Effect of Temperature Changes on Serum Protein Adsorption on Thermoresponsive Cell-Culture Surfaces Monitored by A Quartz Crystal Microbalance with Dissipation
Abstract
:1. Introduction
2. Results
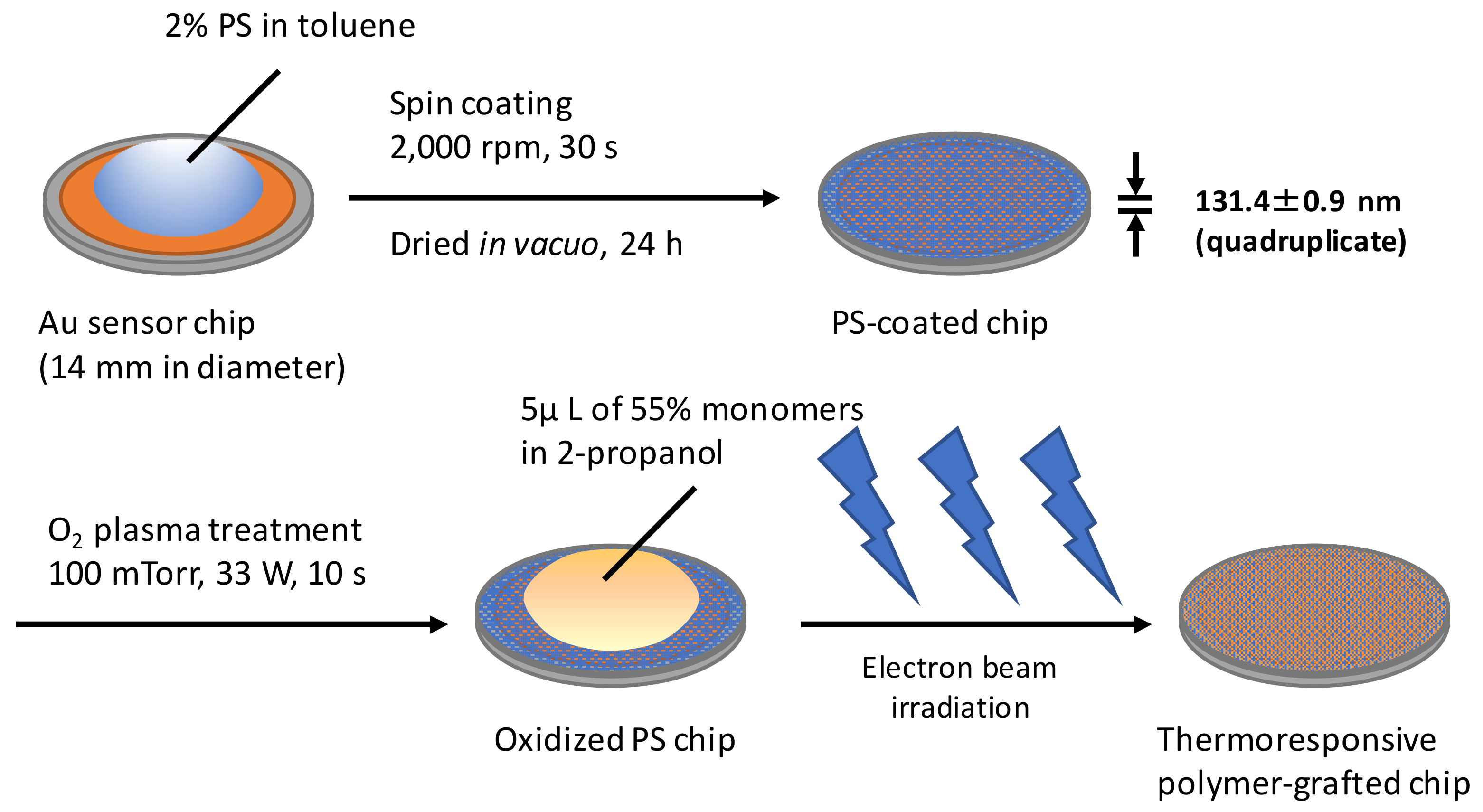
2.1. Preparation of Thermoresponsive Polymer-Grafted PS (Polystyrene) Surfaces on Sensor Chips
2.2. Temperature-Dependent Changes in Δf and ΔD Values on PIPAAm-Grafted Surfaces
2.3. Estimation of Adsorbed Serum Proteins by QCM-D Measurements Using the Voigt Model and Sauerbrey Equation
2.4. Effect of Temperature on the Adsorption of Serum Proteins on Thermoresponsive Polymer-Grafted Surfaces
2.5. Dynamic Monitoring of Adsorbed Serum Proteins on Thermoresponsive Polymer-Grafted Surfaces during Stepwise Temperature Changes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Thermoresponsive Polymer-Grafted PS Surfaces on Sensor Chips
4.3. Heparin Immobilization on Poly(IPAAm-co-CIPAAm)-Grafted Sensor Chips
4.4. Surface Analyses
4.5. Monitoring Temperature-Dependent Changes in Frequency and Dissipation Using the QCM-D System
4.6. Monitoring Serum Protein Adsorption Using the QCM-D System
4.7. Estimation of The Thickness and Mass of The Adsorbed Serum Proteins
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| QCM-D | Quartz crystal microbalance with dissipation |
| PIPAAm | Poly(N-isoprolylacrylamide)-grafted surface |
| Hparin-IC1 | Heparin-immobilized poly(N-isoprolylacrylamide-co-2-carboxyisopropylacrylamide)-grafted surface |
| PS | Polystyrene |
| ECM | Extracellular matrix |
| FN | Fibronectin |
| BSA | Bovine serum albumin |
| XPS | X-ray photoelectron spectroscopy |
References
- Nagase, K.; Kobayashi, J.; Okano, T. Temperature-responsive intelligent interfaces for biomolecular separation and cell sheet engineering. J. R. Soc. Interface 2009, 6, S293–S309. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Yamato, M.; Okano, T. On-off affinity binding modulation on thermoresponsive polymer-grafted surfaces for capture and release of proteins and cells. J. Biomater. Sci. Polym. Ed. 2017, 28, 939–957. [Google Scholar] [CrossRef] [PubMed]
- Heskins, M.; Guillet, J.E. Solution properties of poly(N-isopropylacrylamide). J. Macromol. Sci. Part A Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Makromol. Chem. Rapid Commun. 1990, 11, 571–576. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Sakai, H.; Sakurai, Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J. Biomed. Mater. Res. 1993, 27, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Kushida, A.; Yamato, M.; Konno, C.; Kikuchi, A.; Sakurai, Y.; Okano, T. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J. Biomed. Mater. Res. 1999, 45, 355–362. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Shimizu, T.; Sasagawa, T.; Sekine, H.; Sakaguchi, K.; Kikuchi, T.; Sekine, W.; Sekiya, S.; Yamato, M.; Umezu, M.; et al. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat. Protoc. 2012, 7, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Sawa, Y.; Yoshikawa, Y.; Toda, K.; Fukushima, S.; Yamazaki, K.; Ono, M.; Sakata, Y.; Hagiwara, N.; Kinugawa, K.; Miyagawa, S. Safety and efficacy of autologous skeletal myoblast sheets (TCD-51073) for the treatment of severe chronic heart failure due to ischemic heart disease. Circ. J. 2015, 79, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Ohki, T.; Yamato, M.; Ota, M.; Takagi, R.; Murakami, D.; Kondo, M.; Sasaki, R.; Namiki, H.; Okano, T.; Yamamoto, M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 2012, 143, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Yamato, M.; Washio, K.; Ando, T.; Okano, T.; Ishikawa, I. Cell sheets for periodontal tissue engineering. Curr. Oral Health Rep. 2015, 2, 252–256. [Google Scholar] [CrossRef]
- Kanzaki, M.; Takagi, R.; Washio, K.; Kokubo, M.; Yamato, M. Bio-artificial pleura using an autologous dermal fibroblast sheet. NPJ Regen. Med. 2017, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Yamato, M.; Morino, T.; Sugiyama, H.; Takagi, R.; Yaguchi, Y.; Okano, T.; Kojima, H. Middle ear mucosal regeneration by tissue-engineered cell sheet transplantation. NPJ Regen. Med. 2017, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Yamada, N.; Okuhara, M.; Sakai, H.; Sakurai, Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials 1995, 16, 297–303. [Google Scholar] [CrossRef]
- Yamato, M.; Okuhara, M.; Karikusa, F.; Kikuchi, A.; Sakurai, Y.; Okano, T. Signal transduction and cytoskeletal reorganization are required for cell detachment from cell culture surfaces grafted with a temperature-responsive polymer. J. Biomed. Mater. Res. 1999, 44, 44–52. [Google Scholar] [CrossRef]
- Yamato, M.; Konno, C.; Kushida, A.; Hirose, M.; Mika, U.; Kikuchi, A.; Okano, T. Release of adsorbed fibronectin from temperature-responsive culture surfaces requires cellular activity. Biomaterials 2000, 21, 981–986. [Google Scholar] [CrossRef]
- Arisaka, Y.; Kobayashi, J.; Yamato, M.; Akiyama, Y.; Okano, T. Switching of cell growth/detachment on heparin-functionalized thermoresponsive surface for rapid cell sheet fabrication and manipulation. Biomaterials 2013, 34, 4214–4222. [Google Scholar] [CrossRef] [PubMed]
- Arisaka, Y.; Kobayashi, J.; Ohashi, K.; Tatsumi, K.; Kim, K.; Akiyama, Y.; Yamato, M.; Okano, T. A heparin-modified thermoresponsive surface with heparin-binding epidermal growth factor-like growth factor for maintaining hepatic functions in vitro and harvesting hepatocyte sheets. Regen. Ther. 2016, 3, 97–106. [Google Scholar] [CrossRef]
- Tang, Z.; Akiyama, Y.; Yamato, M.; Okano, T. Comb-type grafted poly(N-isopropylacrylamide) gel modified surfaces for rapid detachment of cell sheet. Biomaterials 2010, 31, 7435–7443. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Akiyama, Y.; Itoga, K.; Kobayashi, J.; Yamato, M.; Okano, T. Shear stress-dependent cell detachment from temperature-responsive cell culture surfaces in a microfluidic device. Biomaterials 2012, 33, 7405–7411. [Google Scholar] [CrossRef] [PubMed]
- Kumashiro, Y.; Fukumori, K.; Takahashi, H.; Nakayama, M.; Akiyama, Y.; Yamato, M.; Okano, T. Modulation of cell adhesion and detachment on thermo-responsive polymeric surfaces through the observation of surface dynamics. Colloids Surf. B Biointerfaces 2013, 106, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Z. Study on conformation change of thermally sensitive linear grafted poly(N-isopropylacrylamide) chains by quartz crystal microbalance. Macromolecules 2004, 37, 6553–6557. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, G. Collapse and swelling of thermally sensitive poly(N-isopropylacrylamide) brushes monitored with a quartz crystal microbalance. J. Phys. Chem. B 2005, 109, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Vashist, P. Recent advances in quartz crystal microbalance-based sensors. J. Sens. 2011, 2011, 571405. [Google Scholar] [CrossRef]
- Bohanon, T.; Elender, G.; Knoll, W.; Köberle, P.; Lee, J.-S.; Offenhäusser, A.; Ringsdorf, H.; Sackmann, E.; Simon, J.; Tovar, G.; et al. Neural cell pattern formation on glass and oxidized silicon surfaces modified with poly(N-isopropylacrylamide). J. Biomater. Sci. Polym. Ed. 1997, 8, 19–39. [Google Scholar] [CrossRef]
- Balamurugan, S.; Mendez, S.; Balamurugan, S.S.; O’Brien, M.J., 2nd; Lopez, G.P. Thermal response of poly(N-isopropylacrylamide) brushes probed by surface plasmon resonance. Langmuir 2003, 19, 2545–2549. [Google Scholar] [CrossRef] [PubMed]
- Okada, F.; Akiyama, Y.; Kobayashi, J.; Ninomiya, H.; Kanazawa, H.; Yamato, M.; Okano, T. Measurement of the dynamic behavior of thin poly(N-isopropylacrylamide) hydrogels and their phase transition temperatures measured using reflectometric interference spectroscopy. J. Nanopart. Res. 2015, 17, 148. [Google Scholar] [CrossRef]
- Fukumori, K.; Akiyama, Y.; Kumashiro, Y.; Kobayashi, J.; Yamato, M.; Sakai, K.; Okano, T. Characterization of ultra-thin temperature-responsive polymer layer and its polymer thickness dependency on cell attachment/detachment properties. Macromol. Biosci. 2010, 10, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A.L.; Gambillara, V.; Grainger, D.W. Correlating fibronectin adsorption with endothelial cell adhesion and signaling on polymer substrates. J. Biomed. Mater. Res. Part A 2003, 64, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Salez, T.; McGraw, J.D.; Dalnoki-Veress, K.; Raphaël, E.; Forrest, J.A. Glass transition at interfaces. Europhys. News 2017, 48, 24–28. [Google Scholar] [CrossRef]
- Satomi, N.; Tanaka, K.; Takahara, A.; Kajiyama, T. Effect of internal bulk phase on surface viscoelastic properties by scanning probe microscopy. Macromolecules 2001, 34, 6420–6423. [Google Scholar] [CrossRef]
- Hall, D.B.; Underhill, P.; Torkelson, J.M. Spin coating of thin and ultrathin polymer films. Polym. Eng. Sci. 1998, 38, 2039–2045. [Google Scholar] [CrossRef]
- Annaka, M.; Yahiro, C.; Nagase, K.; Kikuchi, A.; Okano, T. Real-time observation of coil-to-globule transition in thermosensitive poly(N-isopropylacrylamide) brushes by quartz crystal microbalance. Polymer 2007, 48, 5713–5720. [Google Scholar] [CrossRef]
- Kanazawa, K.K.; Gordon, J.G. Frequency of a quartz microbalance in contact with liquid. Anal. Chem. 1985, 57, 1770–1771. [Google Scholar] [CrossRef]
- Höök, F.; Rodahl, M.; Brzezinski, P.; Kasemo, B. Energy dissipation kinetics for protein and antibody−antigen adsorption under shear oscillation on a quartz crystal microbalance. Langmuir 1998, 14, 729–734. [Google Scholar] [CrossRef]
- Höök, F.; Kasemo, B.; Nylander, T.; Fant, C.; Sott, K.; Elwing, H. Variations in coupled water, viscoelastic properties, and film thickness of a Mefp-1 protein film during adsorption and cross-linking: A quartz crystal microbalance with dissipation monitoring, ellipsometry, and surface plasmon resonance study. Anal. Chem. 2001, 73, 5796–5804. [Google Scholar] [CrossRef] [PubMed]
- Capila, I.; Linhardt, R.J. Heparin-protein interactions. Angew. Chem. Int. Ed. 2002, 41, 390–412. [Google Scholar] [CrossRef]
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Ultrathin poly(N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir 2004, 20, 5506–5511. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Canavan, H.E.; Graham, D.J.; Castner, D.G.; Ratner, B.D. Temperature dependent activity and structure of adsorbed proteins on plasma polymerized N-isopropyl acrylamide. Biointerphases 2006, 1, 61. [Google Scholar] [CrossRef] [PubMed]
- Jackler, G.; Steitz, R.; Czeslik, C. Effect of temperature on the adsorption of lysozyme at the silica/water interface studied by optical and neutron reflectometry. Langmuir 2002, 18, 6565–6570. [Google Scholar] [CrossRef]
- Kiesel, I.; Paulus, M.; Nase, J.; Tiemeyer, S.; Sternemann, C.; Ruster, K.; Wirkert, F.J.; Mende, K.; Buning, T.; Tolan, M. Temperature-driven adsorption and desorption of proteins at solid-liquid interfaces. Langmuir 2014, 30, 2077–2083. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, F.; Feld, M.K. Adsorption characteristics of plasma fibronectin in relationship to biological activity. J. Biomed. Mater. Res. 1981, 15, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.J.; Vega, M.D.; Boettiger, D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol. Biol. Cell 1999, 10, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Modin, C.; Foss, M.; Duch, M.; Simmons, A.; Pedersen, F.S.; Milthorpe, B.K.; Besenbacher, F. Monitoring cell adhesion on tantalum and oxidised polystyrene using a quartz crystal microbalance with dissipation. Biomaterials 2006, 27, 4529–4537. [Google Scholar] [CrossRef] [PubMed]
- Takagi, J.; Strokovich, K.; Springer, T.A.; Walz, T. Structure of integrin alpha5beta1 in complex with fibronectin. EMBO J. 2003, 22, 4607–4615. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Ebara, M.; Sakai, K.; Sakurai, Y.; Okano, T. Novel bifunctional polymer with reactivity and temperature sensitivity. J. Biomater. Sci. Polym. Ed. 2000, 11, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Mosesson, M.W.; Umfleet, R.A. The cold-insoluble globulin of human plasma. I. Purification, primary characterization, and relationship to fibrinogen and other cold-insoluble fraction components. J. Biol. Chem. 1970, 245, 5728–5736. [Google Scholar] [PubMed]
- Rocco, M.; Carson, M.; Hantgan, R.; McDonagh, J.; Hermans, J. Dependence of the shape of the plasma fibronectin molecule on solvent composition. Ionic strength and glycerol content. J. Biol. Chem. 1983, 258, 14545–14549. [Google Scholar] [PubMed]






| Code | Atom (%) 2 | N/C 4 | ||||
|---|---|---|---|---|---|---|
| C | N | O | S | Au | ||
| Oxidized PS | 77.74 ± 0.40 | 1.83 ± 0.30 | 20.42 ± 0.33 | 0.01 ± 0.01 | N.D. 3 | 0.02 ± 0.00 |
| PIPAAm | 80.15 ± 0.69 | 7.19 ± 0.97 | 12.63 ± 0.30 | 0.03 ± 0.02 | N.D. 3 | 0.09 ± 0.01 |
| Heparin-IC1 | 80.60 ± 0.55 | 6.95 ± 0.31 | 12.35 ± 0.28 | 0.09 ± 0.01 | N.D. 3 | 0.09 ± 0.00 |
| Code | Thickness (nm) | |
|---|---|---|
| BSA | FN | |
| Oxidized PS, 20 °C | 4.5 ± 0.4 | 13.8 ± 1.0 ** |
| Oxidized PS, 37 °C | 4.2 ± 0.6 | 10.3 ± 0.1 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, J.; Arisaka, Y.; Yui, N.; Akiyama, Y.; Yamato, M.; Okano, T. Effect of Temperature Changes on Serum Protein Adsorption on Thermoresponsive Cell-Culture Surfaces Monitored by A Quartz Crystal Microbalance with Dissipation. Int. J. Mol. Sci. 2018, 19, 1516. https://doi.org/10.3390/ijms19051516
Kobayashi J, Arisaka Y, Yui N, Akiyama Y, Yamato M, Okano T. Effect of Temperature Changes on Serum Protein Adsorption on Thermoresponsive Cell-Culture Surfaces Monitored by A Quartz Crystal Microbalance with Dissipation. International Journal of Molecular Sciences. 2018; 19(5):1516. https://doi.org/10.3390/ijms19051516
Chicago/Turabian StyleKobayashi, Jun, Yoshinori Arisaka, Nobuhiko Yui, Yoshikatsu Akiyama, Masayuki Yamato, and Teruo Okano. 2018. "Effect of Temperature Changes on Serum Protein Adsorption on Thermoresponsive Cell-Culture Surfaces Monitored by A Quartz Crystal Microbalance with Dissipation" International Journal of Molecular Sciences 19, no. 5: 1516. https://doi.org/10.3390/ijms19051516
APA StyleKobayashi, J., Arisaka, Y., Yui, N., Akiyama, Y., Yamato, M., & Okano, T. (2018). Effect of Temperature Changes on Serum Protein Adsorption on Thermoresponsive Cell-Culture Surfaces Monitored by A Quartz Crystal Microbalance with Dissipation. International Journal of Molecular Sciences, 19(5), 1516. https://doi.org/10.3390/ijms19051516





