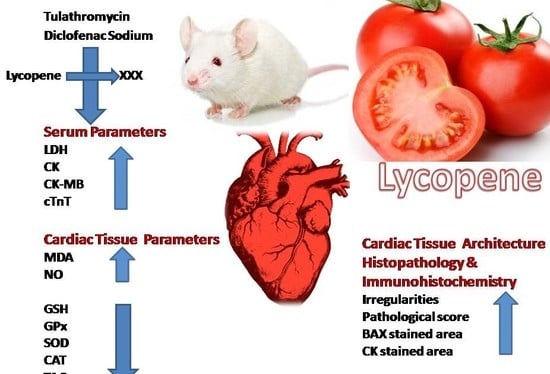
Lycopene Attenuates Tulathromycin and Diclofenac Sodium-Induced Cardiotoxicity in Mice
Abstract
:1. Introduction
2. Results
2.1. Biochemical Analysis
2.2. Histopathology
2.3. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals and Experimental Procedures
4.3. Biochemical Analysis
4.4. Histopathological Examination
4.5. Immunohistochemical Analysis
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CAT | Catalase |
| CK-MB | Creatine kinase-myocardial B fraction |
| CTnT | Cardiac-specific troponin-T |
| DFS | Diclofenac sodium |
| GSH | Reduced glutathione |
| LDH | Lactate dehydrogenase |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| TAC | Total antioxidant capacity |
References
- Abu-Gharbieh, E.; Vasina, V.; Poluzzi, E.; de Ponti, F. Antibacterial macrolides: A drug class with a complex pharmacological profile. Pharmacol. Res. 2004, 50, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Anadon, A.; Reeve-Johnson, L. Macrolide antibiotics, drug interactions and microsomal enzymes: Implications for veterinary medicine. Res. Vet. Sci. 1999, 66, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Burke, J.; Beattie, D. The sensitivity of rat liver and yeast mitochondrial ribosomes to inhibitors of protein synthesis. J. Biol. Chem. 1974, 249, 6806–6811. [Google Scholar] [PubMed]
- Villa, P.; Sassella, D.; Corada, M.; Bartosek, I. Toxicity, uptake, and subcellular distribution in rat hepatocytes of roxithromycin, a new semisynthetic macrolide, and erythromycin base. Antimicrob. Agents Chemother. 1988, 32, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- De Vries, H.; Arendzen, A.; Kroon, A. The interference of the macrolide antibiotics with mitochondrial protein synthesis. Biochim. Biophys. Acta 1973, 331, 264–275. [Google Scholar] [CrossRef]
- Evans, N.A. Tulathromycin: An overview of a new triamilide antibiotic for livestock respiratory disease. Vet. Ther. Res. Appl. Vet. Med. 2005, 6, 83–95. [Google Scholar]
- Hart, F.J.; Kilgore, R.W.; Meinert, T.R.; Nutsch, R.G.; Sunderland, S.J.; Lechtenberg, K.F. Efficacy of tulathromycin in the treatment of respiratory disease in pigs caused by Actinobacillus pleuropneumoniae. Vet. Rec. 2006, 158, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Er, A.; Altan, F.; Cetin, G.; Dik, B.; Elmas, M.; Yazar, E. Assessment of the cardiotoxicity of tulathromycin in rabbits. Acta Vet. Hung. 2011, 59, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Er, A.; Ulutas, E.; Altan, F.; Cetin, G.; Bulbul, A.; Elmas, M.; Yazar, E. Tulathromycin disturbs blood oxidative and coagulation status. Afr. J. Biotechnol. 2011, 10, 3243–3247. [Google Scholar]
- Gan, T.J. Diclofenac: An update on its mechanism of action and safety profile. Curr. Med. Res. Opin. 2010, 26, 1715–1731. [Google Scholar] [CrossRef] [PubMed]
- Lekeux, P. A therapeutic strategy for treatment of the bovine respiratory disease complex: The rationale for the combination of a nonsteroidal anti-inflammatory drug with an antibiotic. Cattle Pract. 2007, 15, 115–119. [Google Scholar]
- Brentnall, C.; Cheng, Z.; McKellar, Q.A.; Lees, P. Pharmacokinetic-pharmacodynamic integration and modelling of oxytetracycline administered alone and in combination with carprofen in calves. Res. Vet. Sci. 2013, 94, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.; Rahme, E.; Richard, H.; Pilote, L. Risk of congestive heart failure with nonsteroidal antiinflammatory drugs and selective cyclooxygenase 2 inhibitors: A class effect? Arthritis Care Res. 2007, 57, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Waksman, J.C.; Brody, A.; Phillips, S.D. Nonselective nonsteroidal antiinflammatory drugs and cardiovascular risk: Are they safe? Ann. Pharmacother. 2007, 41, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.S.; Derbalah, A.E. Impact of diclofenac sodium on tilmicosin-induced acute cardiotoxicity in rats (tilmicosin and diclofenac cardiotoxicity). Cardiovasc. Toxicol. 2018, 18, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Abushouk, A.I.; Ismail, A.; Salem, A.M.A.; Afifi, A.M.; Abdel-Daim, M.M. Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed. Pharmacother. 2017, 90, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Ghazy, E.W.; Fayez, M. Synergistic protective role of mirazid (Commiphora molmol) and ascorbic acid against tilmicosin-induced cardiotoxicity in mice. Can. J. Physiol. Pharmacol. 2014, 93, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Kilany, O.E.; Khalifa, H.A.; Ahmed, A.A.M. Allicin ameliorates doxorubicin-induced cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Cancer Chemother. Pharmacol. 2017, 80, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.E.; Abdel-Daim, M.M. Modulating effects of Spirulina platensis against tilmicosin-induced cardiotoxicity in mice. Cell J. 2015, 17, 137–144. [Google Scholar] [PubMed]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Friedman, M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J. Agric. Food Chem. 2013, 61, 9534–9550. [Google Scholar] [CrossRef] [PubMed]
- Karppi, J.; Nurmi, T.; Kurl, S.; Rissanen, T.H.; Nyyssönen, K. Lycopene, lutein and β-carotene as determinants of LDL conjugated dienes in serum. Atherosclerosis 2010, 209, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.Y.; Yoe, H.Y.; Kim, H.J.; Park, J.Y.; Kim, J.Y.; Lee, S.H.; Lee, J.H.; Lee, K.P.; Jang, Y.; Lee, J.H. Independent inverse relationship between serum lycopene concentration and arterial stiffness. Atherosclerosis 2010, 208, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kurata, M.; Suzuki, K.; Hamajima, N.; Hishida, H.; Aoki, K. Cardiovascular disease mortality and serum carotenoid levels: A Japanese population-based follow-up study. J. Epidemiol. 2006, 16, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Karimi, G.; Ramezani, M.; Abdi, A. Protective effects of lycopene and tomato extract against doxorubicin-induced cardiotoxicity. Phytother. Res. 2005, 19, 912–914. [Google Scholar] [CrossRef] [PubMed]
- Anjos Ferreira, A.L.; Russell, R.M.; Rocha, N.; Placido Ladeira, M.S.; Favero Salvadori, D.M.; Oliveira Nascimento, M.C.; Matsui, M.; Carvalho, F.A.; Tang, G.; Matsubara, L.S.; et al. Effect of lycopene on doxorubicin-induced cardiotoxicity: An echocardiographic, histological and morphometrical assessment. Basic Clin. Pharmacol. Toxicol. 2007, 101, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Mohamadin, A.M.; Elberry, A.A.; Mariee, A.D.; Morsy, G.M.; Al-Abbasi, F.A. Lycopene attenuates oxidative stress and heart lysosomal damage in isoproterenol induced cardiotoxicity in rats: A biochemical study. Pathophysiology 2012, 19, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-N.; Lin, J.; Qin, L.; Xia, J.; Zhu, S.-Y.; Li, J.-L. Efficacy of lycopene to prevent against atrazine-induced cardiotoxicity via modulation of nitric oxide and inflammatory NF-κB pathways. FASEB J. 2017, 31, 635.8. [Google Scholar]
- Kearney, P.M.; Baigent, C.; Godwin, J.; Halls, H.; Emberson, J.R.; Patrono, C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 2006, 332, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Aktan, B.; Taysi, S.; Gumustekin, K.; Uçüncü, H.; Memişoğullari, R.; Save, K.; Bakan, N. Effect of macrolide antibiotics on nitric oxide synthase and xanthine oxidase activities, and malondialdehyde level in erythrocyte of the guinea pigs with experimental otitis media with effusion. Pol. J. Pharmacol. 2003, 55, 1105–1110. [Google Scholar] [PubMed]
- Dogan, M. Cardioprotective effect of clarithromycin on doxorubicin-induced cardiac toxicity in rats. Int. J. Hematol. Oncol. 2014, 24, 30–35. [Google Scholar] [CrossRef]
- Lee, S.B.; Bae, I.H.; Bae, Y.S.; Um, H.-D. Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J. Biol. Chem. 2006, 281, 36228–36235. [Google Scholar] [CrossRef] [PubMed]
- Cave, A.C.; Brewer, A.C.; Narayanapanicker, A.; Ray, R.; Grieve, D.J.; Walker, S.; Shah, A.M. NADPH oxidases in cardiovascular health and disease. Antioxid. Redox Signal. 2006, 8, 691–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Hortmann, M.; Daiber, A.; Oelze, M.; Ostad, M.A.; Schwarz, P.M.; Xu, H.; Xia, N.; Kleschyov, A.L.; Mang, C.; et al. Cyclooxygenase 2-selective and nonselective nonsteroideal anti-inflammatory drugs induce oxidative stress by up-regulating vascular NADPH oxidases. J. Pharmacol. Exp. Ther. 2008, 326, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, S.W. Mitochondrial dysfunction in cardiovascular disease. Free Radic. Biol. Med. 2005, 38, 1278–1295. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Pathan, R.A.; Pillai, K.K.; Haque, S.E.; Dubey, K. Diclofenac sodium, a nonselective nonsteroidal anti-inflammatory drug aggravates doxorubicin-induced cardiomyopathy in rats. J. Cardiovasc. Pharmacol. 2010, 55, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Er, A.; Dik, B.; Corum, O.; Cetin, G. Cardiac safety of diclofenac at a single dose in ram. Sci. World J. 2013, 2013, 808731. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.-L.; Ching, T.-T.; Wang, D.-S.; Song, X.; Rangnekar, V.M.; Chen, C.S. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J. Biol. Chem. 2000, 275, 11397–11403. [Google Scholar] [CrossRef] [PubMed]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-induced oxidative stress and toxicity. J. Toxicol. 2012, 2012, 645460. [Google Scholar] [CrossRef] [PubMed]
- Hickey, E.J.; Raje, R.R.; Reid, V.E.; Gross, S.M.; Ray, S.D. Diclofenac induced in vivo nephrotoxicity may involve oxidative stress-mediated massive genomic DNA fragmentation and apoptotic cell death. Free Radic. Biol. Med. 2001, 31, 139–152. [Google Scholar] [CrossRef]
- Böhm, V. Lycopene and heart health. Mol. Nutr. Food Res. 2012, 56, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, J.; Xia, J.; Qin, L.; Zhu, S.-Y.; Li, J.L. Lycopene mitigates atrazine-induced cardiac inflammation via blocking the NF-κB pathway and NO production. J. Funct. Foods 2017, 29, 208–216. [Google Scholar] [CrossRef]
- He, Q.I.N.; Zhou, W.E.I.; Xiong, C.; Tan, G.; Chen, M. Lycopene attenuates inflammation and apoptosis in post-myocardial infarction remodeling by inhibiting the nuclear factor-κB signaling pathway. Mol. Med. Rep. 2015, 11, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-F.; Huang, T.-F.; Chen, B.-H.; Shieh, J.-M.; Wu, P.-H.; Wu, W.-B. Lycopene inhibits TNF-α-induced endothelial ICAM-1 expression and monocyte-endothelial adhesion. Eur. J. Pharmacol. 2008, 586, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Simone, R.; Catalano, A.; Parrone, N.; Monego, G.; Ranelletti, F.O. Lycopene regulation of cholesterol synthesis and efflux in human macrophages. J. Nutr. Biochem. 2011, 22, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Bose, K.S.C.; Agrawal, B.K. Effect of lycopene from cooked tomatoes on serum antioxidant enzymes, lipid peroxidation rate and lipid profile in coronary heart disease. Singap. Med. J. 2007, 48, 415–420. [Google Scholar]
- Kim, J.Y.; Paik, J.K.; Kim, O.Y.; Park, H.W.; Lee, J.H.; Jang, Y.; Lee, J.H. Effects of lycopene supplementation on oxidative stress and markers of endothelial function in healthy men. Atherosclerosis 2011, 215, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Babson, S.R.; Babson, A.L. An improved amylase assay using dyed amylopectin. Clin. Chim. Acta 1973, 44, 193–197. [Google Scholar] [CrossRef]
- Szasz, G.; Waldenström, J.; Gruber, W. Creatine kinase in serum: 6. Inhibition by endogenous polyvalent cations, and effect of chelators on the activity and stability of some assay components. Clin. Chem. 1979, 25, 446–452. [Google Scholar] [PubMed]
- Würzburg, U.; Hennrich, N.; Lang, H.; Prellwitz, W.; Neumeier, D.; Knedel, M. [Determination of creatine kinase-MB in serum using inhibiting antibodies (author’s transl)]. klinische Wochenschr. 1976, 54, 357–360. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Martín-Burriel, I.; Roome, N.O.; Dorchies, O.; Prenez, A. Histopathological and molecular changes during apoptosis produced by 7H-dibenzo [c,g]-carbazole in mouse liver. Toxicol. Pathol. 2004, 32, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Trovato, F.M.; Szychlinska, M.A.; Loreto, C.; Giunta, S.; Scuderi, S.; Passanisi, R.; Fidone, F.; Fagone, P.; Imbesi, R.; et al. Effects of Synthetic Anti-Inflammatory Sterol in CB3V-Induced Myocarditis: A Morphological Study on Heart Muscle Tissue. J. Funct. Morphol. Kinesiol. 2016, 1, 69–89. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Daim, M.M.; Eltaysh, R.; Hassan, A.; Mousa, S.A. Lycopene Attenuates Tulathromycin and Diclofenac Sodium-Induced Cardiotoxicity in Mice. Int. J. Mol. Sci. 2018, 19, 344. https://doi.org/10.3390/ijms19020344
Abdel-Daim MM, Eltaysh R, Hassan A, Mousa SA. Lycopene Attenuates Tulathromycin and Diclofenac Sodium-Induced Cardiotoxicity in Mice. International Journal of Molecular Sciences. 2018; 19(2):344. https://doi.org/10.3390/ijms19020344
Chicago/Turabian StyleAbdel-Daim, Mohamed M., Rasha Eltaysh, Azza Hassan, and Shaker A. Mousa. 2018. "Lycopene Attenuates Tulathromycin and Diclofenac Sodium-Induced Cardiotoxicity in Mice" International Journal of Molecular Sciences 19, no. 2: 344. https://doi.org/10.3390/ijms19020344
APA StyleAbdel-Daim, M. M., Eltaysh, R., Hassan, A., & Mousa, S. A. (2018). Lycopene Attenuates Tulathromycin and Diclofenac Sodium-Induced Cardiotoxicity in Mice. International Journal of Molecular Sciences, 19(2), 344. https://doi.org/10.3390/ijms19020344