Targeted Metabolomic and Transcriptomic Analyses of “Red Russian” Kale (Brassicae napus var. pabularia) Following Methyl Jasmonate Treatment and Larval Infestation by the Cabbage Looper (Trichoplusia ni Hübner)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Quantification of Insect-Damaged Area
2.2. Effect of MeJA Application and T. ni Treatment on GS, Their Hydrolysis Products, and Myrosinase Activity
2.3. Effect of MeJA Application and T. ni Larva Feeding on the Expression of Genes Related to Indolyl GS Biosynthesis and of Indolyl GS Transcription Factors, Myrosinase, and Specifier Proteins
2.4. Effect of MeJA Application and T. ni Larval Feeding on Myrosinase Activity and Nitrile Formation
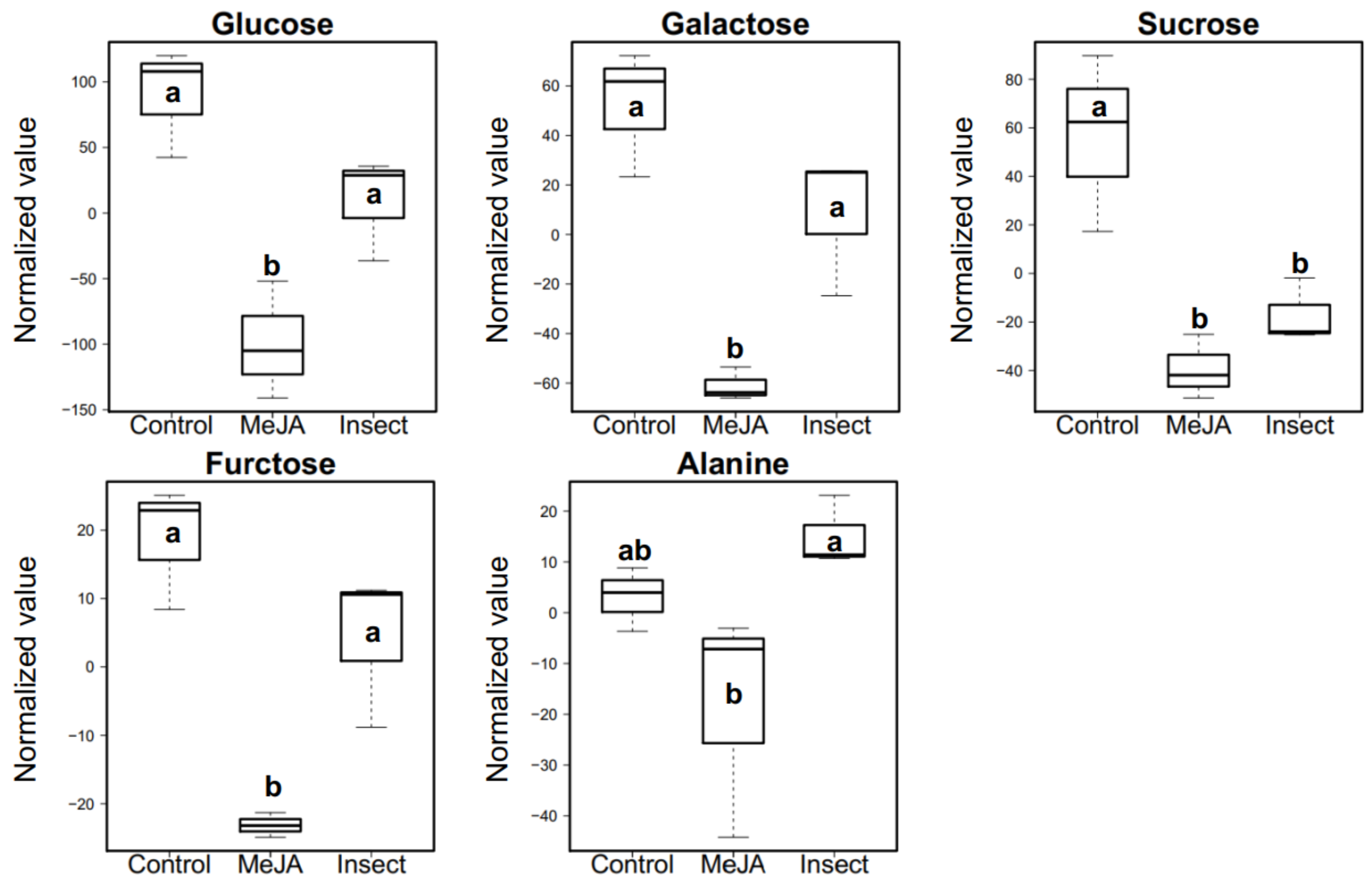
2.5. Effect of MeJA Application and T. ni Treatment on Polar Primary Metabolites
3. Materials and Methods
3.1. Kale Cultivation and Treatments
3.2. Quantification of Insect-Damaged Area Using ImageJ
3.3. Quantification of Glucosinolate Concentrations
3.4. Quantification of Glucosinolate Hydrolysis Products
3.5. Measurement of Myrosinase Activities and Nitrile Formation
3.6. RNA Extraction and Quantitative Real-Time PCR
3.7. Untargeted Metabolomics by GC–MS
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Migliozzi, M.; Thavarajah, D.; Thavarajah, P.; Smith, P. Lentil and Kale: Complementary Nutrient-Rich Whole Food Sources to Combat Micronutrient and Calorie Malnutrition. Nutrients 2015, 7, 9285–9298. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Wehage, S.L.; Holtzclaw, W.D.; Kensler, T.W.; Egner, P.A.; Shapiro, T.A.; Talalay, P. Protection of Humans by Plant Glucosinolates: Efficiency of Conversion of Glucosinolates to Isothiocyanates by the Gastrointestinal Microflora. Cancer Prev. Res. 2012, 5, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.-N.T. Dietary Glucosinolates Sulforaphane, Phenethyl Isothiocyanate, Indole-3-Carbinol/3,3′-Diindolylmethane: Antioxidative Stress/Inflammation, Nrf2, Epigenetics/Epigenomics and In Vivo Cancer Chemopreventive Efficacy. Curr. Pharmacol. Rep. 2015, 1, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.-M.; Jeffery, E.H.; Juvik, J.A. Exogenous Methyl Jasmonate Treatment Increases Glucosinolate Biosynthesis and Quinone Reductase Activity in Kale Leaf Tissue. PLoS ONE 2014, 9, e103407. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, T.A.; Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Chemoprotective Glucosinolates and Isothiocyanates of Broccoli Sprouts Metabolism and Excretion in Humans. Cancer Epidemiol. Biomark. Prev. 2001, 10, 501–508. [Google Scholar]
- Hahn, C.; Müller, A.; Kuhnert, N.; Albach, D. Diversity of Kale (Brassica oleracea var. sabellica): Glucosinolate Content and Phylogenetic Relationships. J. Agric. Food Chem. 2016, 64, 3215–3225. [Google Scholar] [CrossRef] [PubMed]
- Velasco, P.; Cartea, M.E.; González, C.; Vilar, M.; Ordás, A. Factors Affecting the Glucosinolate Content of Kale (Brassica oleracea acephala Group). J. Agric. Food Chem. 2007, 55, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fei, M.; Wang, Y.; Chen, S.; Yan, X. Proteomic investigation of glucosinolate systematically changes in Arabidopsis Rosette leaves to exogenous methyl jasmonate. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2015, 149, 346–353. [Google Scholar] [CrossRef]
- Kim, H.S.; Juvik, J.A. Effect of Selenium Fertilization and Methyl Jasmonate Treatment on Glucosinolate Accumulation in Broccoli Florets. J. Am. Soc. Hortic. Sci. 2011, 136, 239–246. [Google Scholar]
- Ku, K.M.; Choi, J.-H.; Kushad, M.M.; Jeffery, E.H.; Juvik, J.A. Pre-harvest Methyl Jasmonate Treatment Enhances Cauliflower Chemoprotective Attributes Without a Loss in Postharvest Quality. Plant Foods Hum. Nutr. 2013, 68, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zhang, H.; Huang, L.; Wang, F.; Gao, F.; Lv, X.; Yang, J.; Zhu, B.; Hong, S.-B.; Zhu, Z. Glucosinolate enhancement in leaves and roots of pak choi (Brassica rapa ssp. chinensis) by methyl jasmonate. Hortic. Environ. Biotechnol. 2016, 56, 830–840. [Google Scholar] [CrossRef]
- Zang, Y.; Zheng, W.; He, Y.; Hong, S.-B.; Zhu, Z. Global analysis of transcriptional response of Chinese cabbage to methyl jasmonate reveals JA signaling on enhancement of secondary metabolism pathways. Sci. Hortic. 2015, 189, 159–167. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and Biochemistry of Glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates—Gene discovery and beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Auborn, K.J.; Fan, S.; Rosen, E.M.; Goodwin, L.; Chandraskaren, A.; Williams, D.E.; Chen, D.; Carter, T.H. Indole-3-Carbinol Is a Negative Regulator of Estrogen. J. Nutr. 2003, 133, 2470S–2475S. [Google Scholar] [CrossRef] [PubMed]
- Burow, M.; Markert, J.; Gershenzon, J.; Wittstock, U. Comparative biochemical characterization of nitrile-forming proteins from plants and insects that alter myrosinase-catalysed hydrolysis of glucosinolates. FEBS J. 2006, 273, 2432–2446. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zul, S.; Surugau, N. Effects of ascorbic acid and ferum ions concentration on the hydrolysis of flucosinolaye and myrosinase activity in the watercress (Nasturtium officinale sp.). J. Teknol. 2016, 78. [Google Scholar] [CrossRef]
- Williams, D.J.; Critchley, C.; Pun, S.; Nottingham, S.; O’Hare, T.J. Epithiospecifier protein activity in broccoli: The link between terminal alkenyl glucosinolates and sulphoraphane nitrile. Phytochemistry 2008, 69, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ober, J.A.; Kliebenstein, D.J. The Gene Controlling the Quantitative Trait Locus EPITHIOSPECIFIER MODIFIER1 Alters Glucosinolate Hydrolysis and Insect Resistance in Arabidopsis. Plant Cell 2006, 18, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, M.; Van Dam, N.M.; Van Loon, J.J.A.; Dicke, M. Jasmonic Acid-Induced Changes in Brassica oleracea Affect Oviposition Preference of Two Specialist Herbivores. J. Chem. Ecol. 2007, 33, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jander, G. Myzus persicae (green peach aphid) feeding on Arabidopsis induces the formation of a deterrent indole glucosinolate. Plant J. 2007, 49, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Barak, J.D.; Liang, A.; Narm, K.-E. Differential Attachment to and Subsequent Contamination of Agricultural Crops by Salmonella enterica. Appl. Environ. Microbiol. 2008, 74, 5568–5570. [Google Scholar] [CrossRef] [PubMed]
- Beekwilder, J.; van Leeuwen, W.; van Dam, N.M.; Bertossi, M.; Grandi, V.; Mizzi, L.; Soloviev, M.; Szabados, L.; Molthoff, J.W.; Schipper, B.; et al. The Impact of the Absence of Aliphatic Glucosinolates on Insect Herbivory in Arabidopsis. PLOS ONE 2008, 3, e2068. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.F.; Yousef, G.G.; Jeffery, E.H.; Klein, B.P.; Wallig, M.A.; Kushad, M.M.; Juvik, J.A. Glucosinolate Profiles in Broccoli: Variation in Levels and Implications in Breeding for Cancer Chemoprotection. J. Am. Soc. Hortic. Sci. 2002, 127, 807–813. [Google Scholar]
- Ku, K.M.; Jeffery, E.H.; Juvik, J.A. Influence of Seasonal Variation and Methyl Jasmonate Mediated Induction of Glucosinolate Biosynthesis on Quinone Reductase Activity in Broccoli Florets. J. Agric. Food Chem. 2013, 61, 9623–9631. [Google Scholar] [CrossRef] [PubMed]
- Falk, K.L.; Kästner, J.; Bodenhausen, N.; Schramm, K.; Paetz, C.; Vassão, D.G.; Reichelt, M.; von Knorre, D.; Bergelson, J.; Erb, M.; et al. The role of glucosinolates and the jasmonic acid pathway in resistance of Arabidopsis thaliana against molluscan herbivores. Mol. Ecol. 2014, 23, 1188–1203. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.-B.; Liu, Y.-Q.; Chen, D.-Y.; Chen, F.-Y.; Fang, X.; Hong, G.-J.; Wang, L.-J.; Wang, J.-W.; Chen, X.-Y. Jasmonate response decay and defense metabolite accumulation contributes to age-regulated dynamics of plant insect resistance. Nat. Commun. 2017, 8, ncomms13925. [Google Scholar] [CrossRef] [PubMed]
- Schuman, M.C.; Baldwin, I.T. The Layers of Plant Responses to Insect Herbivores. Annu. Rev. Entomol. 2016, 61, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant Defense against Insect Herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.-M.; Becker, T.M.; Juvik, J.A. Transcriptome and Metabolome Analyses of Glucosinolates in Two Broccoli Cultivars Following Jasmonate Treatment for the Induction of Glucosinolate Defense to Trichoplusia ni (Hübner). Int. J. Mol. Sci. 2016, 17, 1135. [Google Scholar] [CrossRef] [PubMed]
- Greene, G.L. Economic Damage Threshold and Spray Interval for Cabbage Looper Control on Cabbage 1 2. J. Econ. Entomol. 1972, 65, 205–208. [Google Scholar] [CrossRef]
- Tak, J.-H.; Isman, M.B. Penetration-enhancement underlies synergy of plant essential oil terpenoids as insecticides in the cabbage looper, Trichoplusia ni. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- HOSTS—A Database of the World’s Lepidopteran Hostplants. Available online: http://www.nhm.ac.uk/our-science/data/hostplants/ (accessed on 19 November 2017).
- Rivera-Vega, L.J.; Galbraith, D.A.; Grozinger, C.M.; Felton, G.W. Host plant driven transcriptome plasticity in the salivary glands of the cabbage looper (Trichoplusia ni). PLoS ONE 2017, 12, e0182636. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, M.; Chen, J.; Liu, P.; Li, Z. Effects of MeJA on Arabidopsis metabolome under endogenous JA deficiency. Sci. Rep. 2016, 6, srep37674. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.L.; Yoshida, Y.; Major, I.T.; de Oliveira Ferreira, D.; Weraduwage, S.M.; Froehlich, J.E.; Johnson, B.F.; Kramer, D.M.; Jander, G.; Sharkey, T.D.; et al. Rewiring of jasmonate and phytochrome B signalling uncouples plant growth-defense tradeoffs. Nat. Commun. 2016, 7, 12570. [Google Scholar] [CrossRef] [PubMed]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth–Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huai, D.; Yang, Q.; Cheng, Y.; Ma, M.; Kliebenstein, D.J.; Zhou, Y. Overexpression of Three Glucosinolate Biosynthesis Genes in Brassica napus Identifies Enhanced Resistance to Sclerotinia sclerotiorum and Botrytis cinerea. PLoS ONE 2015, 10, e0140491. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lou, Y.-R.; Tzin, V.; Jander, G. Alteration of Plant Primary Metabolism in Response to Insect Herbivory. Plant Physiol. 2015, 169, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Houston, K.; Tucker, M.R.; Chowdhury, J.; Shirley, N.; Little, A. The Plant Cell Wall: A Complex and Dynamic Structure As Revealed by the Responses of Genes under Stress Conditions. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S. Functional Studies of Lignin Biosynthesis Genes and Putative Flowering Gene in Miscanthus x Giganteus and Studies on Indolyl Glucosinolate Biosynthesis and Translocation in Brassica oleracea; University of Illinois at Urbana-Champaign: Champaign, IL, USA, 2010. [Google Scholar]
- Kim, M.J.; Chiu, Y.-C.; Kim, N.K.; Park, H.M.; Lee, C.H.; Juvik, J.A.; Ku, K.-M. Cultivar-Specific Changes in Primary and Secondary Metabolites in Pak Choi (Brassica Rapa, Chinensis Group) by Methyl Jasmonate. Int. J. Mol. Sci. 2017, 18, 1004. [Google Scholar] [CrossRef] [PubMed]
- McEwen, C.L. Two Entomological Studies: 1. The Potential of Methyl Jasmonate Applications as a Pest Management Method on Cruciferous Crops 2. Contributions to the Biology of Disholcaspis Quercusmamma (Walsh)(Hymenoptera: Cynipidae). Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2011. [Google Scholar]
- Kliebenstein, D.J.; Kroymann, J.; Brown, P.; Figuth, A.; Pedersen, D.; Gershenzon, J.; Mitchell-Olds, T. Genetic Control of Natural Variation in Arabidopsis Glucosinolate Accumulation. Plant Physiol. 2001, 126, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; de Vos, M.; Sun, J.Y.; Sønderby, I.E.; Halkier, B.A.; Wittstock, U.; Jander, G. Differential Effects of Indole and Aliphatic Glucosinolates on Lepidopteran Herbivores. J. Chem. Ecol. 2010, 36, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Bidart-Bouzat, M.G.; Kliebenstein, D. An ecological genomic approach challenging the paradigm of differential plant responses to specialist versus generalist insect herbivores. Oecologia 2011, 167, 677. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, T.A.; Dinh, S.T.; Baldwin, I.T. JA but not JA-Ile is the cell-nonautonomous signal activating JA mediated systemic defenses to herbivory in Nicotiana attenuata. J. Integr. Plant Biol. 2017, 59, 552–571. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.J.; van Dam, N.M.; Loon, J.J.A. van Role of Glucosinolates in Insect-Plant Relationships and Multitrophic Interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; Peterson, C.J.; Coats, J.R. Glucosinolate breakdown products as insect fumigants and their effect on carbon dioxide emission of insects. BMC Ecol. 2002, 2, 5. [Google Scholar] [CrossRef]
- Badenes-Perez, F.R.; Gershenzon, J.; Heckel, D.G. Insect Attraction versus Plant Defense: Young Leaves High in Glucosinolates Stimulate Oviposition by a Specialist Herbivore despite Poor Larval Survival due to High Saponin Content. PLoS ONE 2014, 9, e95766. [Google Scholar] [CrossRef] [PubMed]
- Wittstock, U.; Kliebenstein, D.J.; Lambrix, V.; Reichelt, M.; Gershenzon, J. Chapter five Glucosinolate hydrolysis and its impact on generalist and specialist insect herbivores. Recent Adv. Phytochem. 2003, 37, 101–125. [Google Scholar] [CrossRef]
- Augustine, R.; Majee, M.; Gershenzon, J.; Bisht, N.C. Four genes encoding MYB28, a major transcriptional regulator of the aliphatic glucosinolate pathway, are differentially expressed in the allopolyploid Brassica juncea. J. Exp. Bot. 2013, 64, 4907–4921. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.-E.; Robin, A.H.K.; Yang, K.; Park, J.-I.; Hwang, B.H.; Nou, I.-S. Exogenous Methyl Jasmonate and Salicylic Acid Induce Subspecies-Specific Patterns of Glucosinolate Accumulation and Gene Expression in Brassica oleracea L. Molecules 2016, 21, 1417. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Wang, J.; Wang, Y.; Zhang, J.; Wang, J.; Pei, X. Comparative analysis of MYB28 homologs and development of a MYB28-specific marker in Brassica napus L. Mol. Breed. 2016, 36. [Google Scholar] [CrossRef]
- Mewis, I.; Tokuhisa, J.G.; Schultz, J.C.; Appel, H.M.; Ulrichs, C.; Gershenzon, J. Gene expression and glucosinolate accumulation in Arabidopsis thaliana in response to generalist and specialist herbivores of different feeding guilds and the role of defense signaling pathways. Phytochemistry 2006, 67, 2450–2462. [Google Scholar] [CrossRef] [PubMed]
- Gigolashvili, T.; Berger, B.; Flügge, U.-I. Specific and coordinated control of indolic and aliphatic glucosinolate biosynthesis by R2R3-MYB transcription factors in Arabidopsis thaliana. Phytochem. Rev. 2009, 8, 3–13. [Google Scholar] [CrossRef]
- Dombrecht, B.; Xue, G.P.; Sprague, S.J.; Kirkegaard, J.A.; Ross, J.J.; Reid, J.B.; Fitt, G.P.; Sewelam, N.; Schenk, P.M.; Manners, J.M.; et al. MYC2 Differentially Modulates Diverse Jasmonate-Dependent Functions in Arabidopsis. Plant Cell Online 2007, 19, 2225–2245. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, M.; Hanschen, F.S.; Schreiner, M.; Glatt, H.; Zrenner, R. Induced Production of 1-Methoxy-indol-3-ylmethyl Glucosinolate by Jasmonic Acid and Methyl Jasmonate in Sprouts and Leaves of Pak Choi (Brassica rapa ssp. chinensis). Int. J. Mol. Sci. 2013, 14, 14996–15016. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.D.; Hansen, C.H.; Wittstock, U.; Halkier, B.A. Cytochrome P450 CYP79B2 from Arabidopsis Catalyzes the Conversion of Tryptophan to Indole-3-acetaldoxime, a Precursor of Indole Glucosinolates and Indole-3-acetic Acid. J. Biol. Chem. 2000, 275, 33712–33717. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hull, A.K.; Gupta, N.R.; Goss, K.A.; Alonso, J.; Ecker, J.R.; Normanly, J.; Chory, J.; Celenza, J.L. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev. 2002, 16, 3100–3112. [Google Scholar] [CrossRef] [PubMed]
- Hentrich, M.; Böttcher, C.; Düchting, P.; Cheng, Y.; Zhao, Y.; Berkowitz, O.; Masle, J.; Medina, J.; Pollmann, S. The jasmonic acid signaling pathway is linked to auxin homeostasis through the modulation of YUCCA8 and YUCCA9 gene expression. Plant J. Cell Mol. Biol. 2013, 74, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, R.; Negri, A.; Ronchi, S.; Palmieri, S. Isolation of the epithiospecifier protein from oil-rape (Brassica napus ssp. oleifera) seed and its characterization. FEBS Lett. 2000, 467, 296–298. [Google Scholar] [CrossRef]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, V.; Gershenzon, J.; Vassão, D.G. Chapter Eight—Insect Detoxification of Glucosinolates and Their Hydrolysis Products. In Advances in Botanical Research; Glucosinolates; Kopriva, S., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 80, pp. 199–245. [Google Scholar]
- Mewis, I.; Appel, H.M.; Hom, A.; Raina, R.; Schultz, J.C. Major Signaling Pathways Modulate Arabidopsis Glucosinolate Accumulation and Response to Both Phloem-Feeding and Chewing Insects. Plant Physiol. 2005, 138, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Choi, J.H.; Kim, H.S.; Kushad, M.M.; Jeffery, E.H.; Juvik, J.A. Methyl Jasmonate and 1-Methylcyclopropene Treatment Effects on Quinone Reductase Inducing Activity and Post-Harvest Quality of Broccoli. PLoS ONE 2013, 8, e77127. [Google Scholar] [CrossRef] [PubMed]
- Textor, S.; Gershenzon, J. Herbivore induction of the glucosinolate-myrosinase defense system: major trends, biochemical bases and ecological significance. Phytochem. Rev. 2009, 8, 149–170. [Google Scholar] [CrossRef]
- Martin, N.; Müller, C. Induction of plant responses by a sequestering insect: Relationship of glucosinolate concentration and myrosinase activity. Basic Appl. Ecol. 2007, 8, 13–25. [Google Scholar] [CrossRef]
- Lambrix, V.; Reichelt, M.; Mitchell-Olds, T.; Kliebenstein, D.J.; Gershenzon, J. The Arabidopsis Epithiospecifier Protein Promotes the Hydrolysis of Glucosinolates to Nitriles and Influences Trichoplusia ni Herbivory. Plant Cell Online 2001, 13, 2793–2807. [Google Scholar] [CrossRef]
- Machado, R.A.R.; Arce, C.C.M.; Ferrieri, A.P.; Baldwin, I.T.; Erb, M. Jasmonate-dependent depletion of soluble sugars compromises plant resistance to Manduca sexta. New Phytol. 2015, 207, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Frier, J.P.D.; Hernández, C.V.S.; Tiessen, A. Friend or Foe? Exploring the Factors that Determine the Difference Between Positive and Negative Effects on Photosynthesis in Response to Insect Herbivory. In Artificial Photosynthesis; InTech: London, UK, 2012. [Google Scholar]
- Havko, N.E.; Major, I.T.; Jewell, J.B.; Attaran, E.; Browse, J.; Howe, G.A. Control of Carbon Assimilation and Partitioning by Jasmonate: An Accounting of Growth–Defense Tradeoffs. Plants 2016, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- ISO. The International Organization of Stanardization ISO 9167-1; ISO: Genera, Switzerland, 1992. [Google Scholar]
- Wathelet, J.-P.; Marlier, M.; Severin, M.; Boenke, A.; Wagstaffe, P.J. Measurement of glucosinolates in rapeseeds. Nat. Toxins 1995, 3, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Kusznierewicz, B.; Iori, R.; Piekarska, A.; Namieśnik, J.; Bartoszek, A. Convenient identification of desulfoglucosinolates on the basis of mass spectra obtained during liquid chromatography–diode array–electrospray ionisation mass spectrometry analysis: Method verification for sprouts of different Brassicaceae species extracts. J. Chromatogr. A 2013, 1278, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.-M.; Kim, M.J.; Jeffery, E.H.; Kang, Y.-H.; Juvik, J.A. Profiles of Glucosinolates, Their Hydrolysis Products, and Quinone Reductase Inducing Activity from 39 Arugula (Eruca sativa Mill.) Accessions. J. Agric. Food Chem. 2016, 64, 6524–6532. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Montone, C.M.; Piovesana, S.; Zenezini Chiozzi, R.; Laganà, A. Chromatographic column evaluation for the untargeted profiling of glucosinolates in cauliflower by means of ultra-high performance liquid chromatography coupled to high resolution mass spectrometry. Talanta 2018, 179, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Dosz, E.B.; Ku, K.-M.; Juvik, J.A.; Jeffery, E.H. Total myrosinase activity estimates in brassica vegetable produce. J. Agric. Food Chem. 2014, 62, 8094–8100. [Google Scholar] [CrossRef] [PubMed]
- Hasperué, J.H.; Gómez-Lobato, M.E.; Chaves, A.R.; Civello, P.M.; Martínez, G.A. Time of day at harvest affects the expression of chlorophyll degrading genes during postharvest storage of broccoli. Postharvest Biol. Technol. 2013, 82, 22–27. [Google Scholar] [CrossRef]
- He, L.; Diedrich, J.; Chu, Y.-Y.; Yates, J.R. Extracting Accurate Precursor Information for Tandem Mass Spectra by RawConverter. Anal. Chem. 2015, 87, 11361–11367. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. In Current Protocols in Bioinformatics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; ISBN 978-0-471-25095-1. [Google Scholar]
- Baggaley, A.R. Deciding on the Ratio of Number of Subjects to Number of Variables in Factor Analysis. Available online: http://psycnet.apa.org.www.libproxy.wvu.edu/record/1983-31730-001 (accessed on 16 March 2018).
- Osborne, J.; Costello, A. Sample Size and Subject to Item Ratio in Principal Components Analy. Available online: http://pareonline.net/htm/v9n11.htm (accessed on 16 March 2018).



| Samples | Glucoraphanin | Gluconapin | Progoitrin | Glucobrassicin | Neoglucobrassicin | 4-Methoxy-glucobrassicin | 1-Hydroxy-glucobrassicin | Total Aliphatic GS z | Total Indolyl GS | Total GS |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1.14 ± 0.16 a | 0.64 ± 0.02 a | 4.26 ± 0.55 ab | 0.31 ± 0.07 c | 0.31 ± 0.07 b | 0.03 ± 0.01 c | 0.17 ± 0.02 a | 6.04 ± 0.40 a | 0.81 ± 0.17 c | 6.85± 0.44 b |
| MeJA | 1.13 ± 0.20 a | 0.67 ± 0.09 a | 4.71 ± 0.40 a | 0.98 ± 0.15 b | 1.65 ± 0.19 a | 0.06 ± 0.01 b | 0.10 ± 0.03 b | 6.50 ± 0.60 a | 2.80 ± 0.31 b | 9.31 ± 0.85 a |
| Insect damage | 0.77 ± 0.14 b | 0.30 ± 0.09 b | 3.44 ± 0.86 b | 1.68 ± 0.31 a | 1.73 ± 0.17 a | 0.09 ± 0.02 a | 0.12 ± 0.04 ab | 4.52 ± 1.04 b | 3.59 ± 0.46 a | 8.11 ± 1.35 ab |
| Samples | Sulforaphane | Sulforaphane Nitrile | 3-butenyl isothiocyanate | 1-cyano-3,4-epithiobutane | Crambene | Goitrin | 1-cyano-2-hydroxy-3,4-epithiobutane | I3A | NMI3C | NM3CA |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0 | 0.08 ± 0.02 a | 0.13 ± 0.04 a | 1.11 ± 0.12 a | 1.48 ± 0.19 a | 0 | 1.30 ± 0.24 a | 0 | 0 | 0 |
| MeJA | 0.03 ± 0.01 a | 0.11 ± 0.02 a | 0.38 ± 0.04 a | 1.37 ± 0.28 a | 1.94 ± 0.14 a | 0.07 ± 0.01 a | 1.70 ± 0.21 a | 0.08 ± 0.02 a | 0.37 ± 0.04 a | 0.05 ± 0.01a |
| Insect damage | 0.19 ± 0.23 a | 0.08 ± 0.03 a | 0.75 ± 0.72 a | 0.56 ± 0.28 b | 1.67 ± 0.58 a | 0.42 ± 0.57 a | 1.28 ± 0.57 a | 0.08 ± 0.03 a | 0.65 ± 0.35 a | 0.05 ± 0.03 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, Y.-C.; Juvik, J.A.; Ku, K.-M. Targeted Metabolomic and Transcriptomic Analyses of “Red Russian” Kale (Brassicae napus var. pabularia) Following Methyl Jasmonate Treatment and Larval Infestation by the Cabbage Looper (Trichoplusia ni Hübner). Int. J. Mol. Sci. 2018, 19, 1058. https://doi.org/10.3390/ijms19041058
Chiu Y-C, Juvik JA, Ku K-M. Targeted Metabolomic and Transcriptomic Analyses of “Red Russian” Kale (Brassicae napus var. pabularia) Following Methyl Jasmonate Treatment and Larval Infestation by the Cabbage Looper (Trichoplusia ni Hübner). International Journal of Molecular Sciences. 2018; 19(4):1058. https://doi.org/10.3390/ijms19041058
Chicago/Turabian StyleChiu, Yu-Chun, John A. Juvik, and Kang-Mo Ku. 2018. "Targeted Metabolomic and Transcriptomic Analyses of “Red Russian” Kale (Brassicae napus var. pabularia) Following Methyl Jasmonate Treatment and Larval Infestation by the Cabbage Looper (Trichoplusia ni Hübner)" International Journal of Molecular Sciences 19, no. 4: 1058. https://doi.org/10.3390/ijms19041058
APA StyleChiu, Y.-C., Juvik, J. A., & Ku, K.-M. (2018). Targeted Metabolomic and Transcriptomic Analyses of “Red Russian” Kale (Brassicae napus var. pabularia) Following Methyl Jasmonate Treatment and Larval Infestation by the Cabbage Looper (Trichoplusia ni Hübner). International Journal of Molecular Sciences, 19(4), 1058. https://doi.org/10.3390/ijms19041058







