of cell viability tests also confirmed the anti-cancer efficacy of the curcumin-loaded PECs. However, the mimetic GI tract conditions in our study did not involve enzymatic effects, and those conditions were also far from simulating both fasted state gastric conditions (FaSSGF) and fasted state conditions in the small intestine (FaSSIF), as recorded in the pharmacopeia [
40]. Future application of curcumin-loaded PECs as a new formulation of oral curcumin solution is still being investigated further but worth expecting.
4. Materials and Methods
4.1. Materials
Type B gelatin (molecular weight: 5 k; bloom number: 225), sodium phosphotungstate (PTA) for TEM negative staining and (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) powder for cell viability assay were all purchased from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). Pectin (methylation ratio: 3%–7%) was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Curcumin was acquired from Merck Life Science (Hohenbrunn, Germany). Dialysis bags (M.W.C.O.: 6–8 k) for drug-release assessment were also purchased from Merck Life Science. Ethanol was purchased from Taiwan Sugar Corp. (Tainan, Taiwan). Methanol for drug-loading test was obtained from Alps Chem Co., Ltd. (Hsinchu, Taiwan). Reagents for pH value adjustment, hydrochloric acid and sodium hydroxide, were purchased from J. T. Baker Inc. (Center valley, PA, USA) and Uniregion Bio-tech Inc. (New Taipei, Taiwan), respectively. Biological agents, including dimethyl sulfoxide for preparing curcumin solution in cell viability tests and a Muse Cell Cycle Kit was purchased from Merck Millipore (Burlington, MA, USA). Fluorescent reagents, including Cy 5.5-NHS ester, Lysotracker DND-Red, and DAPI-containing mounting medium, were acquired from Lumiprobe (Hunt Valley, MD, USA), Thermo Fisher Scientific (Waltham, MA, USA), and Vector Laboratory (Burlingame, CA, USA), respectively.
4.2. Preparation and Characterization of Pectin-Type B Gelatin PECs
Pectin (10 mg) was weighed and dissolved in 50 mL of deionized water. Type B gelatin (50 mg) was also weighed and dissolved in 50 mL of deionized water at 25 °C. After complete dissolution, the pH of the produced type B gelatin was adjusted to 4.0 using 0.1 N HCl. Ethanol (0.1 mL) was mixed with type B gelatin solution under stirring for 30 min. Next, the pectin solution was added into the mixture, blended, and the pH of the mixture was further adjusted to 4.0. Thirty minutes later, PECs were acquired and stored at 4 °C. To optimize the preparation and component of PECs, various volumes of type B gelatin and pectin were added to form the PECs. The type B gelatin/pectin PECs composed of various amounts of pectin and type B gelatin were collected and measured by DLS (Malvern ZS 90, Malvern Instruments, Malvern, UK) for particle size, distribution, and superficial zeta potential (ξ-potential). The hydrodynamic diameter was determined by the Stokes-Einstein equation after DLS measurement. Size distribution was determined through cumulant analysis of the measured intensity autocorrelation function assumed by Gaussian distribution. Zeta potential was obtained through ELS and calculated with Henry’s equation.
4.3. Observation of the Morphology of PECs
The prepared PEC sample was dropped to carbon-coated copper mesh and excess sample was removed after the PEC sample was attached to the mesh. Sodium phosphotungstate aqueous (PTA) solution 1% (w/v) was prepared and dropped to the copper grids for negative staining. Excess PTA solution was then eliminated. Next, the copper grids were placed in a vacuum oven. Twenty-four hours later, the dried copper grids were kept from light exposure at room temperature until observation. The morphology of the PECs was observed using TEM (HT7700, Hitachi, Japan) with an accelerated voltage of 120 V under high contrast mode (HC mode).
Besides, the morphology of PECs was also observed using a thermal field emission scanning electron microscope (Thermal FE-SEM) (JEOL JSM-7800F, JEOL Ltd., Tokyo, Japan). The PEC solutions were first prepared and freeze-dried using a vacuum freeze drier (Uniss MF-280, Uniss Company, New Taipei City, Taiwan). The freeze-dried powder was afterwards coated with platinum and observed by the thermal FE-SEM.
4.4. pH Responsiveness of PECs
PECs (5 mL) were placed into a sample vial and its pH was adjusted to 2.0 using HCl (0.1 N) to mimic the acidic condition in the stomach. Afterward, the PEC solution was incubated at 37 °C for 2 h in a shaker (M.BR-022UP, Taitec, Tokyo, Japan). Next, the pH of the PEC solution was adjusted to 8.0 using NaOH (0.5 N) to simulate the alkaline environment in the small intestine. The adjusted PEC solution was placed at 37 °C for 4 h in a shaker. Particle size and surface zeta potential were measured by DLS at every hour.
4.5. Preparation and Characterization of Curcumin-Loaded Pectin-Type B Gelatin PECs
Different amounts of curcumin (0.5, 1, 1.5, 2, or 2.5 mg) were precisely weighed and placed independently in a vial. Curcumin was first dissolved in ethanol (0.1 mL). Simultaneously, pectin (10 mg) and type B gelatin (50 mg) were respectively weighed and dissolved in 50 mL deionized water as stock solutions. Type B gelatin solution was treated with 0.1 N of HCl until the pH was 4.0. Curcumin alcoholic solution (0.1 mL) and type B gelatin solution (0.2 mL) were first mixed in a sample vial with stirring for 30 min. Thereafter, pectin solution (0.7 mL) was added to the mixture, which was then blended for 30 min to fabricate curcumin-loaded PECs. Particle size and distribution were determined using DLS.
4.6. Drug Contents and Loading Efficiency Determination
Curcumin-loaded PECs were prepared as abovementioned. The prepared curcumin-loaded PECs were freeze-dried using a vacuum freeze drier (Uniss MF-280). The dried sample (0.5 mg) was then weighed and dissolved in 1 mL of methanol for curcumin extraction. The extracted curcumin was quantified by an ELISA reader (BioTek Synergy HT, BioTek Instruments, Inc., Winooski, VT, USA) at 423 nm as the detected wavelength.
The drug content of the curcumin-loaded PCs was calculated according to the formula (1):
Besides, drug loading efficiency was also determined and calculated according to the formula (2):
After calculation, the optimization was analyzed with analysis of variance (ANOVA).
4.7. Determination of Drug-Releasing Profiles
Curcumin-loaded PECs (1 mL) were placed into dialysis bags, which were then immersed into 2.5 mL of methanol/deionized water solution (1:1, v/v) in a sample vial. The pH of the methanol/deionized water solutions in a sample vial was first adjusted to 2.0, and then the sample vial was incubated at 37 °C for 2 h. Afterwards, the pH value of the methanol/deionized water solution in the sample vial was adjusted to 8.0, and the sample vial was further incubated at 37 °C for 4 h. At each hour, 0.1 mL of methanol/deionized water solution was collected and absorbance wavelength at 423 nm was detected to quantify the release of curcumin.
4.8. CLSM Observation
HCT116 cells (1 × 105 cells/mL) were seeded on a slide and incubated at 37 °C with 5% CO2 supply for 12 h. After the cells were attached, they were co-cultured with free curcumin- or Cy5.5-labeled curcumin-loaded PECs, which were pretreated with mimetic GI tract conditions for 12 h at 37 °C with 5% CO2 supply. Excess curcumin or curcumin-loaded PECs were thereafter removed from the cells, which were then washed twice with PBS. The cells were further stained with 1 µM of Lysotracker DND-Red at 37 °C with 5% CO2 for 1 h. Next, the lysotracker DND-Red was removed, and the cells were again washed twice with PBS and fixed with 4% paraformaldehyde for 20 min. After fixation, the cells were washed twice with PBS and the cells and their nuclei were stained and mounted with DAPI-containing mounting medium. Fluorescence was observed and detected using a confocal laser scanning microscope (Leica SP8, Leica Microsystem, Wetzlar, Germany). The fluorescence of Cy 5.5 was detected at excitation and emission wavelengths of 633 and 650 nm, respectively. The fluorescence of Lysotracker DND-Red for locating endosome/lysosomes was detected using excitation and emission wavelengths of 577 and 590 nm, respectively. The fluorescence of curcumin was observed at excitation and emission wavelengths of 488 and 520 nm, respectively. DAPI fluorescence was detected using the preset wavelength of the CLSM equipment.
4.9. Cell Viability Determination
Human colon cancer cells HCT116 (7 × 103 cells/mL) were seeded on a 96-well plate at 37 °C with 5% CO2 supply for 12 h to allow cell attachment. Simultaneously, curcumin-loaded PECs were adjusted with the cell culture medium to the predetermined concentrations at 45 to 1.41 µM. Curcumin solutions at the same concentration series were also prepared with 1% of DMSO and cell culture medium. Afterwards, the pH of both curcumin and curcumin-loaded PECs was adjusted to 2.0 for 2 h, then the pH of the PECs was adjusted to 8.0 for 4 h. The pH of the PECs was re-adjusted to 7.4, and then HCT116 cells were treated with the PECs. The HCT116 cells were incubated at 37 °C with 5% CO2. Twelve hours later, the PECs and cell culture medium were removed. MTT assay was utilized to evaluate cell viability.
In addition, the human normal fibroblast cells Detroit 551 (7 × 103 cells/mL) were also seeded on a 96-well plate and cultured at 37 °C with 5% CO2 for 12 h. Bare PECs at concentrations of 112.5 to 3.52 µM were prepared with serial dilution. The bare PECs were separated into two groups: one for direct treatment with Detroit 551 cells and the other for mimetic GI tract treatment, as described above. Next, the latter bare PECs were transferred to pH 7.4 condition, and the cells were cultured with the bare PECs for 12 h. MTT assay was then applied to determine cell viability.
4.10. Cell Cycle Analysis
HCT116 cells (3 × 106 cells/mL) were seeded on a 6-well plate and cultured for 12 h at 37 °C with 5% CO2 supply. After the cells were attached, they were exposed to curcumin or curcumin-loaded PECs pretreated in mimetic GI tract conditions (pH 2.0 for 2 h, pH 8.0 for 4 h, and back to pH 7.0), as described above, and then the cells were incubated at 37 °C with 5% CO2 supply for 12 h. Afterward, the cells were collected, washed with PBS, and fixed with 70% of ethanol at −20 °C for 6 h. Fixed cells were re-collected using centrifugation and suspended in PBS. The cells were further stained with a flow cell cycle kit in the dark for 30 min. Cell cycles were determined using flow cytometry (BD FACSCanto, BD Biosciences, Franklin Lakes, NJ, USA) and analyzed with the software ModFit LT 3.0 (BD Biosciences, Franklin Lakes, NJ, USA).
4.11. Statistical Analysis
All experiments were repeated at least thrice and the results were averaged and presented as mean ± standard deviation (S.D.). Most results have been statistically compared and analyzed using Student’s t-test (Microsoft Excel 2000). Differences were recognized as statistically significant when the p values were less than 0.05, and significant differences are shown in star marks (* p < 0.05; ** p < 0.01, and *** p < 0.001). Besides that, of all results, only the optimization of drug contents and loading efficiency were analyzed with analysis of variance (ANOVA) using OriginPro 8 (OriginLab, Northampton, MA, USA).