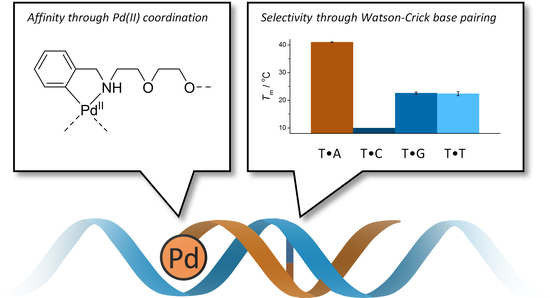
Palladacyclic Conjugate Group Promotes Hybridization of Short Oligonucleotides
Abstract
1. Introduction
2. Results
2.1. Synthesis of the Benzylamine Phosphoramidite Building Block
2.2. Cyclopalladation of 2-[2-(benzylamino)ethoxy]ethanol
2.3. Oligonucleotide Synthesis
2.4. Hybridization Studies
3. Discussion
3.1. Duplex Stabilization by the Palladacyclic “Warhead”
3.2. Impact of the Palladacyclic “Warhead” on Sequence Selectivity
4. Materials and Methods
4.1. General Methods
4.2. N-benzyl-2,2,2-trifluoro-N-[2-(2-hydroxyethoxy)ethyl]acetamide (3)
4.3. 2-[2-(N-benzyl-2,2,2-trifluoroacetamido)ethoxy]ethyl 2-cyanoethyl N,N-diisopropylphosphoramidite (1)
4.4. Bis{N-[2-(2-hydroxyethoxy)ethyl]benzylaminato-C2,N} bis(µ-chloro) dipalladium(II) (4)
4.5. Oligonucleotide Synthesis
4.6. Melting Temperature Measurements
4.7. CD Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CD | circular dichroism |
| DNA | deoxyribonucleic acid |
| PNA | peptide nucleic acid |
| RP-HPLC | reversed-phase high performance liquid chromatography |
| ESI-MS | electrospray ionization mass spectrometry |
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Cano, C.; Hannon, M.J. Novel and emerging approaches for the delivery of metallo-drugs. Dalton Trans. 2009, 10702–10711. [Google Scholar] [CrossRef] [PubMed]
- Ott, I.; Gust, R. Non platinum metal complexes as anti-cancer drugs. Arch. Pharm. 2007, 340, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Bruijnincx, P.C.A.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ott, I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Levina, A.; Mitra, A.; Lay, P.A. Recent developments in ruthenium anticancer drugs. Metallomics 2009, 1, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Berners-Price, S.J.; Filipovska, A. Gold compounds as therapeutic agents for human diseases. Metallomics 2011, 3, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Cutillas, N.; Yellol, G.S.; de Haro, C.; Vicente, C.; Rodríguez, V.; Ruiz, J. Anticancer cyclometalated complexes of platinum group metals and gold. Coord. Chem. Rev. 2013, 257, 2784–2797. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- Bindoli, A.; Rigobello, M.P.; Scutari, G.; Gabbiani, C.; Casini, A.; Messori, L. Thioredoxin reductase: A target for gold compounds acting as potential anticancer drugs. Coord. Chem. Rev. 2009, 253, 1692–1707. [Google Scholar] [CrossRef]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.T.; Lipp, H.-P. Toxicity of platinum compounds. Expert Opin. Pharmacother. 2003, 4, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Daugaard, G.; Abildgaard, U. Cisplatin nephrotoxicity. A review. Cancer Chemother. Pharmacol. 1989, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. Third row transition metals for the treatment of cancer. Philos. Trans. A Math. Phys. Eng. Sci. 2015, 373, 20140185. [Google Scholar] [CrossRef] [PubMed]
- Carreira, M.; Calvo-Sanjuán, R.; Sanaú, M.; Marzo, I.; Contel, M. Organometallic palladium complexes with a water-soluble iminophosphorane ligand as potential anticancer agents. Organometallics 2012, 31, 5772–5781. [Google Scholar] [CrossRef] [PubMed]
- Caires, A.C.F. Recent advances involving palladium (II) complexes for the cancer therapy. Anticancer Agents Med. Chem. 2007, 7, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Taube, H. Rates and mechanisms of substitution in inorganic complexes in solution. Chem. Rev. 1952, 50, 69–126. [Google Scholar] [CrossRef]
- Lippert, B.; Miguel, P.J.S. Comparing PtII- and PdII-nucleobase coordination chemistry: Why PdII not always is a good substitute for PtII. Inorg. Chim. Acta 2018, 472, 207–213. [Google Scholar] [CrossRef]
- Serrano, F.A.; Matsuo, A.L.; Monteforte, P.T.; Bechara, A.; Smaili, S.S.; Santana, D.P.; Rodrigues, T.; Pereira, F.V.; Silva, L.S.; Machado, J.; et al. A cyclopalladated complex interacts with mitochondrial membrane thiol-groups and induces the apoptotic intrinsic pathway in murine and cisplatin-resistant human tumor cells. BMC Cancer 2011, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Marchán, V.; Grandas, A. Platinated oligonucleotides: Synthesis and applications for the control of gene expression. In Metal Complex–DNA Interactions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 273–300. [Google Scholar] [CrossRef]
- Colombier, C.; Lippert, B.; Leng, M. Interstrand cross-linking reaction in triplexes containing a monofunctional transplatin-adduct. Nucleic Acids Res. 1996, 24, 4519–4524. [Google Scholar] [CrossRef] [PubMed]
- Brabec, V.; Reedijk, J.; Leng, M. Sequence-dependent distortions induced in DNA by monofunctional platinum(II) binding. Biochemistry 1992, 31, 12397–12402. [Google Scholar] [CrossRef] [PubMed]
- Algueró, B.; Pedroso, E.; Marchán, V.; Grandas, A. Incorporation of two modified nucleosides allows selective platination of an oligonucleotide making it suitable for duplex cross-linking. J. Biol. Inorg. Chem. 2007, 12, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Algueró, B.; López de la Osa, J.; González, C.; Pedroso, E.; Marchán, V.; Grandas, A. Selective platination of modified oligonucleotides and duplex cross-links. Angew. Chem. Int. Ed. 2006, 45, 8194–8197. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.S.; Boudvillain, M.; Schwartz, A.; van der Marel, G.A.; van Boom, J.H.; Reedijk, J.; Lippert, B. Monofunctionally trans-diammine platinum(II)-modified peptide nucleic acid oligomers: A new generation of potential antisense drugs. Chem. Eur. J. 2002, 8, 5566–5570. [Google Scholar] [CrossRef]
- Maity Sajal, K.; Lönnberg, T. Oligonucleotides incorporating palladacyclic nucleobase surrogates. Chem. Eur. J. 2018, 24, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Cutillas, N.; Vicente, C.; Villa, M.D.; López, G.; Lorenzo, J.; Avilés, F.X.; Moreno, V.; Bautista, D. New palladium(II) and platinum(II) complexes with the model nucleobase 1-methylcytosine: Antitumor activity and interactions with DNA. Inorg. Chem. 2005, 44, 7365–7376. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Lorenzo, J.; Vicente, C.; López, G.; López-de-Luzuriaga, J.M.; Monge, M.; Avilés, F.X.; Bautista, D.; Moreno, V.; Laguna, A. New palladium(II) and platinum(II) complexes with 9-aminoacridine: Structures, luminiscence, theoretical calculations, and antitumor activity. Inorg. Chem. 2008, 47, 6990–7001. [Google Scholar] [CrossRef] [PubMed]
- Fuchita, Y.; Tsuchiya, H. Synthesis of ortho-palladated complexes of n-methylbenzylamine: The first example of ortho-palladation of α-unsubstituted secondary benzylamine. Inorg. Chim. Acta 1993, 209, 229–230. [Google Scholar] [CrossRef]
- Fuchita, Y.; Tsuchiya, H.; Miyafuji, A. Cyclopalladation of secondary and primary benzylamines. Inorg. Chim. Acta 1995, 233, 91–96. [Google Scholar] [CrossRef]
- Selvakumar, K.; Vancheesan, S. Synthesis and characterization of cyclopalladated complexes of secondary benzylamines. Polyhedron 1997, 16, 2405–2411. [Google Scholar] [CrossRef]
- Mergny, J.-L.; Lacroix, L. Analysis of thermal melting curves. Oligonucleotides 2003, 13, 515–537. [Google Scholar] [CrossRef] [PubMed]
- Golubev, O.; Lönnberg, T.; Lönnberg, H. Interaction of Pd2+ complexes of 2,6-disubstituted pyridines with nucleoside 5′-monophosphates. J. Inorg. Biochem. 2014, 139, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.B. Nucleoside sites for transition metal ion binding. Acc. Chem. Res. 1985, 18, 32–38. [Google Scholar] [CrossRef]
- Pneumatikakis, G.; Hadjiliadis, N.; Theophanides, T. Complexes of inosine, cytidine, and guanosine with palladium(II). Inorg. Chem. 1978, 17, 915–922. [Google Scholar] [CrossRef]
- Scharf, P.; Müller, J. Nucleic acids with metal-mediated base pairs and their applications. ChemPlusChem 2013, 78, 20–34. [Google Scholar] [CrossRef]
- Takezawa, Y.; Shionoya, M. Metal-mediated DNA base pairing: Alternatives to hydrogen-bonded Watson–Crick base pairs. Acc. Chem. Res. 2012, 45, 2066–2076. [Google Scholar] [CrossRef] [PubMed]
- Clever, G.H.; Kaul, C.; Carell, T. DNA–metal base pairs. Angew. Chem. Int. Ed. 2007, 46, 6226–6236. [Google Scholar] [CrossRef] [PubMed]
- Clever, G.H.; Shionoya, M. Metal–base pairing in DNA. Coord. Chem. Rev. 2010, 254, 2391–2402. [Google Scholar] [CrossRef]
- Müller, J. Chemistry: Metals line up for DNA. Nature 2006, 444, 698. [Google Scholar] [CrossRef] [PubMed]
- Müller, J. Metal-ion-mediated base pairs in nucleic acids. Eur. J. Inorg. Chem. 2008, 2008, 3749–3763. [Google Scholar] [CrossRef]
- Mandal, S.; Müller, J. Metal-mediated DNA assembly with ligand-based nucleosides. Curr. Opin. Chem. Biol. 2017, 37, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, Y.; Müller, J.; Shionoya, M. Artificial DNA base pairing mediated by diverse metal ions. Chem. Lett. 2017, 46, 622–633. [Google Scholar] [CrossRef]
- Lippert, B.; Sanz Miguel, P.J. The renaissance of metal–pyrimidine nucleobase coordination chemistry. Acc. Chem. Res. 2016, 49, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Taherpour, S.; Golubev, O.; Lönnberg, T. On the feasibility of recognition of nucleic acid sequences by metal-ion-carrying oligonucleotides. Inorg. Chim. Acta 2016, 452, 43–49. [Google Scholar] [CrossRef]
- Clever, G.H.; Söltl, Y.; Burks, H.; Spahl, W.; Carell, T. Metal–salen-base-pair complexes inside DNA: Complexation overrides sequence information. Chem. Eur. J. 2006, 12, 8708–8718. [Google Scholar] [CrossRef] [PubMed]


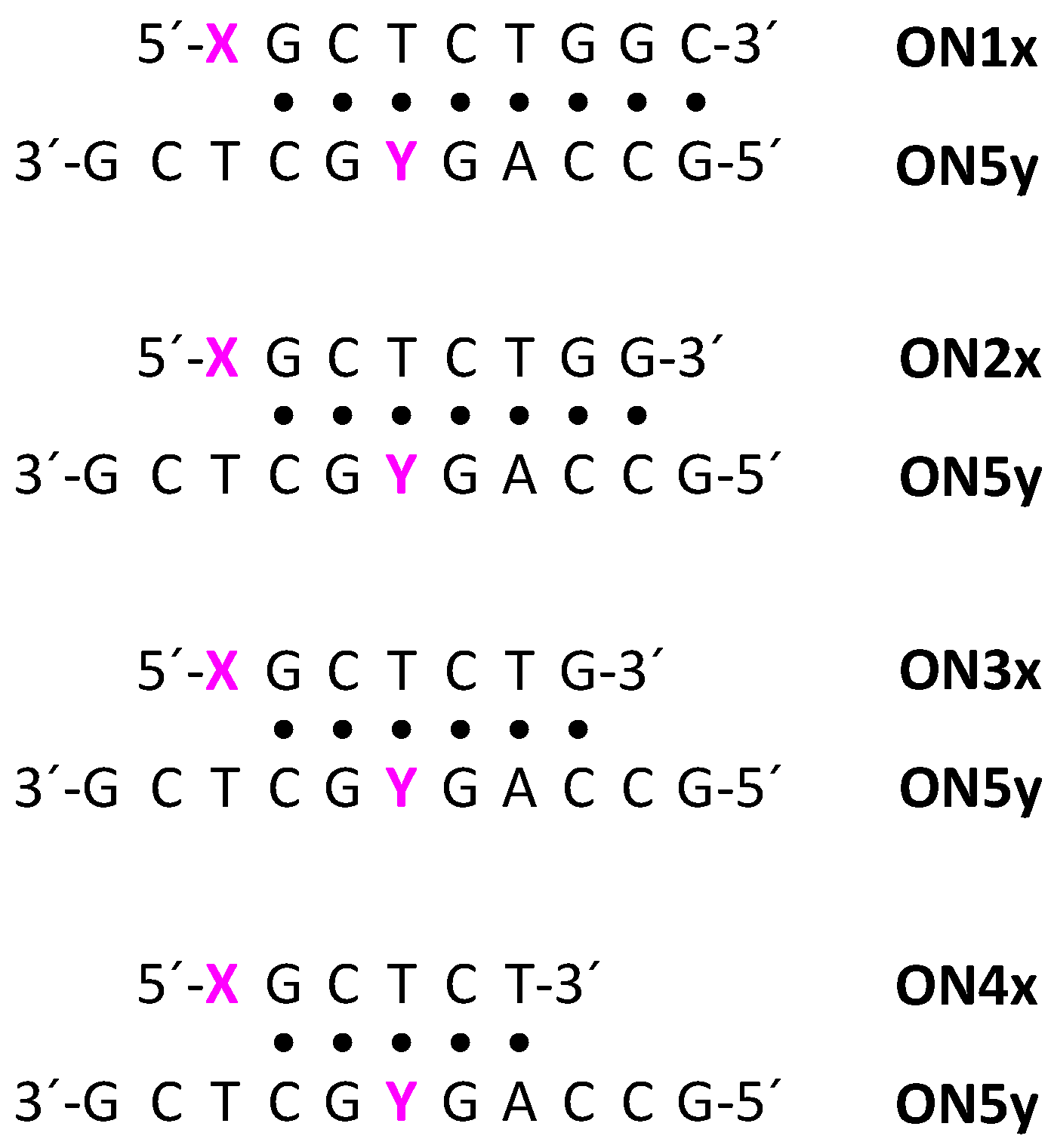



| Sequence 1 | Oligonucleotide |
|---|---|
| ON1a | 5′-AGCTCTGGC-3′ |
| ON2a | 5′-AGCTCTGG-3′ |
| ON3a | 5′-AGCTCTG-3′ |
| ON4a | 5′-AGCTCT-3′ |
| ON1b | 5′-BGCTCTGGC-3′ |
| ON2b | 5′-BGCTCTGG-3′ |
| ON3b | 5′-BGCTCTG-3′ |
| ON4b | 5′-BGCTCT-3′ |
| ON1b-Pd | 5′-BPdGCTCTGGC-3′ |
| ON2b-Pd | 5′-BPdGCTCTGG-3′ |
| ON3b-Pd | 5′-BPdGCTCTG-3′ |
| ON4b-Pd | 5′-BPdGCTCT-3′ |
| ON5a | 5′-GCCAGAGCTCG-3′ |
| ON5c | 5′-GCCAGCGCTCG-3′ |
| ON5g | 5′-GCCAGGGCTCG-3′ |
| ON5t | 5′-GCCAGTGCTCG-3′ |
| Duplex | ΔH°/kJ·mol−1 | ΔS°/J·mol−1·K−1 |
|---|---|---|
| ON1a•ON5a | −200 ± 20 | −530 ± 50 |
| ON1b•ON5a | −200 ± 10 | −550 ± 40 |
| ON1b-Pd•ON5a | −250 ± 20 | −670 ± 50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hande, M.; Maity, S.; Lönnberg, T. Palladacyclic Conjugate Group Promotes Hybridization of Short Oligonucleotides. Int. J. Mol. Sci. 2018, 19, 1588. https://doi.org/10.3390/ijms19061588
Hande M, Maity S, Lönnberg T. Palladacyclic Conjugate Group Promotes Hybridization of Short Oligonucleotides. International Journal of Molecular Sciences. 2018; 19(6):1588. https://doi.org/10.3390/ijms19061588
Chicago/Turabian StyleHande, Madhuri, Sajal Maity, and Tuomas Lönnberg. 2018. "Palladacyclic Conjugate Group Promotes Hybridization of Short Oligonucleotides" International Journal of Molecular Sciences 19, no. 6: 1588. https://doi.org/10.3390/ijms19061588
APA StyleHande, M., Maity, S., & Lönnberg, T. (2018). Palladacyclic Conjugate Group Promotes Hybridization of Short Oligonucleotides. International Journal of Molecular Sciences, 19(6), 1588. https://doi.org/10.3390/ijms19061588