GhHB12, a HD-ZIP I Transcription Factor, Negatively Regulates the Cotton Resistance to Verticillium dahliae
Abstract
:1. Introduction
2. Results
2.1. GhHB12 is Induced by MeJA and V. dahliae Infection
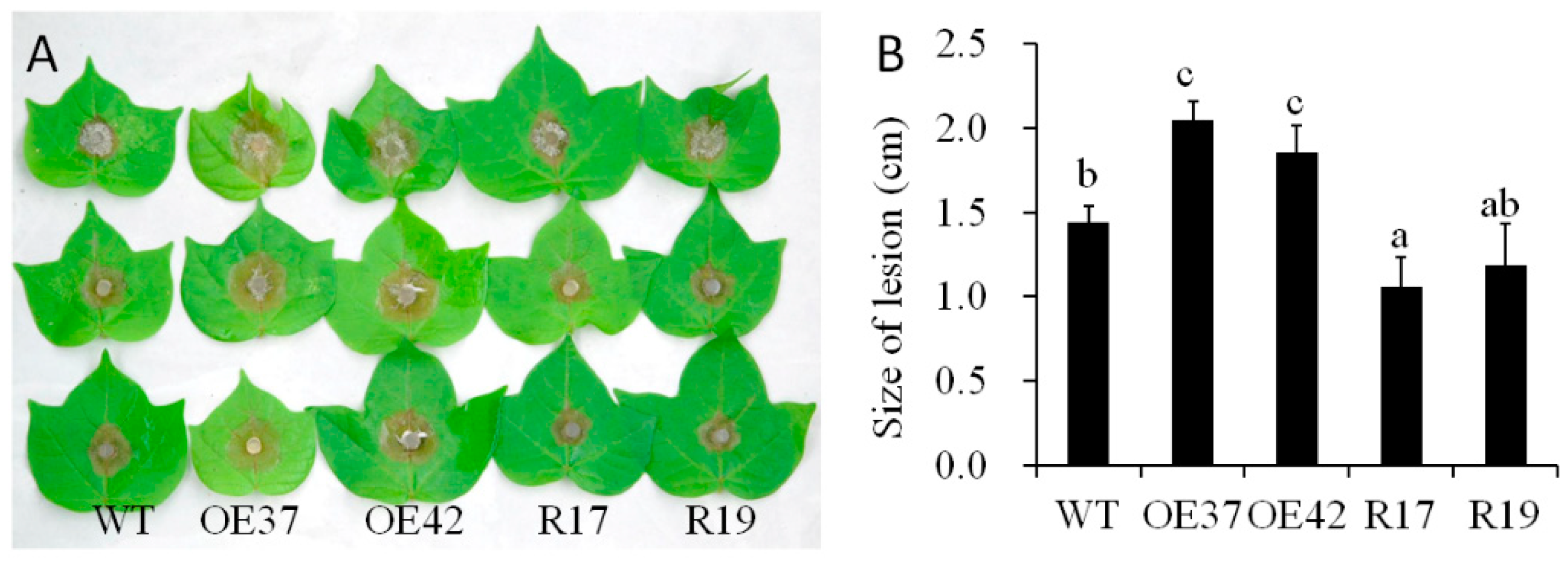
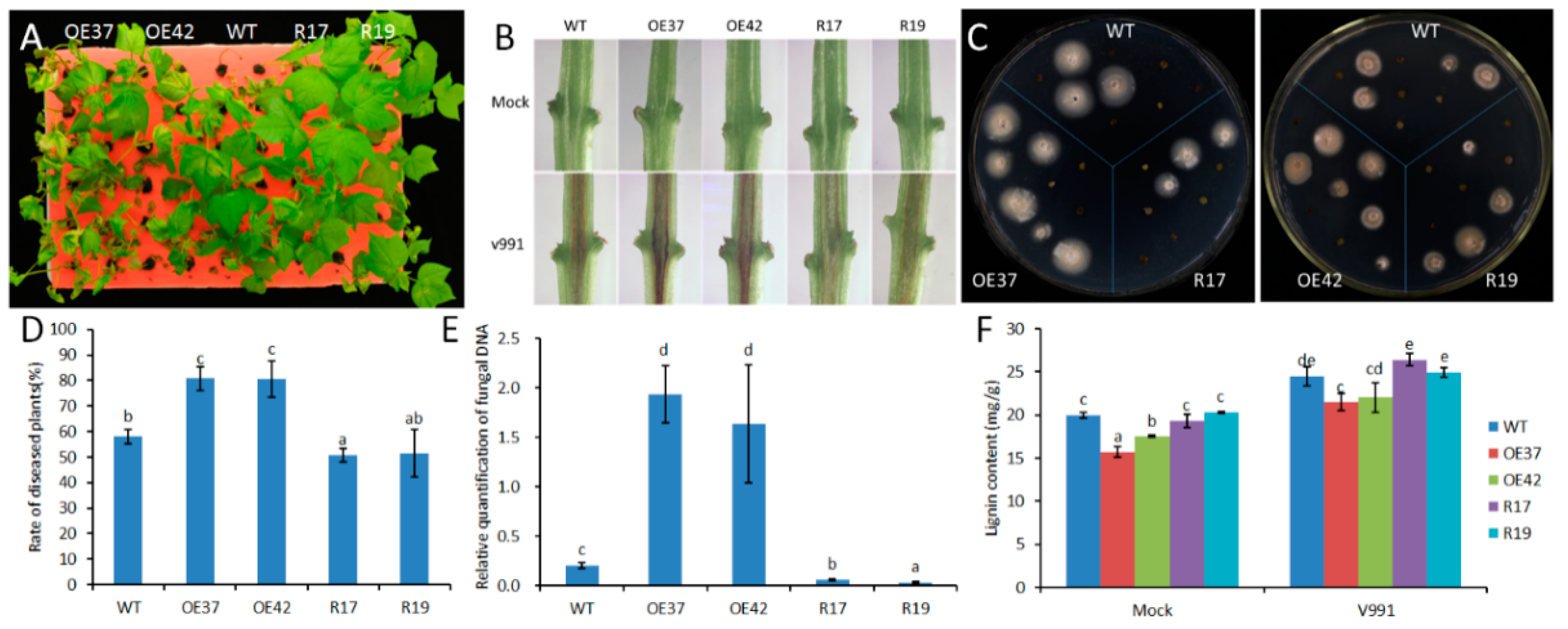
2.2. Reduced Fungal Pathogen Resistance of Transgenic Cotton Overexpressing GhHB12
2.3. Changes in Expression of JA-Responsive Genes in GhHB12 Transgenic Cotton Plants upon V. dahliae Infection
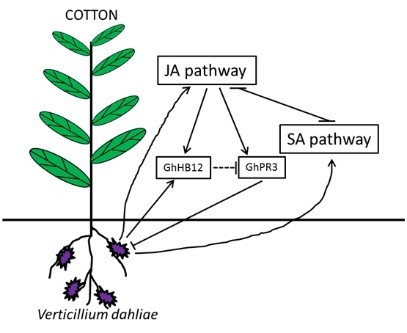
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Stress Treatments
4.2. Expression Analysis
4.3. Promoter Analysis and Histochemical Assay of GUS Activity
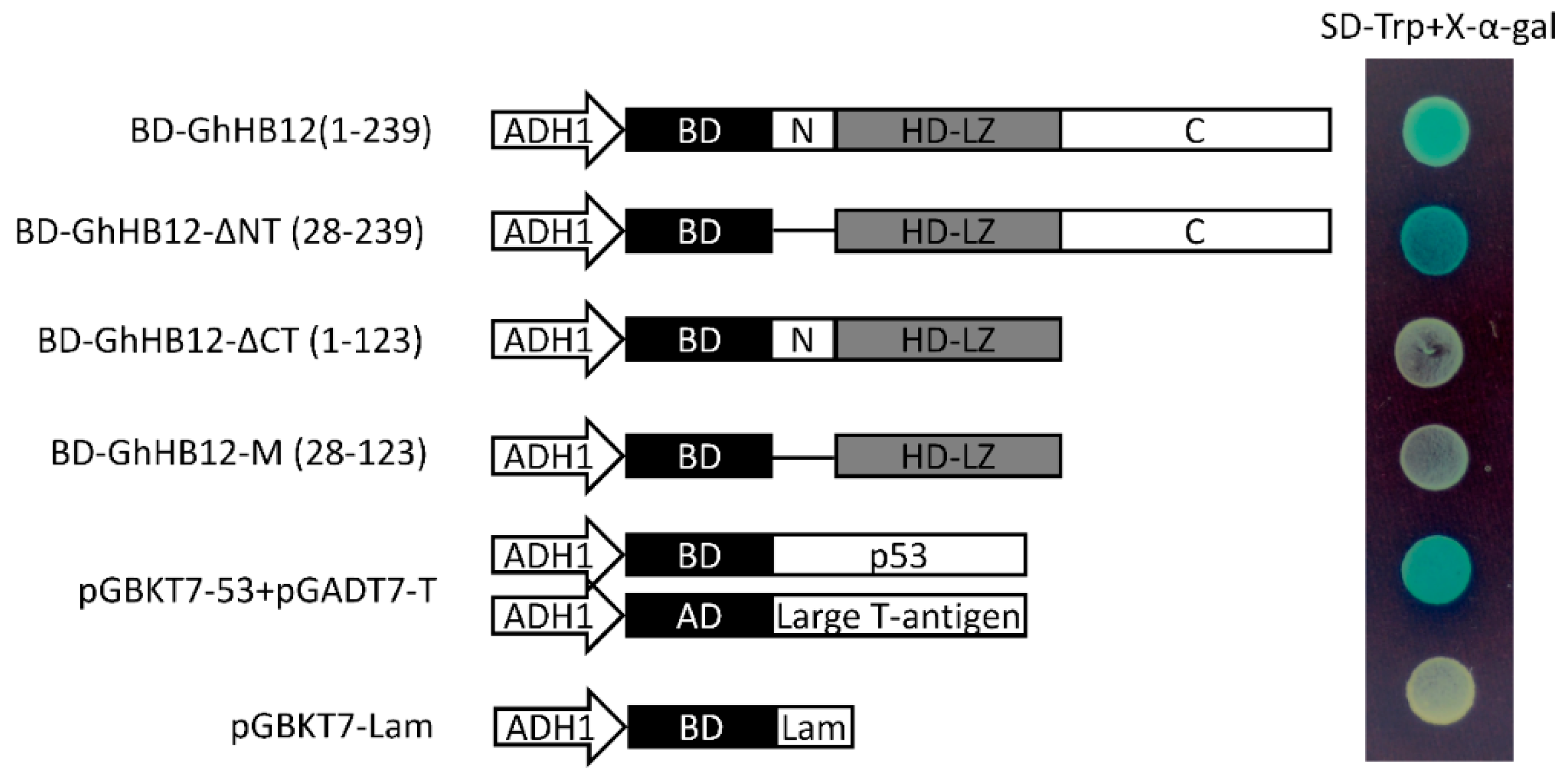
4.4. Transcriptional Activation Activity of the GhHB12 Protein
4.5. Fungal Pathogen Cultivation and Inoculation
4.6. Determination of Lignin Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [PubMed]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, A.; Pieterse, C.M. Cross talk in defense signaling. Plant Physiol. 2008, 146, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Dombrecht, B.; Xue, G.P.; Sprague, S.J.; Kirkegaard, J.A.; Ross, J.J.; Reid, J.B.; Fitt, G.P.; Sewelam, N.; Schenk, P.M.; Manners, J.M.; et al. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 2007, 19, 2225–2245. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef]
- Li, J.; Besseau, S.; Toronen, P.; Sipari, N.; Kollist, H.; Holm, L.; Palva, E.T. Defense-related transcription factors WRKY70 and WRKY54 modulate osmotic stress tolerance by regulating stomatal aperture in Arabidopsis. New Phytol. 2013, 200, 457–472. [Google Scholar] [CrossRef] [Green Version]
- Ariel, F.D.; Manavella, P.A.; Dezar, C.A.; Chan, R.L. The true story of the HD-Zip family. Trends Plant Sci. 2007, 12, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, E.; Olsson, A.S.B.; Johannesson, H.; Johansson, H.; Hanson, J.; Engstrom, P.; Soderman, E. Homeodomain leucine zipper class I genes in Arabidopsis. Expression patterns and phylogenetic relationships. Plant Physiol. 2005, 139, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.E.; Overnas, E.; Johansson, H.; Rada-Iglesias, A.; Engstrom, P. The homeodomain-leucine zipper (HD-Zip) class I transcription factors ATHB7 and ATHB12 modulate abscisic acid signalling by regulating protein phosphatase 2C and abscisic acid receptor gene activities. Plant Mol. Biol. 2012, 80, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, E.; Henriksson, K.N. Salt-stress signalling and the role of calcium in the regulation of the Arabidopsis ATHB7 gene. Plant Cell Environ. 2005, 28, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Olsson, A.S.; Engstrom, P.; Soderman, E. The homeobox genes ATHB12 and ATHB7 encode potential regulators of growth in response to water deficit in Arabidopsis. Plant Mol. Biol. 2004, 55, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Son, O.; Hur, Y.S.; Kim, Y.K.; Lee, H.J.; Kim, S.; Kim, M.R.; Nam, K.H.; Lee, M.S.; Kim, B.Y.; Park, J.; et al. ATHB12, an ABA-inducible homeodomain-leucine zipper (HD-Zip) protein of Arabidopsis, negatively regulates the growth of the inflorescence stem by decreasing the expression of a gibberellin 20-oxidase gene. Plant Cell Physiol. 2010, 51, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, H.J.; Cheon, C.I.; Kim, S.H.; Hur, Y.S.; Auh, C.K.; Im, K.H.; Yun, D.J.; Lee, S.; Davis, K.R. The Arabidopsis thaliana homeobox gene ATHB12 is involved in symptom development caused by geminivirus infection. PLoS ONE 2011, 6, e20054. [Google Scholar] [CrossRef]
- Johannesson, H.; Wang, Y.; Hanson, J.; Engstrom, P. The Arabidopsis thaliana homeobox gene ATHB5 is a potential regulator of abscisic acid responsiveness in developing seedlings. Plant Mol. Biol. 2003, 51, 719–729. [Google Scholar] [CrossRef]
- Re, D.A.; Dezar, C.A.; Chan, R.L.; Baldwin, I.T.; Bonaventure, G. Nicotiana attenuata NaHD20 plays a role in leaf ABA accumulation during water stress, benzylacetone emission from flowers, and the timing of bolting and flower transitions. J. Exp. Bot. 2011, 62, 155–166. [Google Scholar] [CrossRef]
- Cabello, J.V.; Arce, A.L.; Chan, R.L. The homologous HD-Zip I transcription factors HaHB1 and AtHB13 confer cold tolerance via the induction of pathogenesis-related and glucanase proteins. Plant J. 2012, 69, 141–153. [Google Scholar] [CrossRef]
- Yoon, J.; Chung, W.I.; Choi, D. NbHB1, Nicotiana benthamiana homeobox 1, is a jasmonic acid-dependent positive regulator of pathogen-induced plant cell death. New Phytol. 2009, 184, 71–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manavella, P.A.; Dezar, C.A.; Bonaventure, G.; Baldwin, I.T.; Chan, R.L. HAHB4, a sunflower HD-Zip protein, integrates signals from the jasmonic acid and ethylene pathways during wounding and biotic stress responses. Plant J. 2008, 56, 376–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaler, J.S.; Owen, B.; Higgins, V.J. The role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles. Plant Physiol. 2004, 135, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Klosterman, S.J.; Atallah, Z.K.; Vallad, G.E.; Subbarao, K.V. Diversity, pathogenicity, and management of verticillium species. Annu. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, X.; Luo, X.; Xu, L.; Liu, L.; Min, L.; Jin, L.; Zhu, L.; Zhang, X. Cotton WRKY1 mediates the plant defense-to-development transition during infection of cotton by Verticillium dahliae by activating JASMONATE ZIM-DOMAIN1 expression. Plant Physiol. 2014, 166, 2179–2194. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Long, L.; Zhu, L.F.; Xu, L.; Gao, W.H.; Sun, L.Q.; Liu, L.L.; Zhang, X.L. Proteomic and virus-induced gene silencing (VIGS) Analyses reveal that gossypol, brassinosteroids, and jasmonic acid contribute to the resistance of cotton to Verticillium dahliae. Mol. Cell. Proteom. 2013, 12, 3690–3703. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhu, L.F.; Tu, L.L.; Liu, L.L.; Yuan, D.J.; Jin, L.; Long, L.; Zhang, X.L. Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-Seq-dependent transcriptional analysis and histochemistry. J. Exp. Bot. 2011, 62, 5607–5621. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.L.; Song, T.Q.; Zhang, X.; Yuan, H.B.; Su, L.M.; Li, W.L.; Xu, J.; Liu, S.H.; Chen, L.L.; Chen, T.Z.; et al. Unconventionally secreted effectors of two filamentous pathogens target plant salicylate biosynthesis. Nat. Commun. 2014, 5, 4686. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Jin, L.; Miao, Y.; He, X.; Hu, Q.; Guo, K.; Zhu, L.; Zhang, X. An ethylene response-related factor, GbERF1-like, from Gossypium barbadense improves resistance to Verticillium dahliae via activating lignin synthesis. Plant Mol. Biol. 2016, 91, 305–318. [Google Scholar] [CrossRef]
- He, X.; Zhu, L.; Xu, L.; Guo, W.; Zhang, X. GhATAF1, a NAC transcription factor, confers abiotic and biotic stress responses by regulating phytohormonal signaling networks. Plant Cell Rep. 2016, 35, 2167–2179. [Google Scholar] [CrossRef]
- Cheng, H.Q.; Han, L.B.; Yang, C.L.; Wu, X.M.; Zhong, N.Q.; Wu, J.H.; Wang, F.X.; Wang, H.Y.; Xia, G.X. The cotton MYB108 forms a positive feedback regulation loop with CML11 and participates in the defense response against Verticillium dahliae infection. J. Exp. Bot. 2016, 67, 1935–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Zhu, L.; Wassan, G.M.; Wang, Y.; Miao, Y.; Shaban, M.; Hu, H.; Sun, H.; Zhang, X. GhJAZ2 attenuates cotton resistance to biotic stresses via the inhibition of the transcriptional activity of GhbHLH171. Mol. Plant Pathol. 2018, 19, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-F.; He, X.; Yuan, D.-J.; Xu, L.; Xu, L.; Tu, L.-L.; Shen, G.-X.; Zhang, H.; Zhang, X.-L. Genome-Wide Identification of Genes Responsive to ABA and Cold/Salt Stresses in Gossypium hirsutum by Data-Mining and Expression Pattern Analysis. Agric. Sci. China 2011, 10, 499–508. [Google Scholar] [CrossRef]
- He, X.; Wang, T.; Xu, Z.; Liu, N.; Wang, L.; Hu, Q.; Luo, X.; Zhang, X.; Zhu, L. The cotton HD-Zip family transcription factor GhHB12 regulates flowering time and plant architecture via the GhMir157-GhSPL pathway. Commun. Biol. 2018. accepted. [Google Scholar]
- Xu, L.; Zhang, W.; He, X.; Liu, M.; Zhang, K.; Shaban, M.; Sun, L.; Zhu, J.; Luo, Y.; Yuan, D.; et al. Functional characterization of cotton genes responsive to Verticillium dahliae through bioinformatics and reverse genetics strategies. J. Exp. Bot. 2014, 65, 6679–6692. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Xingfen, W.; Wei, R.; Jun, Y.; Zhiying, M. Island Cotton Enhanced Disease Susceptibility 1 Gene Encoding a Lipase-Like Protein Plays a Crucial Role in Response to Verticillium dahliae by Regulating the SA Level and H2O2 Accumulation. Front. Plant Sci. 2016, 7, 1830. [Google Scholar] [CrossRef]
- Boller, T.; Boller, T.; Boller, T.; Boller, T.; Boller, T. Induction of hydrolases as a defense reaction against pathogens. Ucla Symp. Mol. Cell. Biol. 1985, 22, 247–262. [Google Scholar]
- Punja, Z.K.; Zhang, Y.-Y. Plant Chitinases and Their Roles in Resistance to Fungal Diseases. J. Nematol. 1993, 25, 526–540. [Google Scholar]
- Cletus, J.; Balasubramanian, V.; Vashisht, D.; Sakthivel, N. Transgenic expression of plant chitinases to enhance disease resistance. Biotechnol. Lett. 2013, 35, 1719–1732. [Google Scholar] [CrossRef]
- Tohidfar, M.; Mohammadi, M.; Ghareyazie, B. Agrobacterium-mediated transformation of cotton (Gossypium hirsutum) using a heterologous bean chitinase gene. Plant Cell Tissue Organ Cult. 2005, 83, 83–96. [Google Scholar] [CrossRef]
- Tohidfar, M.; Hossaini, R.; Bashir, N.S.; Meisam, T. Enhanced Resistance to Verticillium dahliae in Transgenic Cotton Expressing an Endochitinase Gene from Phaseolus vulgaris. Czech J. Genet. Plant 2012, 48, 33–41. [Google Scholar] [CrossRef]
- Tabaeizadeh, Z.; Agharbaoui, Z.; Harrak, H.; Poysa, V. Transgenic tomato plants expressing a Lycopersicon chilense chitinase gene demonstrate improved resistance to Verticillium dahliae race 2. Plant Cell Rep. 1999, 19, 197–202. [Google Scholar] [CrossRef]
- Chalavi, V.; Tabaeizadeh, Z.; Thibodeau, P. Enhanced resistance to Verticillium dahliae in transgenic strawberry plants expressing a Lycopersicon chilense chitinase gene. J. Am. Soc. Hortic. Sci. 2003, 128, 747–753. [Google Scholar]
- Xu, J.; Xu, X.Y.; Tian, L.L.; Wang, G.L.; Zhang, X.Y.; Wang, X.Y.; Guo, W.Z. Discovery and identification of candidate genes from the chitinase gene family for Verticillium dahliae resistance in cotton. Sci. Rep. 2016, 6, 29022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.F.; Li-Li, T.U.; Zeng, F.C.; Liu, D.Q.; Zhang, X.L. An Improved Simple Protocol for Isolation of High Quality RNA from Gossypium spp. Suitable for cDNA Library Construction. Acta Agron. Sin. 2005, 31, 1657–1659. [Google Scholar]
- Tu, L.; Zhang, X.; Liu, D.; Jin, S.; Cao, J.; Zhu, L.; Deng, F.; Tan, J.; Zhang, C. Suitable internal control genes for qRT-PCR normalization in cotton fiber development and somatic embryogenesis. Chin. Sci. Bull. 2007, 52, 3110–3117. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, X.; Liang, S.; Nie, Y.; Guo, X.; Huang, C. Factors affecting transformation efficiency of embryogenic callus of Upland cotton (Gossypium hirsutum) with Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult. 2005, 81, 229–237. [Google Scholar] [CrossRef]
- Deng, F.; Tu, L.; Tan, J.; Li, Y.; Nie, Y.; Zhang, X. GbPDF1 is involved in cotton fiber initiation via the core cis-element HDZIP2ATATHB2. Plant Physiol. 2012, 158, 890–904. [Google Scholar] [CrossRef]
- Ellendorff, U.; Fradin, E.F.; de Jonge, R.; Thomma, B.P.H.J. RNA silencing is required for Arabidopsis defence against Verticillium wilt disease. J. Exp. Bot. 2009, 60, 591–602. [Google Scholar] [CrossRef]
- Fradin, E.F.; Zhang, Z.; Juarez Ayala, J.C.; Castroverde, C.D.; Nazar, R.N.; Robb, J.; Liu, C.M.; Thomma, B.P. Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol. 2009, 150, 320–332. [Google Scholar] [CrossRef]
- Rodrigues, F.A.; Jurick, W.; Datnoff, L.E.; Jones, J.B.; Rollins, J.A. Silcon influences cytological and molecular events in compatible and incompatible rice-Magnaporthe grisea interactions. Physiol. Mol. Plant Pathol. 2005, 66, 144–159. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Wang, T.; Zhu, W.; Wang, Y.; Zhu, L. GhHB12, a HD-ZIP I Transcription Factor, Negatively Regulates the Cotton Resistance to Verticillium dahliae. Int. J. Mol. Sci. 2018, 19, 3997. https://doi.org/10.3390/ijms19123997
He X, Wang T, Zhu W, Wang Y, Zhu L. GhHB12, a HD-ZIP I Transcription Factor, Negatively Regulates the Cotton Resistance to Verticillium dahliae. International Journal of Molecular Sciences. 2018; 19(12):3997. https://doi.org/10.3390/ijms19123997
Chicago/Turabian StyleHe, Xin, Tianyi Wang, Wan Zhu, Yujing Wang, and Longfu Zhu. 2018. "GhHB12, a HD-ZIP I Transcription Factor, Negatively Regulates the Cotton Resistance to Verticillium dahliae" International Journal of Molecular Sciences 19, no. 12: 3997. https://doi.org/10.3390/ijms19123997