Antimalarial Activity of Plant Metabolites
Abstract
1. Introduction
2. Plant-derived Antimalarial Compounds
2.1. Annonaceae–Asteraceae Families
2.1.1. Annonaceae Family
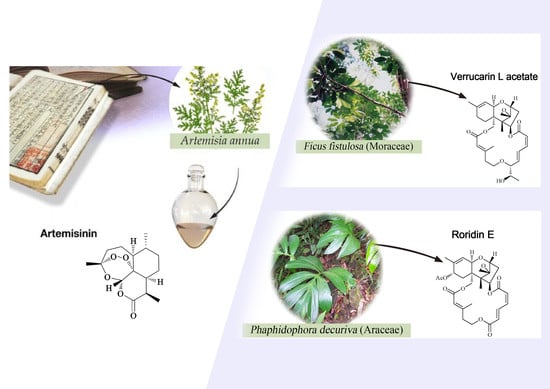
2.1.2. Araceae Family
2.1.3. Asclepiadaceae Family
2.1.4. Asteraceae Family
2.2. Buxaceae Family
2.3. Cecropiaceae–Cucurbitaceae Families
2.3.1. Cecropiaceae Family
2.3.2. Chloranthaceae Family
2.3.3. Chrysobalanaceae Family
2.3.4. Clusiaceae Family
2.3.5. Connaraceae Family
2.3.6. Cornaceae Family
2.3.7. Cucurbitaceae Family
2.4. Ebenaceae–Euphorbiaceae Families
2.4.1. Ebenaceae Family
2.4.2. Euphorbiaceae Family
2.5. Fabaceae–Fagaceae Families
2.5.1. Fabaceae Family
2.5.2. Fagaceae Family
2.6. Hypericaceae Family
2.7. Lamiaceae–Lythraceae Families
2.7.1. Lamiaceae Family
2.7.2. Loganiaceae Family
2.7.3. Lythraceae Family
2.8. Malvaceae–Myristicaceae Families
2.8.1. Malvaceae Family
2.8.2. Monimiaceae Family
2.8.3. Moraceae Family
2.8.4. Myristicaceae Family
2.9. Piperaceae–Platanaceae Families
2.9.1. Piperaceae Family
2.9.2. Platanaceae Family
2.10. Rubiaceae-Rutaceae Families
2.10.1. Rubiaceae Family
2.10.2. Rutaceae Family
2.11. Simaroubaceae Family
2.12. Theaceae–Tiliaceae Families
2.12.1. Theaceae Family
2.12.2. Tiliaceae Family
2.13. Verbenaceae Family
3. Marine Plant-Derived Antimalarial Compounds
4. Ethnologic Antimalarial Compounds
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2017; WHO Press: Geneva, Switzerland, 2017. [Google Scholar]
- Mueller, I.; Zimmerman, P.A.; Reeder, J.C. Plasmodium malariae and Plasmodium ovale—The “bashful” malaria parasites. Trends Parasitol. 2007, 23, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Collins, W.E. Plasmodium knowlesi: A malaria parasite of monkeys and humans. Annu. Rev. Entomol. 2012, 57, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Kajfasz, P. Malaria prevention. Int. Marit. Health 2009, 60, 67–70. [Google Scholar] [PubMed]
- Beare, N.A.; Taylor, T.E.; Harding, S.P.; Lewallen, S.; Molyneux, M.E. Malarial retinopathy: A newly established diagnostic sign in severe malaria. Am. J. Trop. Med. Hyg. 2006, 75, 790–797. [Google Scholar] [PubMed]
- Mehlhorn, H. Encyclopedia of Parasitology, 3rd ed.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Dolabela, M.F.; Oliveira, S.G.; Peres, J.M.; Nascimento, J.M.; Póvoa, M.M.; Oliveira, A.B. In vitro antimalarial activity of six Aspidosperma species from the state of Minas Gerais (Brazil). An. Acad. Bras. Ciênc. 2012, 84, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Boulos, M.; Dutra, A.P.; DiSanti, S.M.; Shiroma, M.; Amato, N.V. The clinical evaluation of quinine for the treatment of Plasmodium falciparum malaria. Rev. Soc. Bras. Med. Trop. 1997, 30, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Guerin, P.J.; Olliaro, P.; Nosten, F.; Druilhe, P.; Laxminarayan, R.; Binka, F.; Kilama, W.L.; Ford, N.; White, N.J. Malaria: Current status of control, diagnosis, treatment, and a proposed agenda for research and development. Lancet Infect. Dis. 2002, 2, 564–573. [Google Scholar] [CrossRef]
- Fidock, D.A.; Rosenthal, P.J.; Croft, L.; Brun, R.; Nwaka, S. Antimalarial drug discovery: Efficacy models for compound screening. Nat. Rev. Drug. Discov. 2004, 3, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Klayman, D. Qinghaosu (artemisinin): An antimalarial drug from China. Science 1985, 228, 1049. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Rao, V.S.; Chandrasekhara, K.V.; Yogeeswari, P. Progress in the research of artemisinin and its analogues as antimalarials: An update. Nat. Prod. Res. 2004, 18, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Ge, M.; Plattner, J.J. Recent Progress in the synthesis of antimalarial agents. Org. Prep. Proced. Int. 2012, 44, 340–374. [Google Scholar] [CrossRef]
- Kappe, S.H.; Vaughan, A.M.; Boddey, J.A.; Cowman, A.F. That was then but this is now: Malaria research in the time of an eradication agenda. Science 2010, 328, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, H.; Atamna, H. The redox status of malaria-infected erythrocytes: An overview with an emphasis on unresolved problems. Parasite 1994, 1, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Zani, B.; Gathu, M.; Donegan, S.; Olliaro, P.L.; Sinclair, D. Dihydroartemisinin-piperaquine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst. Rev. 2014, 1, 1–160. [Google Scholar] [CrossRef] [PubMed]
- Lisewski, A.M.; Quiros, J.P.; Ng, C.L.; Adikesavan, A.K.; Miura, K.; Putluri, N.; Eastman, R.T.; Scanfeld, D.; Regenbogen, S.J.; Altenhofen, L.; et al. Supergenomic network compression and the discovery of EXP1 as a glutathione transferase inhibited by artesunate. Cell 2014, 158, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Schlitzer, M. Antimalarial drugs—What is in use and what is in the pipeline. Arch. Pharm. Chem. Life Sci. 2008, 341, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Li, W.F.; Fong, H.H.S.; Soejarto, D.D. Discovery of bioactive compounds by UIC-ICBG drug discovery program in the 18 years since 1998. Molecules 2016, 21, 1448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Tamez, P.A.; Vu, D.H.; Ghee, T.T.; Nguyen, V.H.; Le, T.X.; Le, M.H.; Nguyen, M.C.; Do, T.T.; Soejarto, D.D.; et al. Antimalarial compounds from Rhaphidophora decursiva. J. Nat. Prod. 2001, 64, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Qiu, S.; Tamez, P.; Tan, G.T.; Aydogmus, Z.; Nguyen, V.H.; Nguyen, M.C.; Angerhofer, C.; Soejarto, D.D.; Pezzuto, J.M.; et al. Antimalarial agents from plants II. Decursivine, a new antimalarial indole alkaloid from Rhaphidophora decursiva. Pharm. Biol. 2002, 40, 221–224. [Google Scholar] [CrossRef]
- Zhang, H.J.; Tamez, P.A.; Aydoqmus, Z.; Tan, G.T.; Saikawa, Y.; Hashimoto, K.; Nakata, M.; Hung, N.V.; Xuan, L.T.; Cuong, N.M.; et al. Antimalarial agents from plants. III. Trichothecenes from Ficus fistulosa and Rhaphidophora decursiva. Planta Med. 2002, 68, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Libman, A.; Zhang, H.; Ma, C.; Southavong, B.; Sydara, K.; Bouamanivong, S.; Tan, G.T.; Fong, H.H.; Soejarto, D.D. A first new antimalarial pregnane glycoside from Gongronema napalense. Asian J. Tradit. Med. 2008, 3, 203–210. [Google Scholar] [PubMed]
- He, Z.D.; Ma, C.Y.; Tan, G.T.; Sydara, K.; Tamez, P.; Southavong, B.; Bouamanivong, S.; Soejarto, D.D.; Pezzuto, J.M.; Fong, H.H.; et al. Rourinoside and rouremin, antimalarial constituents from Rourea minor. Phytochemistry 2006, 67, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Musoke, S.F.; Tan, G.T.; Sydara, K.; Bouamanivong, S.; Southavong, B.; Soejarto, D.D.; Fong, H.H.; Zhang, H.J. Study of antimalarial activity of chemical constituents from Diospyros quaesita. Chem. Biodivers. 2008, 5, 2442–2448. [Google Scholar] [CrossRef] [PubMed]
- He, Z.D.; Ma, C.Y.; Zhang, H.J.; Tan, G.T.; Tamez, P.; Sydara, K.; Bouamanivong, S.; Southavong, B.; Soejarto, D.D.; Pezzuto, J.M.; et al. Antimalarial constituents from Nauclea orientalis (L.) L. Chem. Biodivers. 2005, 2, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, H.J.; Tan, G.T.; Hung, N.V.; Cuong, N.M.; Soejarto, D.D.; Fong, H.H. Antimalarial compounds from Grewia bilamellata. J. Nat. Prod. 2006, 69, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Schwikkard, S.; van Heerden, F.R. Antimalarial activity of plant metabolites. Nat. Prod. Rep. 2002, 19, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Prawat, U.; Phupornprasert, D.; Butsuri, A.; Salae, A.W.; Boonsri, S.; Tuntiwachwuttikul, P. Flavonoids from Friesodielsia discolor. Phytochem. Lett. 2012, 5, 809–813. [Google Scholar] [CrossRef]
- Mueller, D.; Davis, R.A.; Duffy, S.; Avery, V.M.; Camp, D.; Quinn, R.J. Antimalarial activity of azafluorenone alkaloids from the Australian tree Mitrephora diversifolia. J. Nat. Prod. 2009, 72, 1538–1540. [Google Scholar] [CrossRef] [PubMed]
- Promchai, T.; Jaidee, A.; Cheenpracha, S.; Trisuwan, K.; Rattanajak, R.; Kamchonwongpaisan, S.; Laphookhieo, S.; Pyne, S.G.; Ritthiwigrom, T. Antimalarial Oxoprotoberberine Alkaloids from the Leaves of Miliusa cuneata. J. Nat. Prod. 2016, 79, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Beretta, G.; Iriti, M.; Orsenigo, S.; Basilico, N.; Dall’Acqua, S.; Iorizzi, M.; Fico, G. Phenolic compounds from Achillea millefolium L. and their bioactivity. Acta Biochim. Pol. 2011, 58, 203–209. [Google Scholar] [PubMed]
- Chung, I.M.; Seo, S.H.; Kang, E.Y.; Park, W.H.; Park, S.D.; Moon, H.I. Antiplasmodial activity of isolated compounds from Carpesium divaricatum. Phytother. Res. 2010, 24, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Köhlera, I.; Jenett-Siems, K.; Kraft, C.; Siems, K.; Abbiw, D.; Bienzle, U.; Eich, E. Herbal remedies traditionally used against malaria in Ghana: Bioassay-guided fractionation of Microglossa pyrifolia (Asteraceae). Z. Naturforsch. C 2002, 57, 1022–1027. [Google Scholar] [CrossRef]
- Bitew, H.; Mammo, W.; Hymete, A.; Yeshak, M.Y. Antimalatial activity of acetylenic thiophenes from Echinops hoehnelii Schweinf. Molecules 2017, 22, 1965. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Risinger, A.L.; Nair, S.; Peng, J.; Anderson, T.J.; Du, L.; Powell, D.R.; Mooberry, S.L.; Chichewicz, R.H. Identification of compounds with efficacy against malaria parasites from common North American plants. J. Nat. Prod. 2015, 79, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Uchôa, V.T.; de Paula, R.C.; Krettli, L.G.; Santan, A.E.G.; Krettli, A.U. Antimalarial activity of compounds and mixed fractions of Cecropia pachystachya. Drug Dev. Res. 2010, 71, 82–91. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, Y.; Dalal, S.; Merino, E.F.; Liu, Q.F.; Xu, C.H.; Tao, Y.; Ding, J.; Kingston, D.G.I.; Cassera, M.B.; et al. Nanomolar antimalarial agents against chloroquine-resistant Plasmodium falciparum from medicinal plants and their structure-activity relationships. J. Nat. Prod. 2017, 80, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Uys, A.C.; Malan, S.F.; van Dyk, S.; van Zyl, R.L. Antimalarial compounds from Parinari capensis. Bioorg. Med. Chem. Lett. 2002, 12, 2167–2169. [Google Scholar] [CrossRef]
- Auranwiwat, C.; Laphookhieo, S.; Rattanajak, R.; Kamchonwongpaisan, S.; Pyne, S.G.; Ritthiwigrom, T. Antimalarial polyoxygenated and prenylated xanthones from the leaves and branches of Garcinia mckeaniana. Tetrahedron 2016, 72, 6837–6842. [Google Scholar] [CrossRef]
- Graziose, R.; Rojas-Silva, P.; Rathinasabapathy, T.; Dekoc, C.; Grace, M.H.; Poulev, A.; Ann, L.M.; Smith, P.; Raskin, I. Antiparasitic compounds from Cornus florida L. with activities against Plasmodium falciparum and Leishmania tarentolae. J. Ethnopharmacol. 2012, 142, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Banzouzi, J.T.; Soh, P.N.; Mbatchi, B.; Cavé, A.; Ramos, S.; Retailleau, P.; Rakotonandrasana, O.; Berry, A.; Benoit-Vical, F. Cogniauxia podolaena: Bioassay-guided fractionation of defoliated stems, isolation of active compounds, antiplasmodial activity and cytotoxicity. Planta Med. 2008, 74, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Hadi, V.; Hotard, M.; Ling, T.; Salinas, Y.G.; Palacios, G.; Connelly, M.; Rivas, F. Evaluation of Jatropha isabelli natural products and their synthetic analogs as potential antimalarial therapeutic agents. Eur. J. Med. Chem. 2013, 65, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Seephonkai, P.; Sangdee, A.; Bunchalee, P.; Pyne, S.G. Cytotoxic and antiplasmodial compounds from the roots of Strophioblachia fimbricalyx. J. Nat. Prod. 2009, 72, 1892–1894. [Google Scholar] [CrossRef] [PubMed]
- Ajaiyeoba, E.O.; Ogbole, O.O.; Abiodun, O.O.; Ashidi, J.S.; Houghton, P.J.; Wright, C.W. Cajachalcone: An Antimalarial Compound from Cajanus cajan Leaf Extract. J. Parasitol. Res. 2013, 2013, 703781. [Google Scholar] [CrossRef] [PubMed]
- Ramanandraibe, V.; Grellier, P.; Martin, M.T.; Deville, A.; Joyeau, R.; Ramanitrahasimbola, D.; Mouray, E.; Rasoanaivo, P.; Mambu, L. Antiplasmodial phenolic compounds from Piptadenia pervillei. Planta Med. 2008, 74, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Samoylenko, V.; Ashfaq, M.K.; Jacob, M.R.; Tekwani, B.L.; Khan, S.I.; Manly, S.P.; Joshi, V.C.; Walker, L.A.; Muhammad, I. Indolizidine, antiinfective and antiparasitic compounds from Prosopis glandulosa var. Glandulosa. J. Nat. Prod. 2009, 72, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Mbwambo, Z.H.; Apera, S.; Moshi, M.J.; Kapingu, M.C.; Van Miert, S.; Claeys, M.; Brun, R.; Cos, P.; Pieters, L.; Vlietinck, A. Anthranoid compounds with antiprotozoal activity from Vismia orientalis. Planta Med. 2004, 70, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Ndjakou Lenta, B.; Devkota, K.P.; Ngouela, S.; Fekam Boyom, F.; Naz, Q.; Choudhary, M.I.; Tsamo, E.; Rosenthal, P.J.; Sewald, N. Anti-plasmodial and cholinesterase inhibiting activities of some constituents of Psorospermum glaberrimum. Chem. Pharm. Bull. 2008, 56, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S. Small Molecules with Antimalarial Activity. U.S. Patent 2013/0023552 A1, 24 January 2013. [Google Scholar]
- Kirmizibekmez, H.; Calis, I.; Perozzo, R.; Brun, R.; Dönmez, A.A.; Linden, A.; Rüedi, P.; Tasdemir, D. Inhibiting activities of the secondary metabolites of Phlomis brunneogaleata against parasitic protozoa and plasmodial enoyl-ACP Reductase, a crucial enzyme in fatty acid biosynthesis. Planta Med. 2004, 70, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.P.; Van Zyl, R.L.; Davids, H.; Van Heerden, F.R.; Lourens, A.C.U.; Viljoen, A.M. Antimalarial and anticancer activities of selected South African Salviaspecies and isolated compounds from S. radula. S. Afr. J. Bot. 2008, 74, 238–243. [Google Scholar] [CrossRef]
- Tchinda, A.T.; Tamze, V.; Ngono, A.R.N.; Ayimele, G.A.; Cao, M.; Angenot, L.; Frédérich, M. Alkaloids from the stem bark of Strychnos icaja. Phytochem. Lett. 2012, 5, 108–113. [Google Scholar] [CrossRef]
- Upadhyaya, H.C.; Sisodia, B.S.; Agrawal, J.; Pal, A.; Darokar, M.P.; Srivastava, S.K. Antimalarial potential of extracts and isolated compounds from four species of genus Ammannia. Med. Chem. Res. 2014, 23, 870–876. [Google Scholar] [CrossRef]
- Sprogøe, K.; Staek, D.; Ziegler, H.L.; Jensen, T.H.; Holm-Møller, S.B.; Jaroszewski, J.W. Combining HPLC-PDA-MS-SPE-NMR with circular dichroism for complete natural product characterization in crude extracts: Levorotatory gossypol in Thespesia danis. J. Nat. Prod. 2008, 71, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, M.S.; Davis, R.A.; Duffy, S.; Avery, V.M.; Quinn, R.J. Antimalarial benzylisoquinoline alkaloid from the rainforest tree Doryphora sassafras. J. Nat. Prod. 2009, 72, 1541–1543. [Google Scholar] [CrossRef] [PubMed]
- Mbah, J.A.; Tane, P.; Ngadjui, B.T.; Connolly, J.D.; Okunji, C.C.; Iwu, M.M.; Schuster, B.M. Antiplasmodial agents from the leaves of Glossocalyx brevipes. Planta Med. 2004, 70, 437–440. [Google Scholar] [PubMed]
- Kubo, M.; Yatsuzuka, W.; Matsushima, S.; Harada, K.; Inoue, Y.; Miyamoto, H.; Matsumoto, M.; Fukuyama, Y. Antimalarial phenanthroindolizine alkaloids from Ficus septica. Chem. Pharm. Bull. 2016, 64, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Rangkaew, N.; Suttisri, R.; Moriyasu, M.; Kawanishi, K. A new acyclic diterpene acid and bioactive compounds from Knema glauca. Arch. Pharm. Res. 2009, 32, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, T.; Siriwattanakit, P.; Sukcharoenphol, K.; Wongvein, C.; Ruttanaweang, P.; Wongwattanavuch, P.; Suksamrarn, A. Chemical constituents and bioactivity of Piper sarmentosum. J. Ethnopharmacol. 2004, 93, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Sáez Vega, A.; Rojanoa, B.; Blair, S.; Segura, C.; Figadere, B.; Seone, B.; Grellierf, P.; Sáeza, J. Antimalarials and antioxidants compounds from Piper tricuspe (Piperaceae). Pharmacologyonline 2008, 1, 1–8. [Google Scholar]
- Lacroix, D.; Prado, S.; Kamoga, D.; Kasenene, J.; Bodo, B. Structure and in vitro antiparasitic activity of constituents of Citropsis articulata root bark. J. Nat. Prod. 2011, 74, 2286–2289. [Google Scholar] [CrossRef] [PubMed]
- Cebrián-Torrejón, G.; Spelman, K.; Leblanc, K.; Muñoz-Durango, K.; Gutiérrez, S.T.; Ferreira, M.E.; de Arias, A.R.; Figadere, B.; Fournet, A.; Maciuk, A.; et al. The antiplasmodium effects of a traditional South American remedy: Zanthoxylum chiloperone var. angustifolium against chloroquine resistant and chloroquine sensitive strains of Plasmodium falciparum. Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 2011, 21, 652–661. [Google Scholar] [CrossRef]
- Kuo, P.C.; Damu, A.G.; Lee, K.H.; Wu, T.S. Cytotoxic and antimalarial constituents from the roots of Eurycoma longifolia. Bioorg. Med. Chem. 2004, 12, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Yasuda, F.; Otani, K.; Doteuchi, M.; Ishihara, Y.; Shiro, M. New antiulcer quassinoids from Eurycoma longifolia. Eur. J. Med. Chem. 1991, 26, 345–349. [Google Scholar] [CrossRef]
- De Andrade-Neto, V.F.; Pohlit, A.M.; Pinto, A.C.; Silva, E.C.; Nogueir, K.L.; Melo, M.R.; Henrique, M.C.; Amorim, R.C.; Silva, L.F.; Costa, M.R.; et al. In vitro inhibition of Plasmodium falciparum by substances isolated from Amazonian antimalarial plants. Mem. Inst. Oswaldo Cruz 2007, 102, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Tegar, M.; Purnomo, H. Tea leaves extracted as anti-malaria based on molecular docking plants. Procedia Environ. Sci. 2013, 17, 188–194. [Google Scholar] [CrossRef]
- Ludere, M.T.; van Ree, T.; Vleggaa, R. Isolation and relative stereochemistry of lippialactone, a new antimalarial compound from Lippia javanica. Fitoterapia 2013, 86, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Karunamoorthi, K.; Tsehaye, E. Ethnomedicinal knowledge, belief and self-reported practice of local inhabitants on traditional antimalarial plants and phytotherapy. J. Ethnopharmacol. 2012, 141, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Adepiti, A.O.; Elujoba, A.A.; Bolaji, O.O. In vivo antimalarial evaluation of MAMA decoction on Plasmodium berghei in mice. Parasitol. Res. 2014, 113, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Ajaiyeoba, E.O.; Abiodun, O.O.; Falade, M.O.; Ogbole, N.O.; Ashidi, J.S.; Happi, C.T.; Akinboye, D.O. In vitro cytotoxicity studies of 20 plants used in Nigerian antimalarial ethnomedicine. Phytomedicine 2006, 13, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Ogunkunle, A.T.; Oyelakin, T.M.; Enitan, A.O.; Oyewole, F.E. A quantitative documentation of the composition of two powdered herbal formulations (antimalarial and haematinic) using ethnomedicinal information from ogbomoso, Nigeria. Evid. Based Complement. Altern. Med. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rasoanaivo, P.; Petitjean, A.; Ratsimamanga-Urverg, S.; Rakoto-Ratsimamanga, A. Medicinal plants used to treat malaria in Madagascar. J. Ethnopharmacol. 1992, 37, 117–127. [Google Scholar] [CrossRef]
- Ojewole, J.A.; Mawoza, T.; Chiwororo, W.D.; Owira, P.M. Sclerocarya birrea (A. Rich) Hochst. [‘Marula’] (Anacardiaceae): A review of its phytochemistry, pharmacology and toxicology and its ethnomedicinal uses. Phytother. Res. 2010, 24, 633–639. [Google Scholar] [PubMed]
- Qinghaosu Antimalaria Coordinating Research Group. Antimalaria studies on Qinghaosu. Chin. Med. J. 1979, 92, 811–816. [Google Scholar]
- Kitua, A.Y.; Malebo, H.M. Malaria control in Africa and the role of traditional medicine. In Traditional Medicinal Plants and Malaria, 1st ed.; Willcox, M., Bodeker, G., Rasoanaivo, P., Addae-Kyereme, J., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 2–20. [Google Scholar]
- Zeleke, G.; Kebebe, D.; Mulisa, E.; Gashe, F. In vivo antimalarial activity of the solvent fractions of fruit and root of Carica papaya Linn (Caricaceae) against Plasmodium berghei in Mice. Evid. Based Complement. Altern. Med. 2017, 2017, 3121050. [Google Scholar] [CrossRef] [PubMed]
- Muregi, F.W.; Ishih, A.; Miyase, T.; Suzuki, T.; Kino, H.; Amano, T.; Mkoji, G.M.; Terada, M. Antimalarial activity of methanolic extracts from plants used in Kenyan ethnomedicine and their interactions with chloroquine (CQ) against a CQ-tolerant rodent parasite, in mice. J. Ethnopharmacol. 2007, 111, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Abiodun, O.O.; Gbotosho, G.O.; Ajaiyeoba, E.O.; Happi, C.T.; Falade, M.; Wittlin, S.; Sowunmi, A.; Brun, R.; Oduola, A. In vitro antiplasmodial activity and toxicity assessment of some plants from Nigerian ethnomedicine. Pharm. Biol. 2011, 49, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Abiodun, O.O.; Gbotosho, G.O.; Ajaiyeoba, E.O.; Happi, C.T.; Hofer, S.; Wittlin, S.; Sowunmi, A.; Brun, R.; Oduola, A.M. Comparison of SYBR Green I-, PicoGreen-, and [3H]-hypoxanthine-based assays for in vitro antimalarial screening of plants from Nigerian ethnomedicine. Parasitol. Res. 2010, 106, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.R.; Tripathi, P.; Sharma, V.; Chauhan, N.S.; Dixit, V.K. Phyllanthus amarus: Ethnomedicinal uses, phytochemistry and pharmacology: A review. J. Ethnopharmacol. 2011, 138, 286–313. [Google Scholar] [CrossRef] [PubMed]
- Adjobimey, T.; Edaye, I.; Lagnika, L.; Gbenou, J.; Moudachirou, M.; Sanni, A. Activités antiplasmodiales in vitro de quelques plantes antipaludiques de la pharmacopée béninoise. Comptes Rendus Chim. 2004, 7, 1023–1027. [Google Scholar] [CrossRef]
- Upadhyay, B.; Parveen Dhaker, A.K.; Kumar, A. Ethnomedicinal and ethnopharmaco-statistical studies of Eastern Rajasthan. Indian J. Ethnopharmacol. 2010, 129, 64–86. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.B.; Tharaphan, P.; Chotivanich, K.; Tarning, J.; Anal, A.K. In vitro antioxidant and antimalarial activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. BMC Complement. Altern. Med. 2017, 17, 372. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, Q.; Gudise, C.; Wei, L.; Smith, E.; Zeng, Y. Synthesis and biological evaluation of febrifugine analogues as potential antimalarial agents. Bioorg. Med. Chem. 2009, 17, 4496–4502. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Satish, P.V.V.; Sunita, K. Antimalarial efficacy of Pongamia pinnata (L) Pierre against Plasmodium falciparum (3D7 strain) and Plasmodium berghei (ANKA). BMC Complement. Altern. Med. 2017, 17, 458. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Goh, B.H.; Chan, C.K.; Shabab, T.; Kadir, H.A. Biological activities and phytochemicals of Swietenia macrophylla King. Molecules 2013, 18, 10465–10483. [Google Scholar] [CrossRef] [PubMed]
- Falade, M.O.; Akinboye, D.O.; Gbotosho, G.O.; Ajaiyeob, E.O.; Happi, T.C.; Abiodun, O.O.; Oduola, A.M. In Vitro and In Vivo Antimalarial Activity of Ficus thonningii Blume (Moraceae) and Lophira alata Banks (Ochnaceae), Identified from the Ethnomedicine of the Nigerian Middle Belt. J. Parasitol. Res. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Teinkela, J.E.M.; Noundou, X.S.; Nguemfo, E.L.; Meyer, F.; Wintjens, R.; Isaacs, M.; Mpondo Mpondo, A.E.; Hoppe, H.C.; Krause, R.W.M.; Azebaze, A.G.B. Biological activities of plant extracts from Ficus elastica and Selaginella vogelli: An antimalarial, antitrypanosomal and cytotoxity evaluation. Saudi J. Biol. Sci. 2018, 25, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Ansah, C.; Gooderham, N.J. The popular herbal antimalarial, extract of Cryptolepis sanguinolenta, is potently cytotoxic. Toxicol. Sci. 2002, 70, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Kazuki, T.; Yasunori, Y.; Masao, K. Constituents of the leaves and roots of Ligularia stenocephala Matsum. J. Nat. Med. 2006, 60, 329–330. [Google Scholar]
- Lane, A.L.; Stout, E.P.; Lin, A.S.; Prudhomme, J.; le Roch, K.; Fairchild, C.R.; Franzblau, S.G.; Hay, M.E.; Aalbersberg, W.; Kubanek, J. Antimalarial bromophycolides J-Q from the Fijian red alga Callophycus serratus. J. Org. Chem. 2009, 74, 2736–2742. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, M.E.; Prudhomme, J.; Torres, M.; Braley, M.; Cervantes, S.; Bhatia, S.C.; la Clair, J.J.; le Roch, K.; Kubanek, J. Pharmacokinetics, metabolism, and in vivo efficacy of the antimalarial natural product bromophycolide A. ACS Med. Chem. Lett. 2013, 4, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, R.P.; Xu, B.; Yu, H.B.; Ma, G.Y.; Wang, G.F.; Dai, S.W.; Zhang, W.; Jiao, W.H.; Song, S.J.; et al. New antimalarial norterpene cyclic peroxides from Xisha Islands sponge Diacarnus megaspinorhabdosa. Bioorg. Med. Chem. Lett. 2016, 526, 2084–2087. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Report 2002; WHO Press: Geneva, Switzerland, 2002. [Google Scholar]
- De Ridder, S.; van der Kooy, F.; Robert Verpoorte, R. Artemisia annua as a self-reliant treatment for malaria in developing countries. J. Ethnopharmacol. 2008, 120, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Dharani, N.; Rukunga, G.; Abiy Yenesew, A.; Mbora, A.; Mwaura, L.; Dawson, I.; Jamnadass, R. Common Antimalarial Trees and Shrubs of East Africa; World Agroforestry Centre and the Kenya Medical Research Institute: Nairobo, Kenya, 2010. [Google Scholar]
- Loua, J.; Traore, M.S.; Camara, A.; Balde, M.A.; Maes, L.; Pieters, L.; Balde, A.M. Biological and phytochemical investigations on Caesalpinia benthamiana, a plant traditionally used as antimalarial in Guinea. Evid. Based Complement. Altern. Med. 2017, 2017, 9438607. [Google Scholar] [CrossRef] [PubMed]





































| Chemical Class | Generic Names | Chemical Class | Generic Names |
|---|---|---|---|
| 4-Aminoquinolines | chloroquine | Antibiotics | azythromycin |
| amodiaquine | clindamycin | ||
| piperaquine | doxycycline | ||
| 8-Aminoquinoline | primaquine | Artemisinin-based combination therapy (ACT) | artemether-lumefantrine |
| bulaquine | artesunate | ||
| Arylamino-alcohols | quinine | artesunate/sulfadoxine/pyrimethamine | |
| quinidine | artesunate/sulfadoxine-pyrimethamine/primaquine | ||
| mefloquine | artesunate/amodiaquine | ||
| halofantrine | artesunate/mefloquine | ||
| lumefantrine | artesunate/pyronaridine | ||
| Biguanides | proguanil | chloroquine/primaquine | |
| chlorproguanil | dihydroartemisinin/piperaquine | ||
| Glycosylamines | pyrimethamine | Antibiotics-antimalarial drug combination | doxycyclin/quinine |
| proguanil | doxycycline/artesunate | ||
| cycloguanil | doxycyclin/mefloquine | ||
| chlorproguanil | clindamycin/quinine | ||
| chlorcycloguanil | clindamycin/artesunate | ||
| Naphthoquinone | atovaquone | clindamycin/mefloquine | |
| Sesquiterpene lactones | artemisinin | Other combination therapy | sulfadoxine/pyrimethamine |
| arteether | bulaquine/chloroquine | ||
| artemether | dapsone/chlorproguanil | ||
| artesunate | atovaquone/proguanil | ||
| dihydroartemisinin | |||
| Sulfonamides/Sulfones | sulfadoxine | ||
| sulfalene | |||
| dapsone |
| Family | Species | Extract Solvent | Compound | Antiplasmodial IC50 (μM) a (P. falciparum) | Cytotoxicity, ED50 (μM) b (Cell Line) | References |
|---|---|---|---|---|---|---|
| Annonaceae | Friesodielsia discolor | EtOAc | 30-formyl-20,40-dihydroxy-60-methoxychalcone (5) | 9.2 (K1) | 21.8 (KB); 13.9 (MCF-7) | [31] |
| 8-formyl-7-hydroxy-5-methoxyflavanone (6) | 9.3 (K1) | 41.9 (KB); 34.5 (MCF-7) | ||||
| tectochrysin (7) | 7.8 (K1) | 59.1 (KB); 16.8 (MCF-7) | ||||
| Mitrephora diversifolia | CH2Cl2/MeOH | 5-hydroxy-6-methoxyonychine (8) | 9.9 (3D7); 11.4 (Dd2) | 120.0 (HEK293) | [32] | |
| Miliusa cuneata | Acetone | miliusacunines A (9) | 19.3 (TM4) | – | [33] | |
| miliusacunines B (10) | 10.8 (K1) | – | ||||
| Araceae | Rhaphidophora decursiva | MeOH | polysyphorin (1) | 1.7 (D6); 1.5 (W2) | 8.3 (KB) | [22,23] |
| rhaphidecurperoxin (2) | 1.8 (D6); 1.4 (W2) | 13.1 (KB) | ||||
| rhaphidecursinol A (11) | 7.2 (D6); 4.2 (W2) | 28.7 (KB) | ||||
| rhaphidecursinol B (12) | 12.9 (D6); 11.2 (W2) | 23.9 (KB) | ||||
| grandisin (13) | 3.5 (D6); 3.4 (W2) | 32.4 (KB) | ||||
| epigrandisin (14) | >23 (D6); 7.7 (W2) | 37.0 (KB) | ||||
| decursivine (15) | 11.2 (D6); 12.6 (W2) | – | [22,23] | |||
| Roridin E (3) | 0.0004 (D6); 0.001 (W2) | 0.0004 (KB) | [24] | |||
| Asclepiadaceae | Gongronema napalense | EtOH | gongroneside A (16) | 1.6 (D6); 1.4 (W2) | >13.7 (KB) | [25] |
| Asteraceae | Achillea millefolium | MeOH | apigenin 7-O-glucoside (17) | 25.3 (D10); 15.3 (W2) | – | [34] |
| luteolin 7-O-glucoside (18) | 61.1 (D10); 62.5 (W2) | – | ||||
| Carpesium divaricatum | MeOH | 2-isopropenyl-6-acetyl-8-methoxy-1,3-benzodioxin-4-one (19) | 2.3 (D10) | 63.2 (SK-OV-3) | [35] | |
| Microglossa pyrifolia | Petroleum ether-EtOAc (1:1, v/v) | E-phytol (20) | 8.5 (PoW); 11.5 (Dd2) | – | [36] | |
| 6E-geranylgeraniol-19-oic acid (21) | 12.9 (PoW); 15.6 (Dd2) | – | ||||
| Echinops hoehnelii | CH2Cl2 | 5-(penta-1,3-diynyl)-2-(3,4-dihydroxybut-1-ynyl)-thiophene (22) | 50.2% (100 mg/kg) | – | [37] | |
| 5-(penta-1,3-diynyl)-2-(3-chloro-4-acetoxy-but-1-yn)-thiophene (23) | 32.7% (100 mg/kg) | – | ||||
| Buxaceae | Buxus semperviren | MeOH | compound (24) | 0.5–3.0 (HB3) | 7.0 (Hela) | [38] |
| compound (25) | 0.5–3.0 (HB3) | >20 (Hela) | ||||
| 23-O-(trans)-feruloyl-23-hydroxybetulin (26) | 0.5–3.0 (HB3) | >20 (Hela) | ||||
| compound (27–31) | 0.5–3.0 (HB3) | >20 (Hela) | ||||
| Cecropiaceae | Cecropia pachystachya | EtOH | β-sitosterol (32) | >120 (W2) | – | [39] |
| tormentic acid (33) | 19.0–25.2 (W2) | – | ||||
| Chloranthaceae | Chloranthus. fortunei | EtOH | fortunilide A (34) | 0.005 (Dd2) | 8.8 (WI-38) | [40] |
| fortunilide B (35) | 0.02 (Dd2) | 3.1 (WI-38) | ||||
| fortunilide C (36) | 0.2 (Dd2) | - | ||||
| fortunilide D (37) | 0.03 (Dd2) | 0.5 (WI-38) | ||||
| fortunilide E (38) | 0.04 (Dd2) | >100 (WI-38) | ||||
| fortunilide F (39) | 5.3 (Dd2) | - | ||||
| fortunilide G (40) | 0.05 (Dd2) | 1.2 (WI-38) | ||||
| fortunilide H (41) | 0.2 (Dd2) | - | ||||
| fortunilide I (42) | 0.09 (Dd2) | - | ||||
| fortunilide J (43) | 9.9 (Dd2) | - | ||||
| fortunilide K (44) | 4.7 (Dd2) | - | ||||
| fortunilide L (45) | 0.1 (Dd2) | 15.5 (WI-38) | ||||
| sarglabolide I (46) | 4.6 (Dd2) | - | ||||
| sarglabolide J (47) | 0.007 (Dd2) | 4.0 (WI-38) | ||||
| shizukaol K (48) | 0.9 (Dd2) | - | ||||
| shizukaol I (49) | 0.1 (Dd2) | - | ||||
| shizukaol C (50) | 0.02 (Dd2) | 0.8 (WI-38) | ||||
| schizukaol M (51) | 0.10 (Dd2) | 4.5 (WI-38) | ||||
| chlorahololide D (53) | 0.01 (Dd2) | 0.2 (WI-38) | ||||
| C. multisachys | - | chloramultilide B (65) | 7.1 (Dd2) | - | ||
| C. serratus and C. spicatus | - | chlorajaponilide C (52) | 0.001 (Dd2) | 5.4 (WI-38) | ||
| shizukaol N (54) | 0.1 (Dd2) | 10.0 (WI-38) | ||||
| shizukaol E (58) | 1.8 (Dd2) | - | ||||
| shizukaol D (59) | 0.6 (Dd2) | - | ||||
| shizukaol F (60) | 0.01 (Dd2) | 0.2 (WI-38) | ||||
| shizukaol G (61) | 0.01 (Dd2) | 1.7 (WI-38) | ||||
| shizukaol B (62) | 0.03 (Dd2) | 16.7 (WI-38) | ||||
| spicachlorantin D (63) | 0.5 (Dd2) | - | ||||
| shizukaol A (64) | 1.5 (Dd2) | - | ||||
| Sarcandra glabra | - | sarcandrolide B (55) | 0.27 (Dd2) | - | ||
| sarcandrolide A (56) | 0.3 (Dd2) | - | ||||
| sarcandrolide J (57) | 11.4 (Dd2) | - | ||||
| Chrysobalanceae | Parinari capensis | Petroleum ether and CH2Cl2 | 10,13-dihydroxy-9-methyl-15-oxo-20-norkaur-16-en-18-oic acid γ-lactone (66) | 1.7 (FCR-3) | 5.5 (Graham) | [41] |
| 10-hydroxy-13-methoxy-9-methyl-15-oxo-20-norkaur-16-en-18-oic acid γ-lactone (37) | 1.9 (FCR-3) | 3.2 (Graham) | ||||
| 10-hydroxy-9-methyl-15-oxo-20-norkaur-16-en-18-oic acid γ-lactone (68) | 5.0 (FCR-3) | 9.6 (Graham) | ||||
| Clusiaceae | Garcinia mckeaniana | Acetone | mckeanianones A (69) | 6.2 (TM4) | – | [42] |
| mckeanianones B (70) | 6.7 (TM4) | 12.9 (Vero) | ||||
| mckeanianones C (71) | 6.0 (TM4) | 29.5 (Vero) | ||||
| bannaxanthones I (72) | 8.5 (TM4) | – | ||||
| bannaxanthones E (73) | 8.3 (TM4) | – | ||||
| Connaraceae | Rourea minor (Gaertn.) Aubl. | CHCl3 | rourinoside (74) | 3.7 (D6); 2.1 (W2) | KB: ED50: >35.1 | [26] |
| rouremin (75) | 5.1 (D6); 4.5 (W2) | KB: ED50: >25.5 | ||||
| 1-(26-hydroxyhexacosanoyl)-glycerol (76) | 9.5 (D6); 12.7 (W2) | KB: ED50: >41.2 | ||||
| Cornaceae | Cornus florida L. | EtOH | ergosta-4,6,8,22-tetraene-3-one (77) | 61.0 (D10) | 27.0 (L6) | [43] |
| 3-epideoxyflindissol (78) | 128.0 (D10) | 14.7 (L6) | ||||
| 3β-O-cis-coumaroyl betulinic acid (79) | 10.4 (D10) | 5.6 (L6) | ||||
| 3β-O-trans-coumaroyl betulinic acid (80) | 15.3 (D10) | 9.3 (L6) | ||||
| Cucurbitaceae | Cogniauxia podolaena Baill. | CH2Cl2 | cucurbitacin B (81) | 2.9 (FcM29 strain) | 94% inhibition of KB at 1.8 μM | [44] |
| cucurbitacin D (82) | 7.8 (FcM29 strain) | 95% inhibition of KB at 1.9 μM | ||||
| 20-epibryonolic acid (83) | 4.4 (FcM29 strain) | 20% inhibition of KB at 2.2 μM | ||||
| Ebenaceae | Diospyros quaesita Thw. | CHCl3 | betulinic acid 3-caffeate (84) | 1.4 (D6); 1.0 (W2) | 4.0 (KB) | [27] |
| Euphorbiaceae | Jatropha isabelli | - | compound 85 | – | – | [45] |
| compound 86 | – | – | ||||
| Strophioblachia fimbricalyx | MeOH | 9-O-demethyltrigonostemone (87) | 8.7 (K1) | 2.6 (KB) | [46] | |
| 3,6,9-trimethoxyphenanthropolone (88) | 9.9 (K1) | 12.3 (KB) | ||||
| Fabaceae | Cajanus cajan L. | - | cajachalcone (89) | 7.4 (K1) | – | [47] |
| Piptadenia pervillei | EtOAc | (+)-catechin 5-gallate (70) | 1.2 (FcB1) | >75 (MRC-5) | [48] | |
| (+)-catechin 3-gallate (91) | 1.0 (FcB1) | >75 (MRC-5) | ||||
| Prosopis glandulosa var. glandulosa | EtOH | prosopilosidine (92) | 0.1 (D6); 0.3 (W2) | 20.2 (KB) | [49] | |
| isoprosopilosidine (93) | 0.1 (D6); 0.3 (W2) | 18.8 (KB) | ||||
| Fagaceae | Quercus laceyi | MeOH | kaempferol 3-O-glucosides (94–97) | 0.6–2.1 (HB3) | <3.0 (Hela) | [38] |
| Hypericaceae | Vismia orientalis | - | vismione D (98) | 2.4 (K1) | 10.0 (L6 cell) | [50] |
| Psorospermum glaberrimum | Hexane | 3-geranyloxyemodin anthrone (99) | 1.7 (W2) | – | [51] | |
| acetylvismione D (100) | 0.1 (W2) | – | ||||
| Lamiaceae | Ocimum sanctum | EtOAc | compound 101 | 0.1 (3D7) | – | [52] |
| Phlomis brunneogaleata | MeOH | luteolin 7-O-β-d-glucopyranoside (102) | 5.4 (K1) | >200 | [53] | |
| chrysoeriol 7-O-β-d-glucopyranoside (103) | 12.7 (K1) | >194 | ||||
| Salvia radula | MeOH:CHCl3 = 1:1 | betulafolientriol oxide (104) | 10.4 (FCR-3) | – | [54] | |
| salvigenin (105) | 75.0 (FCR-3) | 207 (MCF-7) | ||||
| Loganiaceae | Strychnos icaja | EtOAc-EtOH-NH4OH (96:3:1) | 15-hydroxyvomicine (106) | 101.0 (W2) | – | [55] |
| N-methyl-sec-iso-pseudostrychnine (107) | 110.6 (W2) | – | ||||
| Lythraceae | Ammannia multiflora, A. baccifera | MeOH | 4-hydroxy-α-tetralone (108) | 194.0 (NF-54) | – | [56] |
| tetralone-4-O-β-d-glucopyranoside (109) | 124.0 (NF-54) | – | ||||
| ammaniol (110) | 88.3 (NF-54) | – | ||||
| Malvaceae | Thespesia danis. | Acetone–water (7:3) | (R)-(−)-gossypol (111) | 4.5 (3D7) | – | [57] |
| Monimiaceae | Doryphora sassafras | CH2Cl2/MeOH | 1-(4-hydroxybenzyl)-6,7-methylenedioxy-2-methylisoquinolinium trifluoroacetate (112) | 3.0 (3D7); 4.4 (Dd2) | 120.0 (HEK293) | [58] |
| Glossocalyx brevipes Benth. | CHCl3/MeOH (1/1) | methyl 2-(1′β-geranyl-5′β-hydroxy-2′-oxocyclohex-3′-enyl) acetate (113) | 2.2 (D6); 6.6 (W2) | – | [59] | |
| 2-(1′β-geranyl-5′β-hydroxy-2′-oxocyclohex-3′-enyl) acetic acid (114) | 4.8 (D6); 8.3 (W2) | – | ||||
| Moraceae | Ficus fistulosa | - | verrucarin L acetate (4) | 0.001 (D6); 0.001 (W2) | 0.2 (KB) | [24] |
| F. septica | MeOH | dehydrotylophorine (115) | 0.4 (3D7) | 8.2 (L929) | [60] | |
| dehydroantofine (116) | 0.03 (3D7) | >55 (L929) | ||||
| tylophoridicine D (117) | 0.06 (3D7) | >56 (L929) | ||||
| Myristicaceae | Knema glauca | EtOAc | malabaricone A (118) | 8.5 (K1) | >61 (KB); 55.4 (NCI-H187) | [61] |
| Piperaceae | Piper sarmentosum | Hexane-MeOH | sarmentine (119) | 85.5 (K1) | – | [62] |
| 1-piperettyl pyrrolidine (120) | 21.9 (K1) | – | ||||
| P. tricuspe | Petroleum ether | dictyochromenol (121) | 9.6 (FcB1) | 7.7 (L-6) | [63] | |
| 3-farnesyl-p-hydroxy benzoic acid (122) | 29.8 (FcB1) | 40.9 (L-6) | ||||
| 2′E,6′E 2-farnesyl hydroquinone (123) | 1.4 (FcB1) | 1.1 (L-6) | ||||
| Platanaceae | Platanus occidentalis | MeOH | kaempferol 3-O-rhamnosides (124–127) | 0.5–1.8 (HB3) | 9.3–20.0 (Hela) | [38] |
| Rubiaceae | Nauclea orientalis | MeOH | naucleaorine (128) | 6.9 (D6); 8.0 (W2) | 38.0 (KB) | [28] |
| epimethoxynaucleaorine (129) | 12.4 (D6); 13.2 (W2) | >37.9 (KB) | ||||
| 3α,23-dihydroxyurs-12-en-28-oic acid (130) | 9.7 (D6); 12.7 (W2) | >42.2 (KB) | ||||
| oleanolic acid (131) | 4.6 (D6); 5.1 (W2) | 46.0 (KB) | ||||
| Rutaceae | Citropsis articulata | MeOH | 5-hydroxynoracronycine (132) | 2.8 (FcB1) | 28.8 (Vero) | [64] |
| 1,5-dihydroxy-2,3-dimethoxy-10-methyl-9-acridone (133) | 10.0 (FcB1) | 101 (Vero) | ||||
| Zanthoxylum chiloperone var. angustifolium Engl. | CH2Cl2 | trans-avicennol (134) | 7.8 (K1); 1.5 (F32); 3.5 (PFB); 6.4 (FcB1) | 12.8 (MCR5) | ||
| canthin-6-one (135) | 24.1 (K1); 9.1 (F32); 14.6 (PFB); 18.2 (FcB1) | 42.7 (MCR5) | [65] | |||
| 5-methoxycanthin-6-one (136) | 20.4 (K1); 41.6 (F32) | – | ||||
| Simaroubaceae | Eurycoma longifolia | CH2Cl2 | eurycomanone (137) | 0.06 (D6); 0.04 (W2) | 0.02 (A-549); <0.006 (MCF-7) | [66,67] |
| pasakbumin B (138) | 0.08 (D6); 0.05 (W2) | 0.02 (A-549); <0.006 (MCF-7) | ||||
| Picrolemma sprucei | Hexane/H2O | neosergeolide (139) | 0.002 (K1) | – | [68] | |
| Apocynaceae | Aspidosperma vargasii | EtOH | ellipticine (140) | 0.07 (K1) | – | |
| A. desmanthum | EtOH | aspidocarpine (141) | 0.02 (K1) | – | ||
| Piperaceae | Pothomorphe peltata | CHCl3/EtOH | 4-nerolidylcatechol (142) | 0.7 (K1) | – | |
| Theaceae | Camellia sinensis | mefloquine (143) | – | – | [69] | |
| gallocatecin (144) | – | – | ||||
| Tiliaceae | Grewia bilamellata | MeOH | 3α,20-lupandiol (145) | 19.8 (D6); 19.1 (W2) | >90 (KB) | [29] |
| grewin (146) | 11.2 (D6); 5.5 (W2) | >107.5 (KB) | ||||
| nitidanin (147) | 21.2 (D6); 18.4 (W2) | >90 (KB) | ||||
| 2α,3β-dihydroxyolean-12-en-28-oic acid (148) | 21.1 (D6); 8.6 (W2) | 51.5 (KB) | ||||
| 2,6-dimethoxy-1-acetonylquinol (149) | 42.2 (D6); 23.0 (W2) | 169 (KB) | ||||
| Verbenaceae | Lippia javanica | EtOAc (aerial parts) | lippialactone (150) | 23.8 (D10) | – | [70] |
| Family | Ethnologic Plant | Country | Plant Part | Antiplasmodial Activity (IC50) (μg/mL, Unless Indicated) a (P. falciparum) | Cytotoxicity (CC50 for Cells, LD50 for Brine Shrimp) (μg/mL, Unless Indicated) b,c (Cell Line) | References |
|---|---|---|---|---|---|---|
| Acanthaceae | Justilia schimperand (Hochst ex Nees) T. Alnder | Roots | – | – | [71] | |
| Anacardiaceae | Mangifera indica L. | Africa | Leaves | % parasitaemia reduced from 8.9 at 60 mg/kg to 7.2 at 240 mg/kg (mice) | 208.3 mg/kg (mice) | [72] |
| Nigeria | Leaves | – | 3079.1 (brine shrimp) | [73] | ||
| Nigeria | Stem barks | – | 2456.0 (brine shrimp) | [73,74] | ||
| Pseudoprotorhus longifolius H. Perr. | Madagascar | Leaves | – | – | [75] | |
| Rhus taratana (Bak.) H. Perr. | Madagascar | Leaves | – | – | [75] | |
| Sclerocarya birrea (A. Rich) Hochst. | South Africa | Stem-bark (MeOH) | 5.91 (D6) | – | [76] | |
| S. caffra Sond. | Madagascar | Leaves | – | – | [75] | |
| Annonaceae | Annona senegalensis Rolyns &Gh | Nigeria | Leaves | – | 6811.0 (brine shrimp) | [73] |
| Enantia chlorantha Oliv. | Nigeria | Stem barks | – | 214.3 (brine shrimp) | [73,74] | |
| Apocynaceae | Alstonia boonei DeWild | Nigeria | Leaves; stem barks | % parasitaemia reduced from 19.4% (negative control) to 5.5% at 240 mg/kg (mice) | 78.77 mg/kg (mice) | [72,74] |
| Aspidosperma cylindrocarpon Müll. Arg. | Brazil | Trunk woods (EtOH) | 44.0 (W2); 39.0 (3D7) | >500 (Vero) | [7] | |
| A. parvifolium A. DC. | Brazil | Trunk barks (EtOH) | 32.8 (W2); 20.5 (3D7) | >500 (Vero) | [7] | |
| A. olivaceum Müll. Arg. | Brazil | Leaves (CH2Cl2) | 7.0 (W2); 25.5 (3D7) | >500 (Vero) | [7] | |
| Leaves (EtOH) | 7.0 (W2); 5.0 (3D7) | – | ||||
| Trunk wood (CH2Cl2) | <6 (W2); <6 (3D7) | >500 (Vero) | ||||
| Trunk bark (CH2Cl2) | <6 (W2); <6 (3D7) | – | ||||
| Trunk bark (EtOH) | 5.0 (W2); 7.0 (3D7) | >500 (Vero) | ||||
| A. ramiflorum Müll. Arg. | Brazil | Leaves (EtOH) | 32.8 (W2); 20.5 (3D7) | – | [7] | |
| Leaves (CH2Cl2) | <6 (W2); <6 (3D7) | – | ||||
| Trunk woods (EtOH) | 36.5 (W2); 48.0 (3D7) | – | ||||
| Trunk woods (CH2Cl2) | 9.5 (3D7) | >500 (Vero) | ||||
| Trunk woods (EtOH) | 19.8 (W2); 1.0 (3D7) | – | ||||
| Trunk barks (CH2Cl2) | <6 (W2); <6 (3D7) | >500 (Vero)) | ||||
| A. spruceanum Benth. ex Müll. Arg. | Brazil | Leaves (EtOH) | 65.0 (W2); >100 (3D7) | – | [7] | |
| Leaves (CH2Cl2) | 23.25 (W2); 47.0 (3D7) | – | ||||
| Trunk woods (EtOH) | 29.5 (W2); 41.5 (3D7) | – | ||||
| Trunk woods (CH2Cl2) | <6 (W2); <6 (3D7) | 109.6 (Vero)) | ||||
| Trunk woods (CHCl3) | 37.0 (W2); >100 (3D7) | – | ||||
| Trunk barks (EtOH) | 26.3 (W2); 14.0 (3D7) | – | ||||
| Trunk barks (CH2Cl2) | <6 (W2); <6 (3D7) | – | ||||
| Trunk barks (EtOH) | 28.0 (W2); 19.0 (3D7) | – | ||||
| A. tomentosum Mart. | Brazil | Trunk woods (EtOH) | 26.5 (W2); 25.0 (3D7) | – | [7] | |
| Leaves (EtOH) | 23.8 (W2); 27.0 (3D7) | – | ||||
| Fruits (EtOH) | 20.5 (W2); 38.6 (3D7) | – | ||||
| Seeds (EtOH) | 24.5 (W2); 3.0 (3D7) | >500 (Vero)) | ||||
| Aristolochiaceae | Aristolochia acuminata Lamk. | Madagascar | Roots, stems, leaves | – | – | [75] |
| Asteraceae | Artemisia annua L. | China | Whole plants | – | – | [77] |
| Tithonia diversifolia A. Gray | Nigeria | Leaves | – | 2304 (brine shrimp) | [73] | |
| Vernonia amygdalina Del. | Leaves | – | – | [71] | ||
| Avicenniaceae | Avicennia marina (Forsk) Vierh. | Madagascar | Aerial parts | – | – | [78] |
| A. basilicum L. | Madagascar | Aerial parts | – | – | [75] | |
| Bignoniaceae | Fernandoa sp. | Madagascar | Aerial parts | – | – | [75] |
| Kigelianthe madagascariensis Sprague var. hidebrandtii | Madagascar | Leaves | – | – | [75] | |
| Brassicaceae | Brassica nigra (L.) Koch. | Seeds | – | – | [71] | |
| Caricaceae | Carica papaya L. | Leaves, fruits, roots | – | [71,79] | ||
| Celastraceae | Maytenus acuminata (L.f.) Loes | Kenya | leaves, root barks | 36.6–41.5% | – | [80] |
| Combretaceae | Combretu raimbaulti Heckel | Madagascar | Leaves | – | [75] | |
| Terminalia catappa | Nigeria | Leaves (EtOAc) | 3.1 (K1) | 159.9 μg/L (L6) | [81] | |
| T. latifolia Engl. | Nigeria | leaves | – | 272.9 (brine shrimp) | [73] | |
| Commelinaceae | Commelina benghalensis L. | Madagascar | Aerial parts | – | – | [75] |
| Compositae | Brachylaena ramiflora (DC.) H. Humb | Madagascar | Aerial parts | – | – | [75] |
| Conyza aegytiaca Ait. Var lineariloba | Madagascar | Aerial parts | – | – | [75] | |
| Inula perrieri H. Humb. | Madagascar | Leaves | – | – | [75] | |
| Parthenium hysterophorus L. | Madagascar | Aerial parts | – | – | [75] | |
| Senecio ompricaefolius (ex DC.) H. Humb. | Madagascar | Aerial parts | – | – | [75] | |
| Stenocline inuloides DC. | Madagascar | Leaves | – | – | [75] | |
| Tagetes erecta L. | Madagascar | Leaves | – | – | [75] | |
| T. patula L. | Madagascar | Leaves | – | – | [75] | |
| Vernonia lasiopus O. Hoffm. | Kenya | Root barks | – | – | [75] | |
| V. pectoralis Bak. | Madagascar | Aerial parts | – | – | [75] | |
| V. trichodesma Bak. | Madagascar | Leaves | – | – | [75] | |
| V. chapelieri Drak. | Madagascar | Aerial parts | – | – | [75] | |
| V. sp. (Dr. Hely) | Madagascar | Aerial parts | – | – | [75] | |
| V. ampandrandavensis Bak. | Madagascar | Aerial parts | – | – | [75] | |
| Cucurbitaceae | Momordica charantia L. | Madagascar | Aerial parts | – | – | [75] |
| Zehneria scabra (Lf.) Sond. | Roots | – | – | [71] | ||
| Euphorbiaceae | Bridelia micrantha Benth. | Nigeria | Leaves | – | >90,000 (brine shrimp) | [73] |
| Croton goudoti H. Bn. | Madagascar | Leaves | – | – | [75] | |
| C. macrostachyus Hochst. Ex Del. | Leaves/barks/roots | – | – | [71] | ||
| Euphorbia hirta | Nigeria | Whole plants (Hexane) | 4.3 (K1) | 14.2 (L6) | [81,82] | |
| Flueggea microcarpa Blume | Madagascar | Aerial parts | – | [75] | ||
| Jatropha curcas L. | Nigeria | Leaves (EtOAc) | 2.4 (K1) | 126.5 (L6) | [75,81,82] | |
| Madagascar | leaves, roots | |||||
| Manihot utilisma Pohl. | Madagascar | Leaves | – | – | [75] | |
| Phyllanthus amarus Schum. & Thonn. | Brazil, Cuba, Haiti, Nigeria, Elsewhere | Whole plants (MeOH) | 5.0 (3D7) | – | [83,84] | |
| Whole plants (CH2Cl2) | 14.5 (3D7) | – | ||||
| India | Whole plants | – | – | [85] | ||
| Nigeria | Leaves (EtOAc) | 5.6 (K1) | 77.7 (L6) | [81,82] | ||
| Ghana | Whole plants | – | – | [85] | ||
| West Africa | Aerial parts | – | – | |||
| Phyllanthus sp. | Madagascar | Aerial parts | – | – | [75] | |
| Fabaceae | Acacia nilotica L. | Pakistan | Leaves (EtOH) | 1.3 (3D7) | – | [86] |
| Caesalpinia benthamiana | Guinea | Leaves (MeOH) | 4.0 (Ghana) | 32.0 (MRC-5) | [79] | |
| Cajanus cajan Mill sp. | Nigeria | Leaves | – | 988.5 (brine shrimp) | [73,74] | |
| Calliandra haematocephala Hassk | Nigeria | Roots | – | – | ||
| Calpurna ourea (Ait.) Benth | Leaves | – | – | [71] | ||
| Cassia siamea | Nigeria | Stem barks (EtOAc) | 2.70 (K1) | 988.5 (stem bark), 8232.2 (brine shrimp) | [73] | |
| leaves | – | |||||
| Piliostigma thonnigii Schum | Nigeria | Leaves | – | 7958.0 (brine shrimp) | [73] | |
| Flacourtiaceae | Homalium sp. | Madagascar | Aerial parts | – | – | [75] |
| Gramineae | Phragmites mauritianus Kunth | Madagascar | Aerial parts | – | – | [75] |
| Hydrengeaceae | Dichroa febrifuga | China | Roots | – | – | [87] |
| Icacinaceae | Cassinopsis madagascariensis (Baill.) H. Bn. | Madagascar | Leaves, stem barks | – | – | [75] |
| Lamiaceae | Hyptispectinata Poit. | Madagascar | Leaves | – | – | [75] |
| Ocimum canum Sims. | Nigeria | Leaves (EtOAc) | 1.8 (K1) | 60.1 (L6) | [75,81] | |
| Madagascar | Stems, seeds | – | ||||
| O. lamiifolium Hochst. ex Benth. | Leaves | – | – | [71] | ||
| Cassytha filiformis L. | Nigeria | Vines | – | – | [74] | |
| Cinnamomum camphora (L.) Sieb | Madagascar | Leaves | – | – | [75] | |
| Leguminosae | Abrus precatorius L. | Madagascar | Leaves | – | – | [75] |
| Albizzia lebbek Benth. | Madagascar | Aerial parts | – | – | [75] | |
| Caesalpinia bonducella Fleming | Madagascar | Seeds, roots | – | – | [75] | |
| Cassia occidentalis L. | Madagascar | Aerial parts | – | – | [75] | |
| Crotalaria spinosa Hochst. | Madagascar | Leaves | – | – | [75] | |
| Erythryna indica Lamk. | Madagascar | Aerial parts | – | – | [75] | |
| Piliostigma thonningii | Nigeria | Leaves (EtOAc) | 3.6 (K1) | 56.1 (L6) | [81] | |
| Pongamia pinnata L. | India | Barks (MeOH) | 11.7 (CQ-sensitive) | >200 (THP-1) | [88] | |
| Lilliaceae | Allium sativum L. | Bulbs | – | – | [71] | |
| Loganiaceae | Anthocleista amplexicaulus Bak. | Madagascar | Aerial parts | – | – | [75] |
| A. rhizophoroides Bak. | Madagascar | Roots, leaves | – | – | [75] | |
| Strychnos mostuoides Leeuwenberg | Madagascar | Aerial parts | – | – | [75] | |
| Malvaceae | Gossypium arboreum L. | Nigeria | Leaves | – | 94.1 (brine shrimp) | [73] |
| G. barbadense L. | Nigeria | Leaves | – | 3585.0 (brine shrimp) | [73] | |
| G. hirsitum L. | Nigeria | Leaves | – | 257.2 (brine shrimp) | [73] | |
| Meliaceae | Azadirachta indica A. Juss | Africa | leaves | The percentage parasitaemia reduced from 15.7 % to 4.8 % at 240 mg/kg (in vivo) | 140.0 mg/kg (mice) | [72] |
| Swietenia macrophylla King | Indonesia | Seeds | – | – | [89] | |
| Barks | 78% inhibition at 100 (Indo) | – | [90] | |||
| Melianthaceae | Bersama abyssinica Fresen. | Leaves, root barks and stems | – | – | [71] | |
| Menispermaceae | Burasaia australis Sc. Elliot | Madagascar | Root barks | – | – | [75] |
| B. congesta Decne | Madagascar | Root barks | – | – | [75] | |
| B. gracilis Decne | Madagascar | Root barks | – | – | [75] | |
| Burasaia madagascariensis Thou. | Madagascar | Root barks | – | – | [75] | |
| B. nigrescens R. Cap. | Madagascar | Root barks | – | – | [75] | |
| Chasmanthera uviformis Baill. | Madagascar | Stem barks | – | – | [75] | |
| Cissampelos pareira L. | Madagascar | Roots | – | – | [75] | |
| C. madagascariensis (Baill.) Diels. | Madagascar | Roots | – | – | [75] | |
| Spirospermum penduliflorum Thou. | Madagascar | Roots, stem barks | – | – | [75] | |
| Strychnopsis thouarsii Baill. | Madagascar | Leaves, root barks | – | – | [75] | |
| Triclisia macrocarpa (Baill.) Diels | Madagascar | Root barks, stem barks | – | – | [71] | |
| Mimosaceae | Acacia catechu (L.f.) Willd. | Leaves | – | – | [71] | |
| Moraceae | Ficus elastica Roxb. ex Hornem. | Cameroon | Roots (MeOH) | 9.5 | – | [91] |
| F. sur Forssk. | Kenya | Leaves, stem barks, root barks | 34.1–48.4% Inhibition | – | [80] | |
| F. thonningii Blume | Nigeria | Leaves (Hexane) | 2.7 (NF54); 10.4 (K1) | >20 (KB) | [90] | |
| Myrtaceae | Psidium guajava L. | Nigeria | Stem barks | – | 707.2 (brine shrimp) | [72] |
| Ochnaceae | Lophira alata Banks | Nigeria | Leaves (Hexane) | 2.5 (NF54); 2.5 (K1) | >20 (KB) | [90] |
| Papilionaceae | Pericopsis elata Harms | Nigeria | leaves | – | 601.8 (brine shrimp) | [73] |
| Pterocarpus osun Craib | Nigeria | Stem barks | – | – | [74] | |
| Periplocaceae | Cryptolepts sanguinolenta | West Africa | Roots | – | 13.9 (MCF7) | [92] |
| Parquetina nigrescens (Afz.) Bullock | Nigeria | Root barks | – | – | [74] | |
| Phytolaccacaa | Phytolacca dodecandra L’Hér. | Leaves | – | – | [71] | |
| Polygonaceae | Rumex abyssinicus Jacq. | Leaves and stems | – | – | [71] | |
| Potamogetonaceae | Potamogeton javanicus Hass Karl | Madagascar | Aerial parts | – | – | [75] |
| Ranunculaceae | Clematis mauritiana Lamk. Var. normalis | Madagascar | Aerial parts | – | – | [75] |
| Rhamnaceae | Rhamnus prinoides L’ H′erit | Kenya | Leaves, root barks | 34.1–43.9% Inhibition | – | [80] |
| R. staddo A. Rich. | Kenya | Root barks | 11.1% Inhibition | – | [80] | |
| Rubiaceae | Anthospermum emirnense Bak. | Madagascar | Aerial parts | – | – | [75] |
| Cinchona ledgeriana Muens | Madagascar | Stem barks | – | – | [75] | |
| C. offlcinalis L. | Madagascar | Stem barks | – | – | [75] | |
| C. succirubra Pavon et Kiutzsch | Madagascar | Stem barks | – | – | [75] | |
| Cephalanthus spathelliferus Bak. | Madagascar | Leaves | – | – | [75] | |
| Danais fragrans Gaertn. | Madagascar | Roots | – | – | [75] | |
| D. gerrardii Bak. | Madagascar | Roots | – | – | [75] | |
| D. verticillata Bak. | Madagascar | Roots | – | – | [75] | |
| D. breviflora Bak. | Madagascar | Roots | – | – | [75] | |
| D. cernua Bak. | Madagascar | Roots | – | – | [75] | |
| Hymenodyction lohavato baill. | Madagascar | Root barks, stem barks | – | – | [75] | |
| Morinda lucida Benth | Africa | Leaves | The percentage parasitaemia reduced from 14.0 % to 5.8 % at 240 mg/kg (in vivo) | 134.5 mg/kg (mice) | [72] | |
| Nigeria | Stem barks | P. berghei | 2.6 (brine shrimp) | [73] | ||
| Nigeria | Leaves | – | 383.9 (brine shrimp) | [73] | ||
| Nauclea latifolia S.M. | Nigeria | Stem barks | – | 9368.0 (brine shrimp) | [73] | |
| Saldinia sp. (andriambavifoy) | Madagascar | Aerial part | – | – | [75] | |
| Sarcocephalus latifolius (J. E. Smith) E. A. Bruce | Nigeria | Root barks | – | – | [74] | |
| Schismatoclada concinna Bak. | Madagascar | Root barks | – | – | [75] | |
| S. farahimpensis Bak. | Madagascar | Root barks | – | – | [75] | |
| S. viburnoides Bak. | Madagascar | Root barks | – | – | [75] | |
| Citropsis articulata (Willd. ex Spreng.) Swingle & Kellerman | Uganda | Roots | 77% inhibition at 10 (FcB1) | 12% inhibition at 10 (Vero) | [64] | |
| Demethylsuberosin | 16.7 | >50% inhibition at 16.7 (Vero) | ||||
| 5-hydroxynoracronycine | 0.9 | 9.3% inhibition at 0.9 (Vero) | ||||
| 1,5-dihydroxy-2,3-dimethoxy-10-methyl-9-acridone | 3.0 | 30.5% inhibition at 3.0 (Vero) | ||||
| 7α-obacunyl acetate | 9.3 | >50% inhibition at 9.3 (Vero) | ||||
| Rutaceae | Evodia fatraina H. Perr | Madagascar | Root barks, stem barks | – | – | [75] |
| Toddalia asiatica (L.) Lam. | Kenya; Madagascar | Root barks; root barks, stem barks | – | – | [75,80] | |
| Zanthoxylum tsihanimpotsa H. Perr. | Madagascar | Stem barks | – | – | [75] | |
| Santalaceae | Okoubaka aubrevillei Phelleg & Nomand | Nigeria | Stem barks | – | – | [74] |
| Sapindaceae | Dodonaea viscosa Jacq. | Madagascar | Leaves | – | – | [75] |
| D. madagascariensis Rdlk. | Madagascar | Leaves | – | – | [75] | |
| Selaginellaceae | Salaginella vogelli | Cameroon | Leaves (MeOH) | 32.2 | – | [91] |
| Schizaeaceae | Mohria caffrorum (L.) Desv. | Madagascar | Aerial parts | – | – | [75] |
| Simaroubaceae | Brucea antidysenterica J.F. Mill. | Stems, barks seeds | – | – | [71] | |
| Ulmaceae | Trema commersonii Boj. | Madagascar | Aerial part | – | – | [75] |
| T. orientalis Blume | Madagascar | Root barks | 2.0 (K1) | 32.5 (L6) | [75] | |
| Verbanaceae | Lippia multiflora Moldenke | Nigeria | Aerial part | – | 1.1 (brine shrimp) | [73] |
| Clerodendrum myricoides (Hochst.) Vatke | Kenya | Root barks | 9.8% (Plasmodium berghei NK65) | – | [71,80] | |
| Vitex doniana | Nigeria | Leaves (Hexane) | 3.6 (K1) | 431.4 | [81,82] | |
| Stem barks (Hexane) | 6.8 (K1) | ND | [81] | |||
| Zingiberaceae | Curcuma longa L. | Madagascar | Leaves | – | – | [75] |
| Zingiber officinale Roscoe | Rhizome | – | – | [71] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, W.-H.; Xu, X.-Y.; Shi, N.; Tsang, S.W.; Zhang, H.-J. Antimalarial Activity of Plant Metabolites. Int. J. Mol. Sci. 2018, 19, 1382. https://doi.org/10.3390/ijms19051382
Pan W-H, Xu X-Y, Shi N, Tsang SW, Zhang H-J. Antimalarial Activity of Plant Metabolites. International Journal of Molecular Sciences. 2018; 19(5):1382. https://doi.org/10.3390/ijms19051382
Chicago/Turabian StylePan, Wen-Hui, Xin-Ya Xu, Ni Shi, Siu Wai Tsang, and Hong-Jie Zhang. 2018. "Antimalarial Activity of Plant Metabolites" International Journal of Molecular Sciences 19, no. 5: 1382. https://doi.org/10.3390/ijms19051382
APA StylePan, W.-H., Xu, X.-Y., Shi, N., Tsang, S. W., & Zhang, H.-J. (2018). Antimalarial Activity of Plant Metabolites. International Journal of Molecular Sciences, 19(5), 1382. https://doi.org/10.3390/ijms19051382