Oxaloacetate Ameliorates Chemical Liver Injury via Oxidative Stress Reduction and Enhancement of Bioenergetic Fluxes
Abstract
:1. Introduction
2. Results
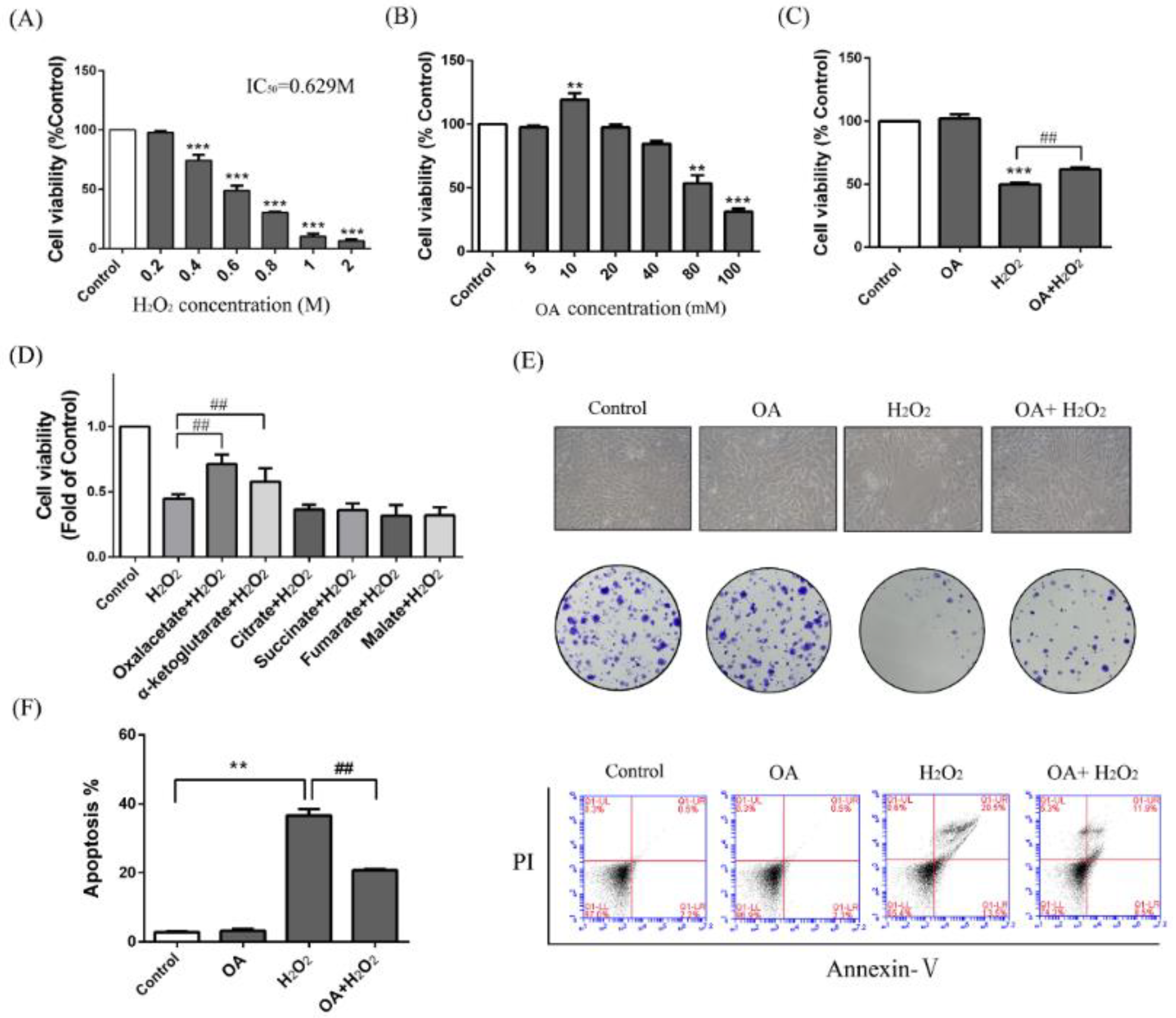
2.1. Protective Effect of Oxaloacetate (OA) on the Human Normal Liver Cells (LO-2 Cells) against Hydrogen Peroxide (H2O2) Injury
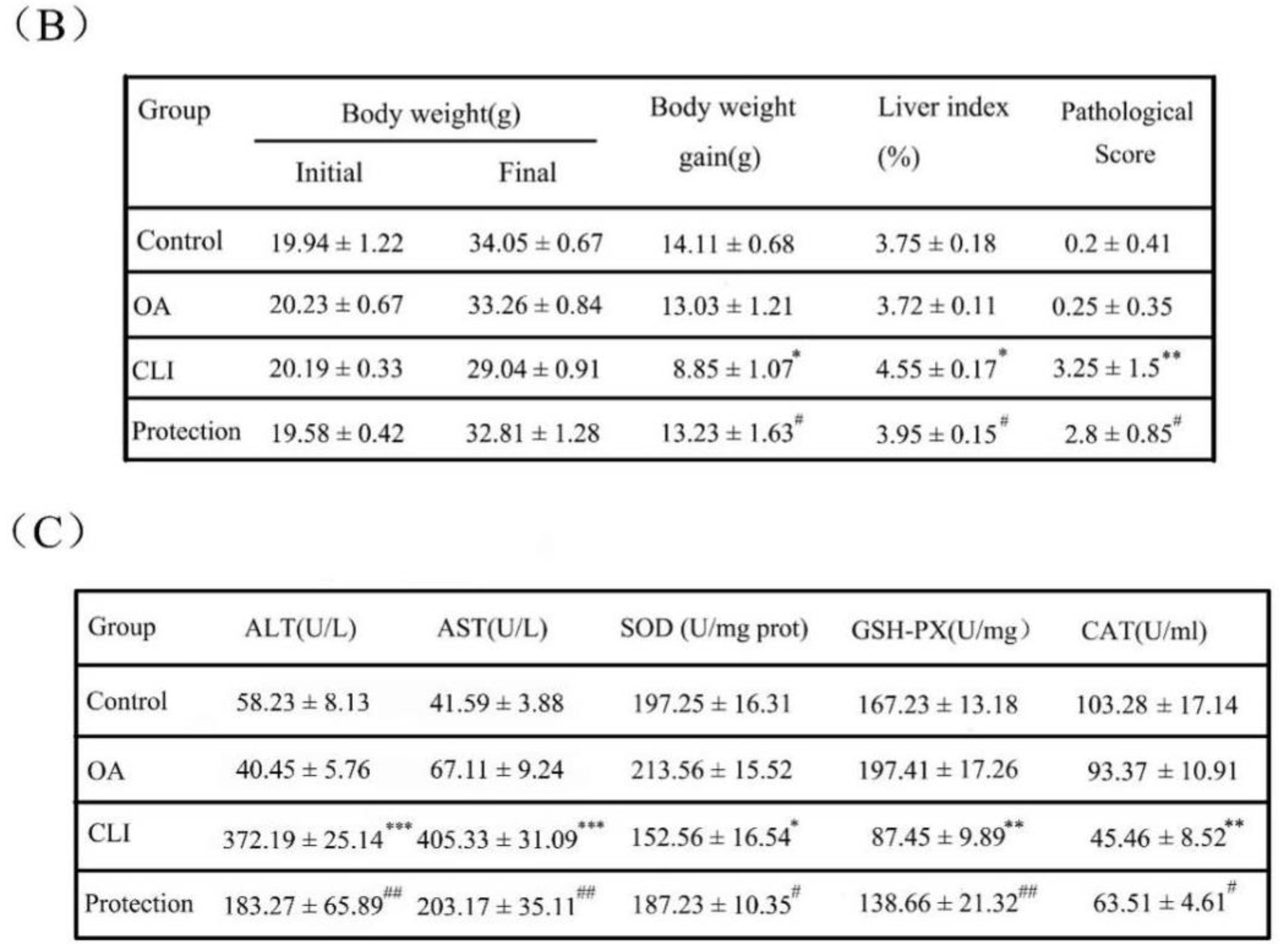
2.2. Effects of OA on Liver Weight and Liver Index
2.3. Hepatoprotective Effect of OA in Liver Histopathology
2.4. Effects of OA on Liver Enzymes
2.5. Protective Effect of OA on Mitochondria
2.6. Effect of OA on Glycolysis
2.7. Effect of OA on Tricarboxylic Acid (TCA) Cycle and Electron Transport Chain
2.8. Effect of OA on Mitogen-Activated Protein Kinase (MAPK) Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Line and Reagents
4.2. Animals
4.3. Cell Viability and Colony Formation
4.4. Analysis of Cell Apoptosis
4.5. Histology and Immunohistochemistry
4.6. Western Blot
4.7. Analysis of Liver Injury
4.8. Analysis of Metabolic Enzymes, Complexes
4.9. Analysis of Adenosine Triphosphate (ATP) Production and Glucose Consumption
4.10. Analysis of IL-8 and IL-10 Level in Serum
4.11. Detection of ROS and Rhodamine 123 Accumulation
4.12. Preparation and Detection of Electron Microscopy Specimens
4.13. Bioinformatics
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Zhu, D.; Ju, B.; Jiang, X.; Hu, J. Hepatoprotective effects of Gentianella turkestanerum extracts on acute liver injury induced by carbon tetrachloride in mice. Am. J. Trans. Res. 2017, 9, 569–579. [Google Scholar]
- Woodhead, J.L.; Watkins, P.B.; Howell, B.A.; Siler, S.Q.; Shoda, L.K.M. The role of quantitative systems pharmacology modeling in the prediction and explanation of idiosyncratic drug-induced liver injury. Drug Metab. Pharmacokinet. 2017, 32, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Alempijevic, T.; Zec, S.; Milosavljevic, T. Drug-induced liver injury: Do we know everything? World J. Hepatol. 2017, 9, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wang, F.; Tang, J.; Mao, A.; Liao, S.; Wang, Q. Dicranostiga leptopodu (Maxim.) Fedde extracts attenuated CCl4-induced acute liver damage in mice through increasing anti-oxidative enzyme activity to improve mitochondrial function. Biomed. Pharmacother. 2017, 85, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.Y.; Jung, Y.S.; Yoon, C.S.; Oh, J.S.; Hong, J.H.; Kim, Y.C.; Kim, S.Y. Fraxin prevents chemically induced hepatotoxicity by reducing oxidative stress. Molecules 2017, 22, 587. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Ghosh, S.; Mukherjee, S.; Gupta, P.; Bhattacharya, S.; Adhikary, A.; Chattopadhyay, S. Pomegranate protects against arsenic-induced p53-dependent ROS-mediated inflammation and apoptosis in liver cells. J. Nutr. Biochem. 2016, 38, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.H.; Hsu, H.H.; Chen, T.S.; Chen, M.C.; Day, C.H.; Tu, C.C.; Lin, Y.M.; Tsai, F.J.; Kuo, W.W.; Huang, C.Y. Phosphorylation of cofilin-1 by ERK confers HDAC inhibitor resistance in hepatocellular carcinoma cells via decreased ROS-mediated mitochondria injury. Oncogene 2017, 36, 1978–1990. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.; Saw, T.Y.; Fong, C.W.; Ho, H.K. Comparative hepatoprotective effects of tocotrienol analogs against drug-induced liver injury. Redox Biol. 2015, 4, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Talwar, S.; Jagani, H.V.; Nayak, P.G.; Kumar, N.; Kishore, A.; Bansal, P.; Shenoy, R.R.; Nandakumar, K. Toxicological evaluation of Terminalia paniculata bark extract and its protective effect against CCl4-induced liver injury in rodents. BMC Complement. Altern. Med. 2013, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.I.E. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Conley, K.E.; Kemper, W.F.; Crowther, G.J. Limits to sustainable muscle performance: Interaction between glycolysis and oxidative phosphorylation. J. Exp. Biol. 2001, 204, 3189–3194. [Google Scholar] [PubMed]
- Brooks, G.A. Cell-cell and intracellular lactate shuttles. J. Physiol. 2009, 587, 5591–5600. [Google Scholar] [CrossRef] [PubMed]
- Vaishnavi, S.N.; Vlassenko, A.G.; Rundle, M.M.; Snyder, A.Z.; Mintun, M.A.; Raichle, M.E. Regional aerobic glycolysis in the human brain. Proc. Natl. Acad. Sci. USA 2010, 107, 17757–17762. [Google Scholar] [CrossRef] [PubMed]
- Mignotte, B.; Vayssiere, J.-L. Mitochondrial control of apoptosis. Adv. Cell Aging Gerontol. 2001, 5, 93–122. [Google Scholar]
- Dolle, C.; Rack, J.G.; Ziegler, M. NAD and ADP-ribose metabolism in mitochondria. FEBS J. 2013, 280, 3530–3541. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, H.M.; Koppel, S. Oxaloacetate enhances neuronal cell bioenergetic fluxes and infrastructure. J. Neurochem. 2016, 137, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, H.M.; Harris, J.L.; Carl, S.M.; Lezi, E.; Lu, J.; Eva Selfridge, J.; Roy, N.; Hutfles, L.; Koppel, S.; Morris, J.; et al. Oxaloacetate activates brain mitochondrial biogenesis, enhances the insulin pathway, reduces inflammation and stimulates neurogenesis. Hum. Mol. Gen. 2014, 23, 6528–6541. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-N.; Chen, H.; Li, Y. Effect of bicyclol on cisplatin-induced hepatotoxicity in the hepatocarcinoma 22 tumour-bearing mice. Basic Clin. Pharmacol. Toxicol. 2009, 104, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Söğüt, S.; Zoroğlu, S.S.; Ozyurt, H.; Yilmaz, H.R.; Ozuğurlu, F.; Sivasli, E.; Yetkin, O.; Yanik, M.; Tutkun, H.; Savaş, H.A.; et al. Changes in nitric oxide levels and antioxidant enzyme activities may have a role in the pathophysiological mechanisms involved in autism. Clin. Chim. Acta 2003, 331, 111–117. [Google Scholar] [CrossRef]
- Baracca, A.; Sgarbi, G.; Solaini, G.; Lenaz, G. Rhodamine 123 as a probe of mitochondrial membrane potential: Evaluation of proton flux through F(0) during ATP synthesis. Biochim. Biophys. Acta 2003, 1606, 137–146. [Google Scholar] [CrossRef]
- Xiong, Y.; Ye, T.; Wang, M.; Xia, Y.; Wang, N.; Song, X.; Wang, F.; Liu, L.; Zhu, Y.; Yang, F.; et al. A novel cinnamide YLT26 induces breast cancer cells apoptosis via ROS-mitochondrial apoptotic pathway in vitro and inhibits lung metastasis in vivo. Cell. Physiol. Biochem. 2014, 34, 1863–1876. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.J.; Hayward, J.A.; Huang, C.E.; Huma, Z.; Sanchez, J. Mechanisms of regulation of the chemokine-receptor network. Int. J. Mol. Sci. 2017, 18, 342. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Lotrich, F.E. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Ainscow, E.K.; Brand, M.D. Internal regulation of ATP turnover, glycolysis and oxidative phosphorylation in rat hepatocytes. Eur. J. Biochem. 1999, 266, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Korzeniewski, B.; Liguzinski, P. Theoretical studies on the regulation of anaerobic glycolysis and its influence on oxidative phosphorylation in skeletal muscle. Biophys. Chem. 2004, 110, 147–169. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, S.S.; Balakireva, A.V.; Markova, O.V.; Severin, F.F. Negative Feedback of Glycolysis and Oxidative Phosphorylation: Mechanisms of and Reasons for It. Biochem. Biokhimiia 2015, 80, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, T.; Schuster, S.; Bonhoeffer, S. Cooperation and competition in the evolution of ATP-producing pathways. Science 2001, 292, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, J.M.; Shi, L.Z.; Tam, J.; Chandsawangbhuwana, C.; Durrant, B.; Botvinick, E.L.; Berns, M.W. Comparison of glycolysis and oxidative phosphorylation as energy sources for mammalian sperm motility, using the combination of fluorescence imaging, laser tweezers, and real-time automated tracking and trapping. J. Cell. Phys. 2008, 217, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, K.; Miwa, H.; Imai, N.; Shikami, M.; Gotou, M.; Goto, M.; Mizuno, S.; Takahashi, M.; Yamamoto, H.; Hiramatsu, A.; et al. Energy metabolism of leukemia cells: Glycolysis versus oxidative phosphorylation. Leuk. Lymphoma 2010, 51, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Ravan, A.P.; Bahmani, M.; Ghasemi Basir, H.R.; Salehi, I.; Oshaghi, E.A. Hepatoprotective effects of Vaccinium arctostaphylos against CCl4-induced acute liver injury in rats. J. Basic Clin. Phys. Pharmacol. 2017, 28, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Li, J.; Li, Y.; Song, H. Antioxidant and Hepatoprotective Effect of Swertiamarin on Carbon Tetrachloride-Induced Hepatotoxicity via the Nrf2/HO-1 Pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 41, 2242–2254. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Nishida, K.; Sasaki, E.; Kongo, M.; Ishiguro, I. Attenuation of disrupted hepatic active oxygen metabolism with the recovery of acute liver injury in rats intoxicated with carbon tetrachloride. Res. Commun. Mol. Pathol. Pharmacol. 1997, 95, 191–207. [Google Scholar] [PubMed]
- Sanmugapriya, E.; Venkataraman, S. Studies on hepatoprotective and antioxidant actions of Strychnos potatorum Linn. seeds on CCl4-induced acute hepatic injury in experimental rats. J. Ethnopharmacol. 2006, 105, 154–160. [Google Scholar] [PubMed]
- Gygi, S.P.; Rochon, Y.; Franza, B.R.; Aebersold, R. Correlation between protein and mRNA abundance in yeast. Mol. Cell. Biol. 1999, 19, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sattler, G.L.; Pitot, H.C. Oxaloacetate induces DNA synthesis and mitosis in primary cultured rat hepatocytes in the absence of EGF. Biochem. Biophys. Res. Commun. 1993, 193, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Ji, L.; Kuang, Y.; Yang, Q. Cytotoxic effect of oxaloacetate on HepG2-human hepatic carcinoma cells via apoptosis and ROS accumulation. Neoplasma 2017, 64, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.; Sobrino, T.; Ramos-Cabrer, P.; Castillo, J. Oxaloacetate: A novel neuroprotective for acute ischemic stroke. Int. J. Biochem. Cell Biol. 2012, 44, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.; Berkutzki, T.; Cooper, I.; Mohar, B.; Teichberg, V.I. Blood glutamate scavengers prolong the survival of rats and mice with brain-implanted gliomas. Investig. New Drugs 2012, 30, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.; Cohen-Kashi Malina, K.; Cooper, I.; Graubardt, N.; Babakin, L.; Jona, G.; Teichberg, V.I. Combined Treatment of an Amyotrophic Lateral Sclerosis Rat Model with Recombinant GOT1 and Oxaloacetic Acid: A Novel Neuroprotective Treatment. Neurodegener. Dis. 2015, 15, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, A.; Sinelnikov, I.; Gruenbaum, B.F.; Gruenbaum, S.E.; Dubilet, M.; Dubilet, E.; Leibowitz, A.; Ohayon, S.; Regev, A.; Boyko, M.; et al. Effect of glutamate and blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome and pathohistology of the hippocampus after traumatic brain injury in rats. Anesthesiology 2012, 116, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H.; Bothwell, R.; Hutfles, L.; Burns, J.M.; Reed, G.A. Tolerability and pharmacokinetics of oxaloacetate 100 mg capsules in Alzheimer’s subjects. BBA Clin. 2016, 5, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K. Studies on the anti-diabetic effect of sodium oxaloacetate. Tohoku J. Exp. Med. 1968, 96, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Hong, D.P.; Kim, H.J.; Jang, K.S.; Shin, D.M.; Ahn, J.I.; Lee, Y.S.; Kong, G. Differential gene expression profiles in the steatosis/fibrosis model of rat liver by chronic administration of carbon tetrachloride. Toxicol. Appl. Pharmacol. 2005, 208, 242–254. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, Y.; Han, X.; Xu, M.; Wang, Y.; Zhao, Y.; Yang, Q. Oxaloacetate Ameliorates Chemical Liver Injury via Oxidative Stress Reduction and Enhancement of Bioenergetic Fluxes. Int. J. Mol. Sci. 2018, 19, 1626. https://doi.org/10.3390/ijms19061626
Kuang Y, Han X, Xu M, Wang Y, Zhao Y, Yang Q. Oxaloacetate Ameliorates Chemical Liver Injury via Oxidative Stress Reduction and Enhancement of Bioenergetic Fluxes. International Journal of Molecular Sciences. 2018; 19(6):1626. https://doi.org/10.3390/ijms19061626
Chicago/Turabian StyleKuang, Ye, Xiaoyun Han, Mu Xu, Yue Wang, Yuxiang Zhao, and Qing Yang. 2018. "Oxaloacetate Ameliorates Chemical Liver Injury via Oxidative Stress Reduction and Enhancement of Bioenergetic Fluxes" International Journal of Molecular Sciences 19, no. 6: 1626. https://doi.org/10.3390/ijms19061626
APA StyleKuang, Y., Han, X., Xu, M., Wang, Y., Zhao, Y., & Yang, Q. (2018). Oxaloacetate Ameliorates Chemical Liver Injury via Oxidative Stress Reduction and Enhancement of Bioenergetic Fluxes. International Journal of Molecular Sciences, 19(6), 1626. https://doi.org/10.3390/ijms19061626