On the Metal Cofactor in the Tyrosinase Family
Abstract
:1. Introduction
1.1. Cloning and Characterization of the Animal Tyrosinase Gene Family
1.2. On the Function of Trp2 and Trp1
1.3. Metal Proteins
1.4. Intracellular Availability of Copper and Zinc
1.5. Tyrosinase Models for Trps: Consequences of the Metal Cofactor
1.6. Some Other Considerations about Factors Affecting the Structure of the Tyrosinase Family
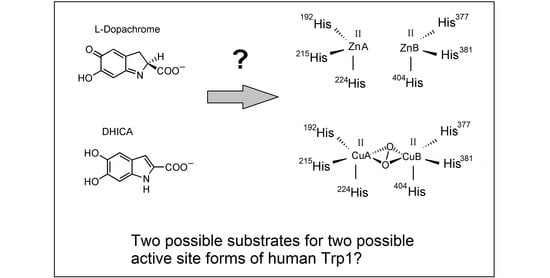
2. Final Remarks and Tentative Hypothesis
Acknowledgments
Conflicts of Interest
Abbreviations
| DHI | 5,6-dihydroxyindole |
| DHICA | 5,6-dihydroxyindole-2-carboxylic acid |
| EGF | Epidermal Growth Factor |
| IQCA | 5,6-dihydroxyindole-2-carboxylic acid |
| MeA | Metal ion binding site A |
| MeB | Metal ion binding site B |
| OCA | Oculocutaneous albinism |
| Trp | Tyrosinase-related protein |
References
- Prota, G. Recent Advances in the Chemistry of Melanogenesis in Mammals. J. Investig. Dermatol. 1980, 75, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J.; Jiménez, M. Analysis of Mammalian Pigmentation at the Molecular Level. Pigment Cell Melanoma Res. 1989, 2, 75–85. [Google Scholar] [CrossRef]
- Solano, F. Melanins: Skin pigments and much more—Types, structural models, biological functions, and formation routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Mayer, A. Polyphenol oxidases in plants-recent progress. Phytochemistry 1986, 26, 11–20. [Google Scholar] [CrossRef]
- Zekiri, F.; Molitor, C.; Mauracher, S.G.; Michael, C.; Mayer, R.L.; Gerner, G.; Rompel, A. Purification and characterization of tyrosinase from walnut leaves (Juglans regia). Phytochemistry 2014, 101, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Solem, E.; Tuczek, F.; Decker, H. Tyrosinase versus Catechol Oxidase: One Asparagine Makes the Difference. Angew. Chem. Int. Ed. 2016, 55, 2884–2888. [Google Scholar] [CrossRef] [PubMed]
- Decker, H.; Ryan, M.; Jaenicke, E.; Terwilliger, N. SDS-induced Phenoloxidase Activity of Hemocyanins from Limulus polyphemus, Eurypelma californicum, and Cancer magister. J. Biol. Chem. 2001, 276, 17796–17799. [Google Scholar] [CrossRef] [PubMed]
- Goldfeder, M.; Kanteev, M.; Isaschar-Ovdat, S.; Adir, N.; Fishman, A. Determination of tyrosinase substrate-binding modes reveals mechanistic differences between type-3 copper proteins. Nat. Commun. 2014, 5, 4505. [Google Scholar] [CrossRef] [PubMed]
- Decker, H.; Even, S.; Tuczek, F. Are glutamate and asparagine necessary for tyrosinase activity of type-3 copper proteins? Inorg. Chim. Acta 2017, in press. [Google Scholar] [CrossRef]
- Pretzler, M.; Rompel, A. What causes the different functionality in type-III-copper enzymes? A state of the art perspective. Inorg. Chim. Acta 2018, in press. [Google Scholar] [CrossRef]
- Logan, A.; Weatherhead, B. Pelage Color Cycles and Hair Follicle Tyrosinase Activity in the Siberian Hamster. J. Investig. Dermatol. 1978, 71, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.; Weatherhead, B. Post-tyrosinase Inhibition of Melanogenesis by Melatonin in Hair Follicles in Vitro. J. Investig. Dermatol. 1980, 74, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Körner, A.M.; Pawelek, J. Dopachrome Conversion: A Possible Control Point in Melanin Biosynthesis. J. Investig. Dermatol. 1980, 75, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Aroca, P.; García-Borrón, J.C.; Solano, F.; Lozano, J.A. Regulation of mammalian melanogenesis, I. Partial purification and characterization of a dopachrome converting factor: Dopachrome tautomerase. Biochim. Biophys. Acta 1990, 1035, 266–275. [Google Scholar] [CrossRef]
- Hearing, V.J.; Tsukamoto, K. Enzymatic control of pigmentation in mammals. FASEB J. 1991, 5, 2902–2909. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Toyoda, R.; Katsuyama, Y.; Saiga, H.; Numakunai, T.; Ikeo, K.; Gojobori, T.; Yajima, I.; Yamamoto, H. Structure and developmental expression of the ascidian TRP gene: Insights into the evolution of pigment cell–specific gene expression. Dev. Dyn. 1999, 215, 225–237. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Jackson, I.J.; Urabe, K.; Montague, P.M.; Hearing, V.J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 1992, 11, 519–526. [Google Scholar] [PubMed]
- García-Borrón, J.C.; Solano, F. Molecular anatomy of tyrosinase and its related proteins: Beyond the histidine-bound metal catalytic center. Pigment Cell Res. 2002, 15, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.; Tsukamoto, K.; Hearing, V.J. Tyrosinases from two different loci are expressed by normal and by transformed melanocytes. J. Biol. Chem. 1991, 266, 1147–1156. [Google Scholar] [PubMed]
- Shibahara, S.; Torruta, Y.; Sakakura, T.; Nager, C.; Bhabatosh, C.; Muller, R. Cloning and expression of cDNA encoding mouse tyrosinase. Nucleic Acids Res. 1986, 14, 2413–2427. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Takeuchi, S.; Kudo, T.; Makino, K.; Nakata, A.; Shinoda, T.; Takeuchi, T. Cloning and sequencing of mouse tyrosinase cDNA. Jpn. J. Genet. 1987, 62, 271–274. [Google Scholar] [CrossRef]
- Jackson, I.J. A cDNA encoding tyrosinase-related protein maps to the brown locus in mouse. Proc. Natl. Acad. Sci. USA 1988, 85, 4392–4396. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.S.; Haq, A.K.; Pomerantz, S.H.; Halaban, R. Isolation and sequence of a cDNA clone for human tyrosinase that maps at the mouse c-albino locus. Proc. Natl. Acad. Sci. USA 1987, 84, 7473–7477. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.; Muller, R.M.; Tomita, Y.; Shibahara, S. Nucleotide sequence of the cDNA encoding human tyrosinase-related protein. Nucleic Acids Res. 1990, 18, 2807–2808. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.J.; Chambers, D.M.; Budd, P.S.; Johnson, R. The tyrosinase-related protein-1 gene has a structure and promoter sequence very different from tyrosinase. Nucleic Acids Res. 1991, 19, 3799–3804. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.J.; Chambers, D.M.; Tsukamoto, K.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Hearing, V. A second tyrosinase-related protein, TRP-2, maps to and is mutated at the mouse slaty locus. EMBO J. 1992, 11, 527–535. [Google Scholar] [PubMed]
- Yokoyama, K.; Yasumoto, K.; Suzuki, H.; Shibahara, S. Cloning of the human DOPAchrome tautomerase/tyrosinase-related protein 2 gene and identification of two regulatory regions required for its pigment cell-specific expression. J. Biol. Chem. 1994, 269, 27080–27087. [Google Scholar] [PubMed]
- Kwon, B.S.; Chintamaneni, C.; Kozak, C.A.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.; Barton, D.; Francke, U.; Kobayashi, Y.; Kim, K.K. A melanocyte-specific gene, Pmel 17, maps near the silver coat color locus on mouse chromosome 10 and is in a syntenic region on human chromosome 12. Proc. Natl. Acad. Sci. USA 1991, 88, 9228–9232. [Google Scholar] [CrossRef] [PubMed]
- Aroca, P.; Solano, F.; García-Borrón, J.C.; Lozano, J.A. Specificity of dopachrome tautomerase and inhibition by carboxylated indoles. Considerations on the enzyme active site. Biochem. J. 1991, 277, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Urabe, K.; Aroca, P.; Tsukamoto, K.; Mascagna, D.; Palumbo, A.; Prota, G.; Hearing, V.J. The inherent cytotoxicity of melanin precursors: A revision. Biochim. Biophys. Acta 1994, 1221, 272–278. [Google Scholar] [CrossRef]
- Michard, Q.; Commo, S.; Rocchetti, J.; Houari, F.E.; Alleaume, A.; Wakamatsu, K.; Ito, S.; Bernard, B.A. TRP-2 expression protects HEK cells from dopamine and hydroquinone-induced toxicity. Free Rad. Biol. Med. 2008, 45, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Vijayasaradhi, S.; Doskoch, P.M.; Houghton, A.N. Biosynthesis and intracellular movement of the melanosomal membrane glycoprotein gp75, the human b (brown) locus product. Exp. Cell Res. 1991, 196, 233–240. [Google Scholar] [CrossRef]
- Halaban, R.; Moellmann, G. Murine and human b locus pigmentation genes encode a glycoprotein (gp75) with catalase activity. Proc. Natl. Acad. Sci. USA 1990, 87, 4809–4813. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Imokawa, G.; Bennett, D.C.; Hearing, V.J. Tyrosinase stabilization by Tyrp1 (the brown locus protein). J. Biol. Chem. 1998, 273, 31801–31805. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Cervantes, C.; Solano, F.; Kobayashi, T.; Urabe, K.; Hearing, V.J.; Lozano, J.A.; García-Borrón, J.C. A new enzymatic function in the melanogenic pathway: The DHICA oxidase activity of tyrosinase related protein-1 (TRP1). J. Biol. Chem. 1994, 269, 17993–18001. [Google Scholar] [PubMed]
- Kobayashi, T.; Urabe, K.; Winder, A.J.; Jiménez-Cervantes, C.; Imokawa, G.; Brewington, T.; Solano, F.; García-Borrón, J.C.; Hearing, V.J. Tyrosinase-related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 1994, 13, 5818–5825. [Google Scholar] [PubMed]
- Winder, A.J.; Wittbjer, A.; Rosengren, E.; Rorsman, H. The mouse brown (b) locus protein has dopachrome tautomerase activity and is located in lysosomes in transfected fibroblasts. J. Cell Sci. 1993, 106, 153–166. [Google Scholar] [PubMed]
- Winder, A.J.; Wittbjer, A.; Odh, G.; Rosengren, E.; Rorsman, H. The mouse brown (b) locus protein functions as a dopachrome tautomerase. Pigment Cell Res. 1994, 7, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Boissy, R.E.; Sakai, C.; Zhao, H.; Kobayashi, T.; Hearing, V.J. Human tyrosinase related protein-1 (TRP-1) does not function as a DHICA oxidase activity in contrast to murine TRP-1. Exp. Dermatol. 1998, 7, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Olivares, C.; Jiménez-Cervantes, C.; Lozano, J.A.; Solano, F.; García-Borrón, J.C. The 5,6-dihydroxyindole-2-carboxylic acid (DHICA) oxidase activity of human tyrosinase. Biochem. J. 2001, 354, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.B. Biological inorganic chemistry at the beginning of the 21st century. Proc. Natl. Acad. Sci. USA 2003, 100, 3563–3568. [Google Scholar] [CrossRef] [PubMed]
- Palm-Espling, M.E.; Niemiec, M.S. Wittung-Stafshede, P. Role of metal in folding and stability of copper proteins in vitro. Biochim. Biophys. Acta 2012, 1823, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Fitzpatrick, T.B.; Calkins, E.; Summerson, W.H. Mammalian tyrosinase; the relationship of copper to enzymatic activity. J. Biol. Chem. 1950, 187, 793–802. [Google Scholar] [PubMed]
- Matoba, Y.; Kumagai, T.; Yamamoto, A.; Yoshitsu, H.; Sugiyama, M. Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis. J. Biol. Chem. 2006, 281, 8981–8990. [Google Scholar] [CrossRef] [PubMed]
- Kaintz, C.; Mauracher, S.G.; Rompel, A. Type-3 copper proteins: Recent advances on polyphenol oxidases. Adv. Protein Chem. Struct. Biol. 2014, 97, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Huang, W.; Zhao, G.; Wan, X.; Hu, X.; Jin, Y.; Li, J.; Liu, J. Determination of the Bridging Ligand in the Active Site of Tyrosinase. Molecules 2017, 22, 1836. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.A.; Riley, P.A. Tyrosinase: The four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorg. Med. Chem. 2014, 22, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.K.; Orlow, S.J.; Pawelek, J.M. Evidence that dopachrome tautomerase is a ferrous iron-binding glycoprotein. FEBS Lett. 1992, 302, 126–128. [Google Scholar] [CrossRef]
- Solano, F.; Jiménez-Cervantes, C.; Martínez-Liarte, J.H.; García-Borrón, J.C.; Jara, J.R.; Lozano, J.A. Molecular mechanism for catalysis by a new-enzyme, dopachrome tautomerase. Biochem. J. 1996, 313, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Olivares, C.; Solano, F. New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment Cell Melanoma Res. 2009, 22, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Furumura, M.; Solano, F.; Matsunaga, N.; Sakai, C.; Spritz, R.A.; Hearing, V.J. Metal ligand-binding specificities of the tyrosinase-related proteins. Biochem. Biophys. Res. Commun. 1998, 242, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Olivares, C.; Solano, F.; Garcı́a-Borrón, J.C. Conformation-dependent Post-translational Glycosylation of Tyrosinase. Requirement of a specific interaction involving the CuB metal binding site. J. Biol. Chem. 2003, 278, 15735–15743. [Google Scholar] [CrossRef] [PubMed]
- Osredkar, J.; Sustar, N. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clin. Toxicol. 2011, S3. [Google Scholar] [CrossRef]
- Lippard, S.J. Free copper ions in the cells? Science 1999, 284, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Kozyreva, T.; Zovo, K.; Palumaa, P. Affinity gradients drive copper to cellular destinations. Nature 2010, 465, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, A.C.; O’Halloran, T.V. Structure and chemistry of the copper chaperone proteins. Curr. Opin. Chem. Biol. 2000, 4, 140–147. [Google Scholar] [CrossRef]
- Petris, M.J.; Stransak, D.; Mercer, J.F. The Menkes copper transporter is required for the activation of tyrosinase. Hum. Mol. Genet. 2000, 9, 2845–2851. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc Biochemistry: From a Single Zinc Enzyme to a Key Element of Life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Kuo, W.Y.; Jinn, T.L. Models for the mechanism for activating copper-zinc superoxide dismutase in the absence of the CCS Cu chaperone in Arabidopsis. Plant Signal Behav. 2012, 7, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Suzuki, N.; Takebayashi, S.; Commo, S.; Wakamatsu, K. Neutral pH and copper ions promote eumelanogenesis after the dopachrome stage. Pigment Cell Melanoma Res. 2013, 26, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Plonka, P.M.; Handjiski, B.; Michalczyk, D.; Popik, M.; Paus, R. Oral zinc sulphate causes murine hair hypopigmentation and is a potent inhibitor of eumelanogenesis in vivo. Br. J. Dermatol. 2006, 155, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, D.A.; Lewis, P.A.; MacKay, E.; Whitley, R.D. The influence of aging and low zinc nutrition on the choroid in the pig: II. The melanosome. Vet. Ophthalmol. 1999, 2, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Faller, P.; Hureau, C.; La Penna, G. Metal Ions and Intrinsically Disordered Proteins and Peptides: From Cu/Zn Amyloid-β to General Principles. Acc. Chem. Res. 2014, 47, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Klabunde, T.; Eicken, C.; Sacchettini, J.C.; Krebs, B. Crystal structure of a plant catechol oxidase containing a dicopper center. Nat. Struct. Biol. 1998, 5, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Fujieda, N.; Yabuta, S.; Ikeda, T.; Oyama, T.; Muraki, N.; Kurisu, G.; Itoh, S. Crystal structures of copper-depleted and copper-bound fungal pro-tyrosinase: Insights into endogenous cysteine-dependent copper incorporation. J. Biol. Chem. 2013, 288, 22128–22140. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Large-Scale Recombinant Expression and Purification of Human Tyrosinase Suitable for Structural Studies. PLoS ONE 2016, 11, e0161697. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure of Human Tyrosinase Related Protein 1 Reveals a Binuclear Zinc Active Site Important for Melanogenesis. Angew. Chem. Int. Ed. 2017, 56, 9812–9815. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure and Function of Human Tyrosinase and Tyrosinase-related Proteins. Chem. Eur. J. 2017, 24, 47–55. [Google Scholar] [CrossRef] [PubMed]
- ExPASy. Available online: www.expasy.org (accessed on 19 January 2018).
- Decker, H.; Tuczek, F. The Recent Crystal Structure of Human Tyrosinase Related Protein 1 (HsTYRP1) Solves an Old Problem and Poses a New One. Angew. Chem. Int. Ed. 2017, 56, 14352–14354. [Google Scholar] [CrossRef] [PubMed]
- Han, H.Y.; Zou, H.C.; Jeon, J.Y.; Wang, Y.J.; Xu, W.A.; Yang, J.M.; Park, Y.D. The inhibition kinetics and thermodynamic changes of tyrosinase via the zinc ion. Biochim. Biophys. Acta 2007, 1774, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Sendovski, M.; Kanteev, M.; Shuster Ben-Yosef, V.; Adir, N.; Fishman, A. First structures of an active bacterial tyrosinase reveal copper plasticity. J. Mol. Biol. 2011, 405, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, C.; Lerch, K. Cobalt tyrosinase: Replacement of the binuclear copper of Neurospora tyrosinase by cobalt. Biochemistry 1981, 20, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Ischia, M.; Misuraca, G.; De Martino, L.; Prota, G. A new dopachrome-rearranging enzyme from the ejected ink of the cuttlefish Sepia officinalis. Biochem. J. 1994, 299, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Barek, H.; Sugumaran, M.; Ito, S.; Wakamatsu, K. Insect cuticular melanins are distinctly different from those of mammalian epidermal melanins. Pigment Cell Melanoma Res. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, M.; Nellaiappan, K.; Amaratunga, C.; Cardinale, S.; Scott, T. Insect melanogenesis III. Metabolon formation in melanogenic pathway—Regulation of phenoloxidase activity by endogenous dopachrome isomerase (decarboxylating) from Manduca sexta. Arch. Biochem. Biophys. 2000, 378, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Negroiu, G.; Branza-Nichita, N.; Petrescu, A.J.; Dwek, R.A.; Petrescu, S.M. Protein specific N-glycosylation of tyrosinase and tyrosinase-related protein-1 in B16 mouse melanoma cells. Biochem. J. 1999, 344, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Negroiu, G.; Dwek, R.A.; Petrescu, S.M. Folding and maturation of tyrosinase-related protein-1 are regulated by the post-translational formation of disulfide bonds and by N-glycan processing. J. Biol. Chem. 2000, 275, 32200–32207. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.M.; Englander, S.W. The N-terminal to C-terminal truncations motif in protein folding and function. Proc. Natl. Acad. Sci. USA 2005, 102, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Fairhead, M.; Thony-Meyer, L. Bacterial tyrosinases: Old enzymes with new relevance to biotechnology. N. Biotechnol. 2012, 29, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Do, H.; Kang, E.; Yang, B.; Cha, H.J.; Choi, Y.S. A tyrosinase, mTyr-CNK, that is functionally available as a monophenol monooxygenase. Sci. Rep. 2017, 7, 17267. [Google Scholar] [CrossRef] [PubMed]
- Berson, J.F.; Frank, D.W.; Calvo, P.A.; Bieler, B.M.; Marks, M.S. A common temperature-sensitive allelic form of human tyrosinase is retained in the endoplasmic reticulum at the nonpermissive temperature. J. Biol. Chem. 2000, 275, 12281–12289. [Google Scholar] [CrossRef] [PubMed]
- Montoliu, L.; Grønskov, K.; Wei, A.H.; Martínez-García, M.; Fernández, A.; Arveiler, B.; Morice-Picard, F.; Riazuddin, S.; Suzuki, T.; Ahmed, Z.M.; et al. Increasing the complexity: New genes and new types of albinism. Pigment Cell Melanoma Res. 2014, 27, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Huizing, M.; Sarangarajan, R.; Strovel, E.; Zhao, Y.; Gahl, W.A.; Boissy, R.E. AP-3-dependent vesicles carry tyrosinase, but not TRP-1 in cultured human melanocytes. Mol. Biol. Cell 2001, 12, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Setaluri, V. Sorting and targeting of melanosomal membrane proteins: Signals, pathways and mechanisms. Pigment Cell Res. 2000, 13, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Simmen, T.; Schmidt, A.; Hunziker, W.; Beerman, F. The tyrosinase tail mediates sorting to the lysosomal compartment in MDCK cells via a di-leucine and a tyrosine-based signal. J. Cell Sci. 1999, 112, 45–53. [Google Scholar] [PubMed]
- Watabe, H.; Valencia, J.C.; Yasumoto, K.; Kushimoto, T.; Ando, H.; Muller, J.; Vieira, W.D.; Mizoguchi, M.; Appella, E.; Hearing, V.J. Regulation of Tyrosinase Processing and Trafficking by Organellar pH and by Proteasome Activity. J. Biol. Chem. 2004, 279, 7971–7981. [Google Scholar] [CrossRef] [PubMed]
- Beermann, F.; Orlow, S.J.; Boissy, R.E.; Schmidt, A.; Boissy, Y.L.; Lamoreaux, M.L. Misrouting of tyrosinase with a truncated cytoplasmic tail as a result of the murine platinum (cp) mutation. Exp. Eye Res. 1995, 61, 599–607. [Google Scholar] [CrossRef]
- Vijayasaradhi, S.; Xu, Y.; Bouchard, B.; Houghton, A.N. Intracellular sorting and targeting of melanosomal membrane proteins: Identification of signals for sorting of the human brown locus protein, gp75. J. Cell Biol. 1995, 130, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.F.; Kandala, G.; Setaluri, V. PDZ-domain protein Interacts with the cytoplasmic tail of melanosomal membrane protein gp75 (tyrosinase related protein-1). J. Biol. Chem. 2001, 276, 35768–35777. [Google Scholar] [CrossRef] [PubMed]
- Gomez, P.F.; Luo, D.; Hirosaki, K.; Shinoda, K.; Yamashita, T.; Suzuki, J.J.; Otsu, K.; Ishikawa, K.; Jimbow, K. Identification of rab7, as a Melanosome-associated protein involved in intracellular transport of tyrosinase-related protein 1. J. Investig. Dermatol. 2001, 117, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Setaluri, V.; Takechi, Y.; Houghton, A.N. Sorting and secretion of a melanosome membrane protein, gp75/TRP1. J. Investig. Dermatol. 1997, 109, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Kenny, E.E.; Timpson, N.J.; Sikora, M.; Yee, M.C.; Moreno-Estrada, A.; Eng, C.; Huntsman, S.; Burchard, E.G.; Stoneking, M.; Bustamante, C.D.; et al. Melanesian blond hair is caused by an amino acid change in TYRP1. Science 2012, 336, 554. [Google Scholar] [CrossRef] [PubMed]
- Branza-Nichita, N.; Petrescu, A.J.; Dwek, R.A.; Wormald, M.R.; Platt, F.M.; Petrescu, S.M. Tyrosinase folding and copper loading in vivo: A crucial role for calnexin and α-glucosidase II. Biochem. Biophys. Res. Commun. 1999, 261, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Branza-Nichita, N.; Negroiu, G.; Petrescu, A.J.; Garman, E.F.; Platt, F.M.; Wormald, M.R.; Dwek, R.A.; Petrescu, S.M. Mutations at critical N-glycosylation sites reduce tyrosinase activity by altering folding and quality control. J. Biol. Chem. 2000, 275, 8169–8175. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, S.M.; Branza-Nichita, N.; Negroiu, G.; Petrescu, A.J.; Dwek, R.A. Tyrosinase and glycoprotein folding: Roles of chaperones that recognize glycans. Biochemistry 2000, 39, 5229–5237. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bartido, S.; Setaluri, V.; Qin, J.; Yang, G.; Houghton, A.N. Diverse roles of conserved asparagine-linked glycan sites on tyrosinase family glycoproteins. Exp. Cell Res. 2001, 267, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Sinha, S.; Mitra, N.; Surolia, A. Probing into the role of conserved N-glycosylation sites in the Tyrosinase glycoprotein family. Glycoconj. J. 2009, 26, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Olivares, C.; García-Borrón, J.C.; Solano, F. Identification of active site residues involved in metal cofactor binding and stereospecific substrate recognition in mammalian tyrosinase. Implications to the catalytic cycle. Biochemistry 2002, 41, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, I.; Von Heijne, G. Glycosylation efficiency of Asn-Xaa-Thr sequons depends both on the distance from the C-terminus and on the presence of a downstream transmembrane segment. J. Biol. Chem. 2000, 275, 17338–17343. [Google Scholar] [CrossRef] [PubMed]
- Berryere, T.G.; Schmutz, S.M.; Schimpf, R.J.; Cowan, C.M.; Potter, J. TYRP1 is associated with dun coat colour in Dexter cattle or how now brown cow? Anim. Genet. 2003, 34, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Costin, G.E.; Valencia, J.C.; Wakamatsu, K.; Ito, S.; Solano, F.; Milac, A.L.; Vieira, W.D.; Yamaguchi, Y.; Rouzaud, F.; Petrescu, A.; et al. Mutations in dopachrome tautomerase (Dct) affect eumelanin/pheomelanin synthesis, but do not affect intracellular trafficking of the mutant protein. Biochem. J. 2005, 391, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Hirobe, T.; Wakamatsu, K.; Ito, S.; Kawa, Y.; Soma, Y.; Mizoguchi, M. The slaty mutation affects eumelanin and pheomelanin synthesis in mouse melanocytes. Eur. J. Cell Biol. 2006, 85, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Guyonneau, L.; Murisier, F.; Rossier, A.; Alexandre Moulin, A.; Beermann, F. Melanocytes and Pigmentation Are Affected in Dopachrome Tautomerase Knockout Mice. Mol. Cell. Biol. 2004, 24, 3396–3403. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solano, F. On the Metal Cofactor in the Tyrosinase Family. Int. J. Mol. Sci. 2018, 19, 633. https://doi.org/10.3390/ijms19020633
Solano F. On the Metal Cofactor in the Tyrosinase Family. International Journal of Molecular Sciences. 2018; 19(2):633. https://doi.org/10.3390/ijms19020633
Chicago/Turabian StyleSolano, Francisco. 2018. "On the Metal Cofactor in the Tyrosinase Family" International Journal of Molecular Sciences 19, no. 2: 633. https://doi.org/10.3390/ijms19020633
APA StyleSolano, F. (2018). On the Metal Cofactor in the Tyrosinase Family. International Journal of Molecular Sciences, 19(2), 633. https://doi.org/10.3390/ijms19020633