Structure-Activity Relationships of Thiazolyl Resorcinols, Potent and Selective Inhibitors of Human Tyrosinase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Motifs Essential for Inhibition
2.2. Steric Factors
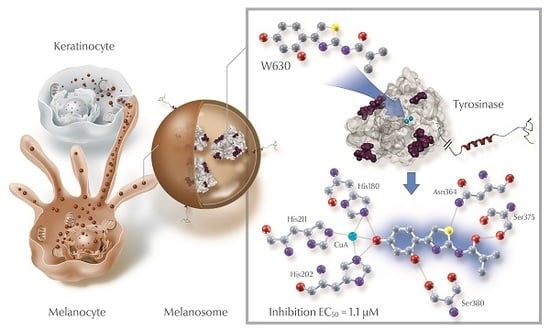
2.3. Homology Modeling
2.4. Molecular Docking
2.5. Role of the Thiazole Ring
3. Materials and Methods
3.1. Human Tyrosinase
3.2. Sources of Inhibitors
3.3. Tyrosinase Assay and Dose-Response Profiles
3.4. Molecular Modeling and Simulation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| bTyr | bacterial tyrosinase from B. megaterium |
| hTyr | human tyrosinase |
| mTyr | mushroom tyrosinase from A. bisporus |
| sTyr | bacterial tyrosinase from S. castaneoglobisporus |
| hTrp-1 | human tyrosinase-related protein 1 |
| TM-score | template modelling score |
| MVD | Molegro Virtual Docker |
| nrSc | negative rerank score as calculated by MVD |
| RSQ | residue-specific quality of an I-Tasser model |
| RMSD | root mean square deviation |
References
- Fenoll, L.G.; Rodriguez-Lopez, J.N.; Varon, R.; Garcia-Ruiz, P.A.; Garcia-Canovas, F.; Tudela, J. Kinetic characterisation of the reaction mechanism of mushroom tyrosinase on tyramine/dopamine and l-tyrosine methyl esther/l-dopa methyl esther. Int. J. Biochem. Cell Biol. 2002, 34, 1594–1607. [Google Scholar] [CrossRef]
- Kondo, T.; Hearing, V.J. Update on the regulation of mammalian melanocyte function and skin pigmentation. Expert Rev. Dermatol. 2011, 6, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, H.R.; Kim, D.S.; Park, K.C. Topical hypopigmenting agents for pigmentary disorders and their mechanisms of action. Ann. Dermatol. 2012, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Espin, J.C.; Varon, R.; Fenoll, L.G.; Gilabert, M.A.; Garcia-Ruiz, P.A.; Tudela, J.; Garcia-Canovas, F. Kinetic characterization of the substrate specificity and mechanism of mushroom tyrosinase. Eur. J. Biochem. 2000, 267, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J., Jr.; Ekel, T.M.; Montague, P.M.; Nicholson, J.M. Mammalin tyrosinase. Stoichiometry and measurement of reaction products. Biochim. Biophys. Acta 1980, 611, 251–268. [Google Scholar] [CrossRef]
- Nishioka, K. Particulate tyrosinase of human malignant melanoma. Solubilization, purification following trypsin treatment, and characterization. Eur. J. Biochem. 1978, 85, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; Schallreuter, K.U. Studies on the reactions between human tyrosinase, superoxide anion, hydrogen peroxide and thiols. Biochim. Biophys. Acta 1991, 1074, 378–385. [Google Scholar] [CrossRef]
- Yurkow, E.J.; Laskin, J.D. Purification of tyrosinase to homogeneity based on its resistance to sodium dodecyl sulfate-proteinase K digestion. Arch. Biochem. Biophys. 1989, 275, 122–129. [Google Scholar] [CrossRef]
- Cordes, P.; Sun, W.; Wolber, R.; Kolbe, L.; Klebe, G.; Röhm, K.H. Expression in non-melanogenic systems and purification of soluble variants of human tyrosinase. Biol. Chem. 2013, 394, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Soler-Lopez, M.; Wichers, H.J.; Dijkstra, B.W. Large-scale recombinant expression and purification of human tyrosinase suitable for structural studies. PLoS ONE 2016, 11, e0161697. [Google Scholar] [CrossRef] [PubMed]
- Dolinska, M.B.; Wingfield, P.T.; Sergeev, Y.V. Purification of recombinant human tyrosinase from insect larvae infected with the baculovirus vector. Curr. Protoc. Protein Sci. 2017, 89, 6.15.1–6.15.12. [Google Scholar] [PubMed]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismaya, W.T.; Tandrasasmita, O.M.; Sundari, S.; Lai, X.; Retnoningrum, D.S.; Dijkstra, B.W.; Tjandrawinata, R.R.; Rachmawati, H. The light subunit of mushroom Agaricus bisporus tyrosinase: Its biological characteristics and implications. Int. J. Biol. Macromol. 2017, 102, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Matoba, Y.; Kumagai, T.; Yamamoto, A.; Yoshitsu, H.; Sugiyama, M. Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis. J. Biol. Chem. 2006, 281, 8981–8990. [Google Scholar] [CrossRef] [PubMed]
- Sendovski, M.; Kanteev, M.; Ben-Yosef, V.S.; Adir, N.; Fishman, A. First structures of an active bacterial tyrosinase reveal copper plasticity. J. Mol. Biol. 2011, 405, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure of human tyrosinase related protein 1 reveals a binuclear zinc active site important for melanogenesis. Angew. Chem. 2017, 56, 9812–9815. [Google Scholar] [CrossRef] [PubMed]
- Kanteev, M.; Goldfeder, M.; Fishman, A. Structure-function correlations in tyrosinases. Protein Sci. 2015, 24, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure and function of human tyrosinase and tyrosinase-related proteins. Chemistry 2018, 24, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Solem, E.; Tuczek, F.; Decker, H. Tyrosinase versus catechol oxidase: One asparagine makes the difference. Angew. Chem. Int. Ed. 2016, 55, 2884–2888. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stäb, F.; Hearing, V.J.; Röhm, K.H.; Kolbe, L. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J. Investig. Dermatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kondo, R.; Sakai, K. Inhibition of tyrosinase by flavonoids, stilbenes and related 4-substituted resorcinols: Structure-activity investigations. Planta Med. 2000, 66, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, L.; Mann, T.; Gerwat, W.; Batzer, J.; Ahlheit, S.; Scherner, C.; Wenck, H.; Stäb, F. 4-n-Butylresorcinol, a highly effective tyrosinase inhibitor for the topical treatment of hyperpigmentation. J. Eur. Acad. Dermatol. Venereol. 2013, 27, S19–S23. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.Y.; Shin, J.W.; Na, J.I.; Huh, C.H.; Youn, S.W.; Park, K.C. The efficacy and safety of 4-n-butylresorcinol 0.1% cream for the treatment of melasma: A randomized controlled split-face trial. Ann. Dermatol. 2010, 22, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kim, S.Y.; Park, S.H.; Choi, Y.G.; Kwon, S.B.; Kim, M.K.; Na, J.I.; Youn, S.W.; Park, K.C. Inhibitory effects of 4-n-butylresorcinol on tyrosinase activity and melanin synthesis. Biol. Pharm. Bull. 2005, 28, 2216–2219. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.X.; Ke, L.N.; Song, K.K.; Huang, H.; Liu, X.D. Inhibitory effects of hexylresorcinol and dodecylresorcinol on mushroom (Agaricus bisporus) tyrosinase. Protein J. 2004, 23, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Schmaus, G.; Vielhaber, G.; Jacobs, K.; Franke, H. 4-(1-phenylethyl) 1,3-benzenediol: A new highly potent lightening agent. J. Cosmet. Sci. 2006, 57, 197–198. [Google Scholar] [PubMed]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-Tasser suite: Protein structure and function prediction. Nat. Meth. 2015, 12, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Ashraf, Z.; Abbas, Q.; Raza, H.; Seo, S.Y. Exploration of novel human tyrosinase inhibitors by molecular modeling, docking and simulation studies. Interdiscip. Sci. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Favre, E.; Daina, A.; Carrupt, P.A.; Nurisso, A. Modeling the met form of human tyrosinase: A refined and hydrated pocket for antagonist design. Chem. Biol. Drug Des. 2014, 84, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Sinha, S.; Mitra, N.; Surolia, A. Probing into the role of conserved N-glycosylation sites in the tyrosinase glycoprotein family. Glycoconj. J. 2009, 26, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Cioaca, D.; Ghenea, S.; Spiridon, L.N.; Marin, M.; Petrescu, A.J.; Petrescu, S.M. C-terminus glycans with critical functional role in the maturation of secretory glycoproteins. PLoS ONE 2011, 6, e19979. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.; Christensen, M.H. Moldock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. Ligplot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gong, Z.; Li, J.; Lu, T. Intermolecular sulfur...oxygen interactions: Theoretical and statistical investigations. J. Chem. Inf. Model. 2015, 55, 2138–2153. [Google Scholar] [CrossRef] [PubMed]
- Koebel, M.R.; Cooper, A.; Schmadeke, G.; Jeon, S.; Narayan, M.; Sirimulla, S. S...O and S...N sulfur bonding interactions in protein-ligand complexes: Empirical considerations and scoring function. J. Chem. Inf. Model. 2016, 56, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- Winder, A.J.; Harris, H. New assays for the tyrosine hydroxylase and dopa oxidase activities of tyrosinase. Eur. J. Biochem. 1991, 198, 317–326. [Google Scholar] [CrossRef] [PubMed]






| (a) | (b) | ||||
|---|---|---|---|---|---|
| Code | Structure | EC50 (µM) | Code | Structure | EC50 (µM) |
| W311 |  | 3.2 ± 0.1 | W547 |  | 3.2 ± 0.6 |
| W452 |  | 19 ± 1 | W633 |  | 750 ± 60 |
| W539 |  | >3000 | W548 |  | 4.6 ± 0.2 |
| W568 |  | >3000 | W632 |  | >3000 |
| W480 |  | >3000 | W607 |  | 56 ± 3 |
| W624 |  | >3000 | W701 |  | >3000 |
| W056 |  | 520 ± 50 | |||
| Code | Core Structure | EC50 (µM) | nrSc | ||||
|---|---|---|---|---|---|---|---|
| W495 |  | 51 ± 2 | 86 | ||||
| (a) | |||||||
| Code | Amines | EC50 (µM) | nrSc | Code | Amides | EC50 (µM) | nrSc |
| W366 |  | 33 ± 1 | - | W547 |  | 3.2 ± 0.6 | 102 |
| W538 |  | 5.6 ± 1.4 | - | W577 |  | 3.5 ± 0.5 | - |
| W533 |  | 6.2 ± 0.2 | - | W630 |  | 1.1 ± 0.1 | 114 |
| W534 |  | 25 ± 1 | - | W687 |  | 1.4 ± 0.1 | - |
| W688 |  | 60 ± 2 | - | W695 |  | 15 ± 1 | - |
| W694 |  | 16 ± 1 | 93 | W548 |  | 4.6 ± 0.2 | - |
| W367 |  | 10 ± 1 | - | W498 |  | 2.5 ± 0.1 | - |
| W693 |  | 5.7 ± 0.2 | - | W619 |  | 1.6 ± 0.4 | - |
| (b) | |||||||
| Code | R | EC50 (µM) | nrSc | Code | R | EC50 (µM) | nrSc |
| W688 |  | 60 ± 2 | - | W692 |  | 6.9 ± 0.2 | - |
| W669 |  | 81 ± 5 | W680 |  | 3.2 ± 0.2 | - | |
| W619 |  | 1.6 ± 0.4 | - | W696 |  | 6.5 ± 0.2 | - |
| W542 |  | 9.8 ± 0.3 | 105 | W693 |  | 5.7 ± 0.2 | - |
| W367 |  | 11 ± 1 | - | W496 |  | 140 ± 6 | 86 |
| W537 |  | 160 ± 2 | - | ||||
| W529 |  | 2.6 ± 0.1 | - | W570 |  | 3.5 ± 0.1 | 115 |
| (a) | (b) | |||||
|---|---|---|---|---|---|---|
| Code | Structure | EC50 (µM) | nrSc | Code | Structure | EC50 (µM) |
| W089 |  | >3000 | 48 | W548 |  | 4.6 ± 0.2 |
| W039 |  | 21 ± 4 | 92 | W605 |  | 1400 ± 160 |
| W785 |  | 560 ± 90 | 66 | W625 |  | >3000 |
| W072 |  | 93 ± 21 | 88 | W681 |  | >3000 |
| W038 |  | 130 ± 10 | 84 | W694 |  | 16 ± 1 |
| W639 |  | 220 ± 10 | - | W646 |  | 40 ± 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mann, T.; Scherner, C.; Röhm, K.-H.; Kolbe, L. Structure-Activity Relationships of Thiazolyl Resorcinols, Potent and Selective Inhibitors of Human Tyrosinase. Int. J. Mol. Sci. 2018, 19, 690. https://doi.org/10.3390/ijms19030690
Mann T, Scherner C, Röhm K-H, Kolbe L. Structure-Activity Relationships of Thiazolyl Resorcinols, Potent and Selective Inhibitors of Human Tyrosinase. International Journal of Molecular Sciences. 2018; 19(3):690. https://doi.org/10.3390/ijms19030690
Chicago/Turabian StyleMann, Tobias, Cathrin Scherner, Klaus-Heinrich Röhm, and Ludger Kolbe. 2018. "Structure-Activity Relationships of Thiazolyl Resorcinols, Potent and Selective Inhibitors of Human Tyrosinase" International Journal of Molecular Sciences 19, no. 3: 690. https://doi.org/10.3390/ijms19030690
APA StyleMann, T., Scherner, C., Röhm, K.-H., & Kolbe, L. (2018). Structure-Activity Relationships of Thiazolyl Resorcinols, Potent and Selective Inhibitors of Human Tyrosinase. International Journal of Molecular Sciences, 19(3), 690. https://doi.org/10.3390/ijms19030690