Abstract
TSPO (18 kDa translocator protein) was identified decades ago in a search for peripheral tissue binding sites for benzodiazepines, and was formerly called the peripheral benzodiazepine receptor. TSPO is a conserved protein throughout evolution and it is implicated in the regulation of many cellular processes, including inflammatory responses, oxidative stress, and mitochondrial homeostasis. TSPO, apart from its broad expression in peripheral tissues, is highly expressed in neuroinflammatory cells, such as activated microglia. In addition, emerging studies employing the ligands of TSPO suggest that TSPO plays an important role in neuropathological settings as a biomarker and therapeutic target. However, the precise molecular function of this protein in normal physiology and neuropathology remains enigmatic. This review provides an overview of recent advances in our understanding of this multifaceted molecule and identifies the knowledge gap in the field for future functional studies.
1. Introduction
Translocator protein (18 kDa, TSPO), which was initially designated as the peripheral-type benzodiazepine receptor (PBR), was identified in 1977 when investigators were searching for peripheral tissue binding sites for benzodiazepines, one of the most widely available class of drugs prescribed to treat patients with anxiety, convulsions, or insomnia [1]. TSPO was discovered in the kidney as a diazepam-binding site [1], and the initial characterization of these benzodiazepine-binding sites outside the brain led to their assignment as ‘peripheral-type’ benzodiazepine receptors (PBR), to distinguish them from the central benzodiazepine receptors (CBR). Two types of benzodiazepine binding receptors have been identified in mammalian tissues, the central-type receptors (CBR) and peripheral-type benzodiazepine receptors (PBR). CBR are located on neurons and found coupled to GABA receptors, regulating the GABA-regulated chloride channels, [2,3] while PBR have a much more ubiquitous distribution [4,5]. PBR is primarily located on the outer membrane of mitochondria and found to be abundant in steroid-synthesizing cells [4]. It is also reported that PBR is pharmacologically and structurally distinct from the CBR [6,7]. Although the name ‘PBR’ was widely accepted previously, multiple other names have also been used to refer this protein such as mitochondrial benzodiazepine receptor, mitochondrial diazepam-binding inhibitor (DBI) receptor complex and PK11195-binding sites [8,9]. However, regardless of its interactions with other proteins or ligands, PBR was renamed as 18 kDa Translocator Protein (TSPO) in 2006 by the HUGO Gene Nomenclature Committee [4] reflecting its putative function in protein or ligand transport/translocation.
2. Evolutionary Conservation of TSPO
The primary coding sequence of TSPO is highly conserved throughout evolution, from bacteria to humans [8,10,11,12], and predicts a tryptophan-rich hydrophobic protein with five transmembrane domains. The functional TSPO protein occurs throughout the phylogenetic spectrum and the cDNA for TSPO has been cloned from various species including humans [10,13]. Notably, the rat TSPO replaced the activity of its bacterial homolog in Rhodobacter sphaeroides [14], indicating evolutionarily conserved functions of TSPO, although these proteins share only about 30% amino acid identity. In the human genome, the TSPO gene is localized to the chromosome 22, within the band 22q13.31 as a single copy and the mRNAs of human and mouse TSPO translate to closely related proteins having 169-amino acid residues with 81% sequence similarity [15,16,17]. TSPO is widely expressed throughout the body and the binding sites for TSPO ligands have been identified in tissues such as heart, kidney, and liver [7]. Further, TSPO is found enriched in tissues in which steroids are synthesized such as adipose tissue and adrenal cortex. In the CNS, the basal expression of TSPO is low and is restricted mostly to glial cells [18,19]. TSPO is a nuclear-encoded protein and at the subcellular level, TSPO is mainly localized in the outer mitochondrial membrane [20], reflecting a key role of TSPO in cellular functions related to mitochondria. Of note, TSPO has been implicated in a wide range of cellular processes including, but not limited to, proliferation and differentiation, apoptosis, immunomodulation, oxidative stress, and mitochondrial physiology [20,21,22,23,24]. A recent study employing microarray analysis of gene expression in a glioblastoma cell line, U118MG, upon treatment with TSPO ligand, PK11195 demonstrated that the mitochondrial expression of TSPO could be a part of mitochondria-to-nucleus signaling pathway resulting in modulation of nuclear gene/transcription factor expression and altered cellular functions [24]. Furthermore, apart from the expression of TSPO in the mitochondrial membrane, it has also been localized in the plasma membrane as well as in nuclear/peri-nuclear areas [25,26,27]. However, the precise molecular function of TSPO as well as its mode of action, whether it operates as molecular receptor or sensor remains largely unclear. In addition, the role of TSPO specific to a particular subcellular localization in normal physiology and pathology needs investigation.
3. TSPO and Steroidogenesis
Consistent with the abundance of TSPO expression in steroid producing tissues, TSPO was considered to be essential for the translocation of cholesterol from the outer mitochondrial membrane to the inner mitochondrial membrane, which is regarded as a rate-limiting step for steroidogenesis [21,28,29,30]. It was proposed that TSPO transports cholesterol across the outer mitochondrial membrane to a steroidogenic enzyme, CYP11A1 [31], which converts cholesterol into pregnenolone, a common precursor for steroids. Of note, synthetic ligands of TSPO such as PK11195 and Ro5-4864 stimulated steroidogenesis and neuro steroidogenesis both in vitro and in vivo [21,28,29,32,33,34]. Further, recent studies demonstrated a positive correlation between the TSPO ligand residence time (the period for which the ligand interacts with its target, TSPO) and its neurosteroidogenic efficacy [35,36]. In addition, the benzodiazepine, Ro5-4864 and the isoquinoline carboxamide, PK11195 exhibit nanomolar affinity for the TSPO and have distinct binding sites on TSPO [37,38]. The thermodynamic studies indicated that the [3H]-PK11195 binding to TSPO is entropy driven, in contrast, the [3H]-RO5-4864 binding is enthalpy driven [39]. Therefore, PK-11195 is being considered as an antagonist of TSPO, and RO5-4864, an agonist or a partial agonist [39] and both have been utilized extensively as prototypical pharmacological tools for characterizing TSPO and its molecular function. Apart from a putative role of TSPO in steroidogenesis, earlier studies have also reported embryonic lethality of TSPO knockout mice [40] implicating a key role of TSPO in normal physiology and development. In contrast, recent studies demonstrated that genetic deletion of TSPO in different cell types had no effect on cellular viability [41]. More importantly, the global TSPO knockout mice that were developed by two independent research groups by Cre-lox technology were viable and exhibited unaltered steroidogenesis [42,43]. Additionally, studies employing a transgenic mouse with conditional TSPO deletion in Leydig cells demonstrated that TSPO was not essential for testosterone production [44]. However, very recent studies demonstrated that global TSPO deletion alter adrenocorticotropic hormone-induced plasma corticosteroid concentrations [45] and TSPO deletion-mediated effects exacerbate with aging [46]. Altogether, the emerging evidence suggests elusive and conflicting roles of TSPO in mammalian cells that warrant further investigation. TSPO ligands augmented steroid hormone production in several different steroidogenic cell types [22,32,47]. Given the cytoprotective effects of steroids, the TSPO ligands have been proposed as therapeutic agents to augment steroid levels in the brain as well as in the reproductive system and the pharmacological agents have been extensively used to elucidate the physiological relevance of TSPO. However, genetic studies showed that the pharmacological effect of TSPO ligand, PK11195 on the induction of steroidogenesis is not mediated through TSPO in MA-10 mouse Leydig tumor cells [48] implicating the possible off-target effects of synthetic ligands and thereby emphasizing the essentiality of genetic cellular and animal model systems in elucidating the TSPO ligand-mediated effects on cellular functions.
4. TSPO and Mitochondrial Functions
Notably, recent studies employing genetic approach demonstrated a significant shift in mitochondrial homeostasis in Tspo−/− fibroblasts that could affect multiple mitochondrial functions [49]. Of note, the ligands of TSPO regulate the mitochondrial permeability transition pore (MPTP) functioning and an association of TSPO with MPTP had been suggested previously [50]. However, recent studies demonstrate that TSPO plays no role in the regulation of MPTP. Further, both endogenous, as well as synthetic ligands of TSPO, do not regulate MPTP activity through TSPO implicating that TSPO-mediated modulation of mitochondrial functions could be independent of the regulation of MPTP [51]. Recent studies have also reported that genetic deletion of TSPO results in an increase in mitochondrial fatty acid oxidation in steroidogenic cells [52] and a decrease in oxygen consumption rate (OCR) in microglia [43] and fibroblasts [49] but the underlying molecular mechanisms of these findings and their relevance to the overall mitochondrial function require further investigation.
5. TSPO and Endogenous Ligands
Various endogenous TSPO ligands have been proposed such as cholesterol, Diazepam Binding Inhibitor (DBI), and porphyrin and the endogenous ligands bind TSPO with different affinities. Cholesterol is a potent ligand of TSPO with nanomolar affinity [53] and it binds to the cholesterol recognition amino acid consensus sequence in the carboxyl-terminal end of TSPO. In contrast, DBI has micromolar affinities for both TSPO and CBR and was initially described based on its ability to interact with CBR and regulate GABAergic transmission [54]. DBI and its proteolytic products can stimulate steroidogenesis by interacting with TSPO [55] and are widely distributed in the CNS and peripheral steroidogenic cells [56,57,58,59,60].
TSPO from all species studied bind porphyrins or cyclic tetrapyrroles (protoporphyrin IX (PPIX), mesoporphyrin IX, deuteroporphyrin IX, heme, and hemin) [61,62,63,64] and porphyrins exhibit high (nM) affinity for TSPO but not for CBR [62,65]. The concept that porphyrins are endogenous ligands is consistent with the mitochondrial location of TSPO since the mitochondria play a key role in porphyrin metabolism [66]. Many porphyrins are naturally occurring and one of the best-known porphyrins is heme, the red blood cell pigment and a cofactor of oxygen- binding protein, hemoglobin. In both eukaryotes and prokaryotes, TSPO interacts with heme and its immediate precursor PPIX [67]. Though it has been suggested that TSPO is a porphyrin transporter, no published experimental evidence supports this claim. Instead of acting as a transporter it is proposed that TSPO binds PPIX as a regulatory cellular protection mechanism against oxidative stress otherwise generated by the free form of these porphyrins. Detergent-purified TSPO can bind porphyrins in vitro, including PPIX and hemin (oxidized heme) [68,69]. Further, Rat TSPO can bind PPIX in vivo and the binding was demonstrated with positron emission tomography [70]. However, the mechanisms involved in the porphyrin/heme binding to the TSPO remain to be determined.
It was proposed that TSPO could sequester free heme in cells and catabolize/degrade excess PPIX in conjugation with ROS generation. Of note, the genetic knockdown of TSPO in human U118MG glioma cell line resulted in mitochondrial PPIX accumulation upon exposure of cells to PPIX, suggesting a role of TSPO in preventing intracellular accumulation of PPIX [71]. In addition, TSPO could act as a scavenger of porphyrin-based compounds in a eukaryotic model such as human colonic epithelial cell line (Caco-2) and may contribute to protecting cells from potential toxic compounds such as free tetrapyrroles [72]. Notably, TSPO ligands could antagonize the functions of the endogenous PPIX and for instance, PK11195 could counteract the cytotoxic effects of hemin in Caco-2 cells [72]. Employing detergent-purified bacterial Chlorobium tepidum TSPO, it was demonstrated that TSPO could induce rapid spectral changes to added PPIX indicative of chemical catalysis [73]. Moreover, in Bacillus cereus, TSPO mediated a light-induced degradation of PPIX [74]. Further, TSPO ligands were able to partially rescue cells from porphyrin-induced phototoxicity [75]. In Arabidopsis thaliana, it was observed that TSPO (AtTSPO) attenuated ALA-induced porphyria through a potential scavenging mechanism [64]. AtTSPO could possibly be involved in the transient clearance of excess cytosolic unbound heme and thereby it could modulate redox homeostasis [64]. However, the analysis of PPIX elimination in Tspo−/− mouse tissues and plasma suggests that TSPO is not a critical regulator of PPIX levels in mammalian systems in normal physiological conditions [49]. Further, PPIX-mediated phototoxic cell death was not different between Tspofl/fl and Tspo−/− fibroblasts [49]. In addition, early studies using TSPO-binding pharmacological agents have suggested a functional link between mammalian TSPO and the induction of hemoglobin synthesis [76,77]. However, recent studies using Tspo−/− mice and cell lines have established that TSPO is not involved in heme biosynthesis [49]. Though these recent observations rule out the role of TSPO in PPIX biosynthesis, given the evolutionarily conserved interaction between TSPO and porphyrins further studies are warranted elucidating the role of TSPO in oxidative stress associated with porphyrins including heme.
6. TSPO and Oxidative Stress
TSPO appears to be an essential participant in the regulation of mitochondrial reactive oxygen species (ROS) levels [78,79,80] and the mitochondrial location of the TSPO is interesting as mitochondria are the main source of cellular ROS [81]. Also, the exposure of neuronal cells to TSPO ligands in vitro generates oxygen-free radicals [82]. In the liver, TSPO was found in colocalization with the mitochondrial manganese-dependent superoxide dismutase, a ROS scavenger [83]. It has also been demonstrated that increased TSPO expression is associated with resistance against ROS and hydrogen peroxide cytotoxicity [84]. Along these lines, Jurkat cells transfected with Tspo cDNA exhibited higher resistance to free radical-mediated damage than controls [84]. Conversely, knockdown of TSPO augmented ROS production [25], suggesting that TSPO may participate in an antioxidant response pathway. Also, it has been suggested that TSPO could act to neutralize ROS. Along these lines, the tryptophan residues in TSPO might react with ROS to generate tryptophan radicals [74]. In MA-10 Leydig cells, CRISPR/Cas9-mediated deletion of TSPO resulted in a modest increase in ROS production compared to controls [52]. Further, it has been demonstrated that oxidative stress modulates both the structure and function of TSPO. Along these lines, increased ROS levels resulted in TSPO polymerization and enhanced ligand binding [85]. However, the precise role of TSPO in the regulation of cellular ROS levels or vice versa requires further studies.
7. TSPO and Neuroinflammation
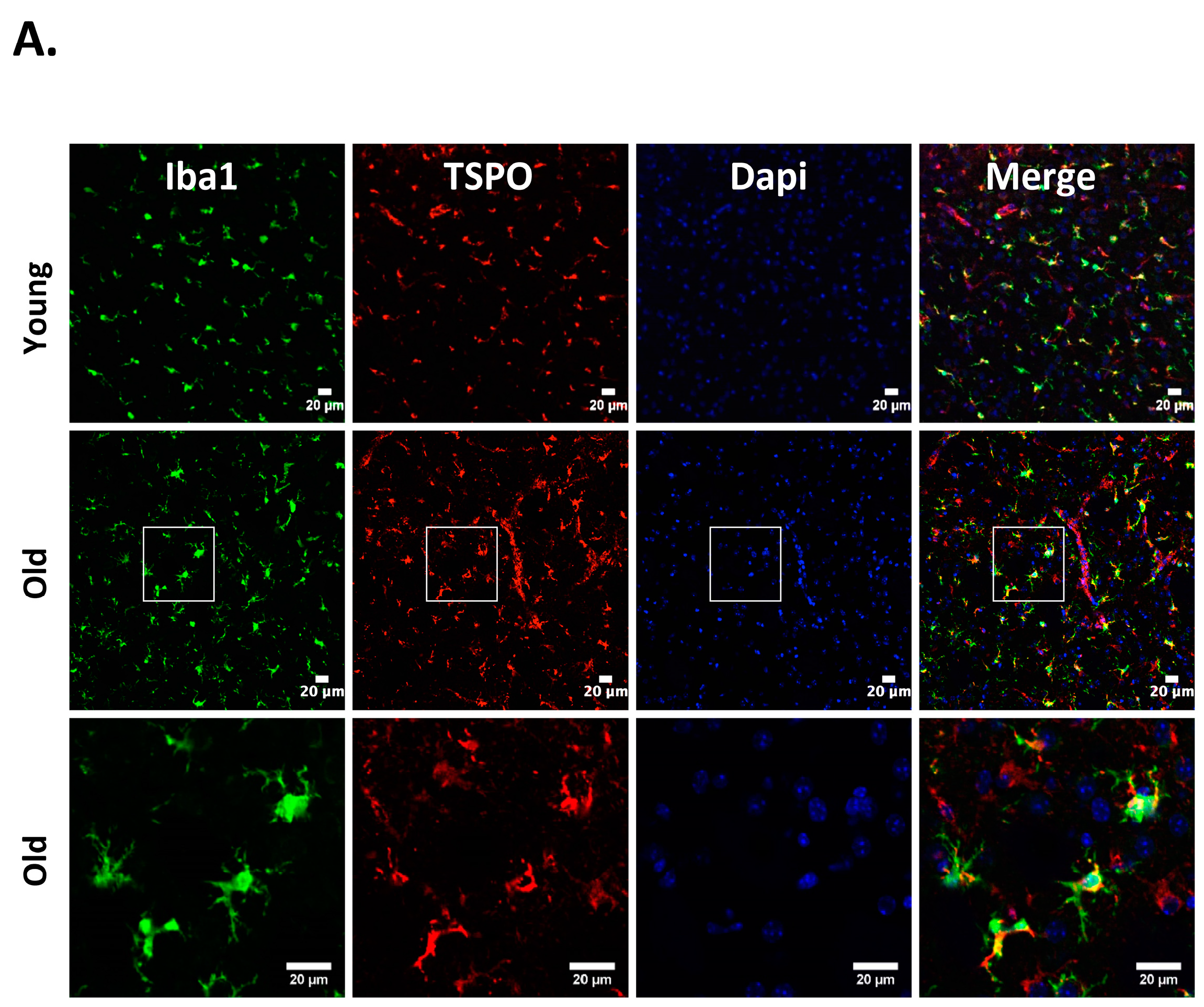
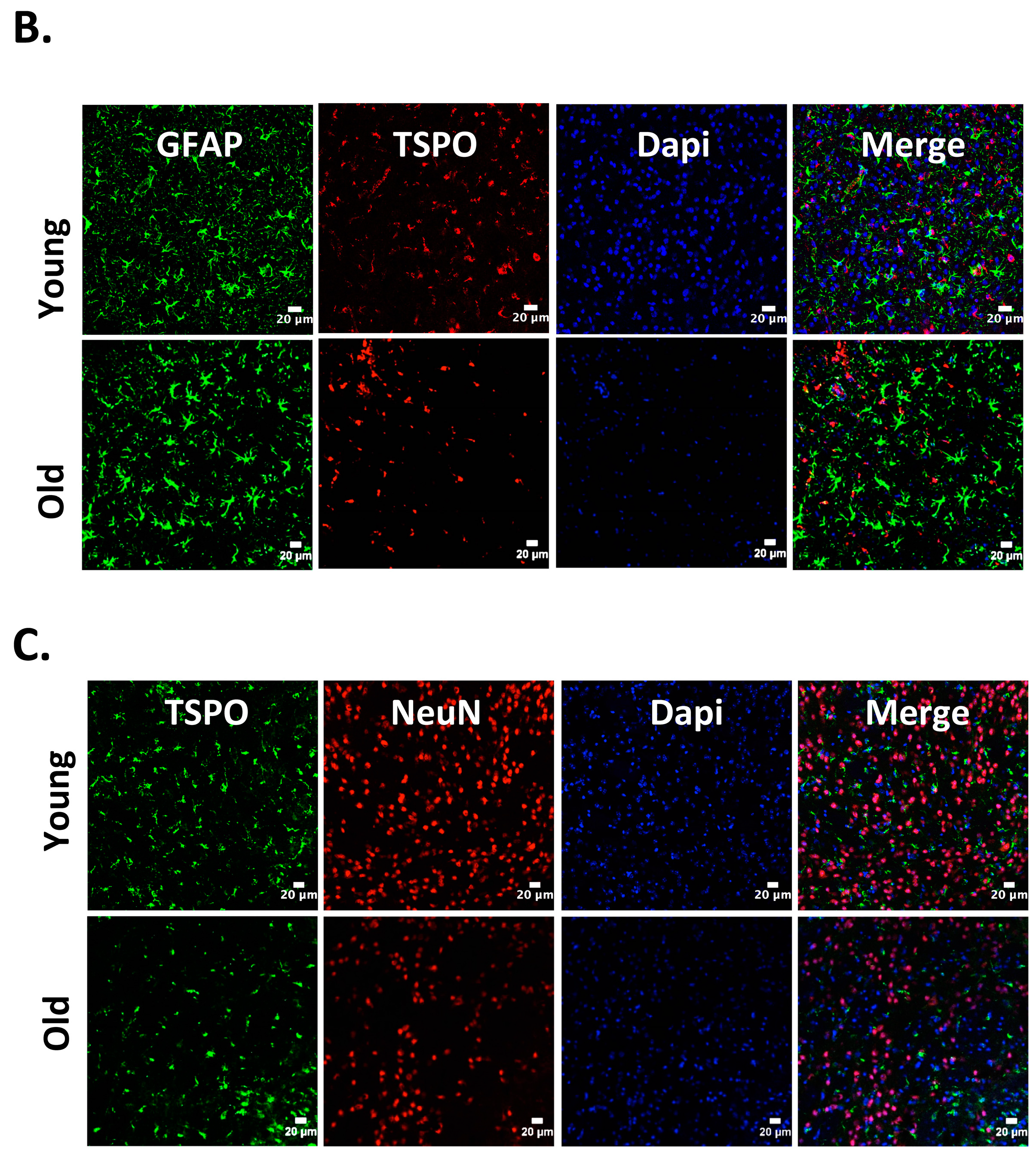
Neuroinflammation characterized by the activation of neuroimmune cells has been implicated as a pathological contributor to several neurodegenerative diseases. Under normal conditions, TSPO expression is low in immune-competent cells, macrophages, and leukocytes in the periphery, as well as in microglia and astrocytes [86]. In response to brain injury, the glial cells become activated and activated microglia/macrophage are often associated with increased expression of TSPO [18,19,87]. Therefore, TSPO is considered as a relevant molecular marker of neuroinflammation and could be an attractive therapeutic target. Though neuroinflammation is closely related to brain injuries and various neurodegenerative disorders such as Huntington’s disease, Dementia, Parkinson’s disease, and Multiple sclerosis, the precise functional consequences of microglial activation in these diseases are unclear. It is proposed that TSPO may regulate the release of pro-inflammatory cytokines during inflammation [27,88,89]. Consistently, genetic knockdown of TSPO in RAW 264.7 cells augmented hemin-induced release of proinflammatory cytokines revealing a negative regulatory role of TSPO in inflammation [27]. Further, our recent preclinical studies demonstrated an augmented expression of TSPO after intracerebral hemorrhage (ICH), a neuropathological condition that mostly afflicts the elderly population [27]. Importantly, irrespective of age, ≈85% TSPO expressing cells in the brain after ICH, co expressed Iba1 (microglia/macrophage marker) (Figure 1), implicating a possible role of TSPO in neuroinflammatory responses. Consistently, the expression of immune response genes was affected in TSPO1−/− tissues [42]. Further, TSPO expression was also observed in activated microglia/macrophages of phagocytic phenotypes [27] and TSPO ligands induced the phagocytic capacity of microglia [90] suggesting an unexplored role of TSPO in microglia/macrophage-mediated phagocytosis in pathological conditions. Altogether, these studies suggest that TSPO may modulate microglia/macrophage, the inflammatory cells of the CNS, at multiple functional levels. Although both astrocytic, as well as microglial expression of TSPO, has been observed in various neuropathological conditions in rodents [26,91,92], microglia/macrophages but not astrocytes are the significant contributors of TSPO binding sites in human neuropathologies [93,94]. Of note, radiolabeled-TSPO ligands have been widely used for monitoring the augmented brain expression of TSPO in neuropathological conditions since TSPO-dependent enhanced binding of the radiotracer can be detected and quantified using non–invasive neuroimaging techniques such as positron emission tomography (PET) or Single-photon emission computed tomography (SPECT). Given the enhanced expression of TSPO in brain inflammatory cells, the neuroimaging employing the radioligands of TSPO provides a valuable tool allowing us to track and quantify the brain inflammation, and thereby ascertain the effectiveness of therapeutic interventions in a real-time manner. Along these lines, [11] C-labeled PK11195 is the first radiotracer that was used for the evaluation of activated microglia/macrophages and neuroinflammation in vivo. However, owing to the high lipophilicity, radiolabeled PK11195 exhibited high non-specific binding and a poor signal-to-noise ratio, complicating its quantification [95,96]. This has prompted the search for radiotracers with improved capacities to quantify TSPO expression. Along these lines, several new TSPO radioligands have been developed [97], and most of them have lower lipophilicity than radiolabelled-PK11195 and improved specific-to-nonspecific binding. Though microglia/macrophages are the most prominent cell type expressing TSPO in diseased brains, the mechanisms regulating augmented TSPO expression in microglia/macrophage, as well as the precise role of TSPO in microglia/macrophage functions in neuropathological conditions, remains largely unknown.
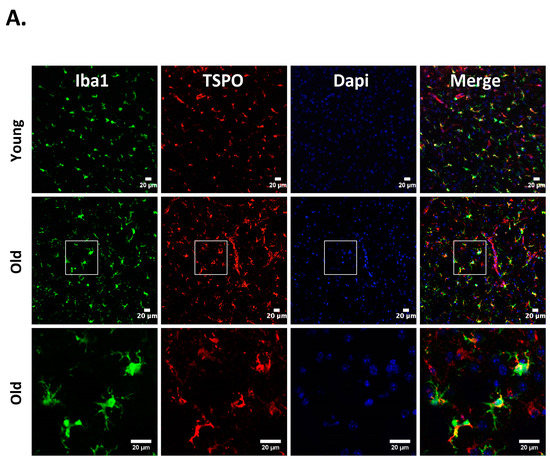
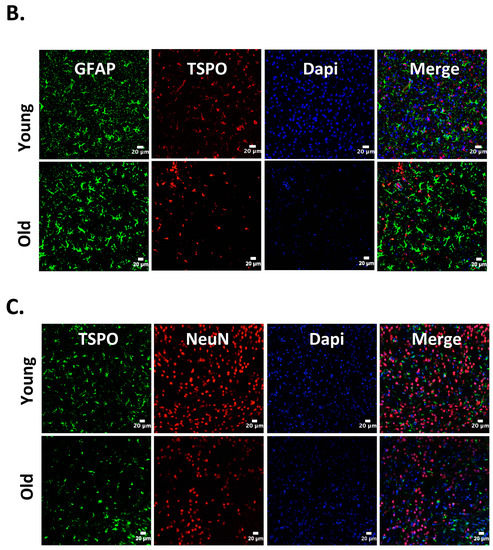
Figure 1.
Cellular localization of 18kDa translocator protein (TSPO) expression in old vs. young mice after intracerebral hemorrhage (ICH). ICH was induced in male mice (20 months or 8 weeks old), as reported previously [27] and the brain sections were subjected to immunohistochemistry [27]. Briefly, brain sections were immunolabeled for (A) TSPO and Iba1 (microglia/macrophage marker); (B) TSPO and GFAP, (astrocyte marker) and (C) TSPO and NeuN (neuronal marker), 3 days-post ICH induction. A remarkable co-localization was observed between TSPO and Iba1, whereas no TSPO expression was observed in either NeuN or GFAP positive cells in both young as well as old mice. Scale Bar = 20 μM; n = 3 mice per group.
8. TSPO as a Therapeutic Target
Accumulating evidence implicate that synthetic TSPO ligands are neuroprotective in various CNS disorders [98] and thereby, TSPO is regarded as a therapeutic target for neurologic disorders [23,99]. Along these lines, Etifoxine, a TSPO ligand and clinically approved drug for the treatment of anxiety disorders, promoted axonal regeneration and functional recovery in an animal model of peripheral nerve freeze injury [100]. Further, both first and second-generation TSPO ligands, PK11195 and DPA-713 respectively, conferred neuroprotection against quinolinic acid injection into the rat striatum [101]. Interestingly, TSPO-agonist, Ro5-4864 significantly reversed the pathology associated with Alzheimer’s disease in vivo [102]. Though, one of the key mechanisms underlying the neuroprotective effects mediated by TSPO ligands has been implicated as the stimulation of mitochondrial steroid synthesis, the precise cellular and molecular mechanisms underlying TSPO ligand-mediated neuroprotection in neuropathological conditions are not well defined. However, in contrast to the neuroprotective role of ligand-mediated TSPO signaling as outlined above, the hGFAP-driven-conditional TSPO knockout mice exhibited reduced astrogliosis and experimental autoimmune encephalomyelitis clinical scoring in a preclinical mouse model of multiple sclerosis (MS) [103] and this discrepancy between the genetic and pharmacological studies demands a thorough investigation. Notably, the widely used TSPO agonist, Etifoxine binds and modulates GABAA receptors further implicating the need to establish the therapeutic potential of TSPO. Altogether, it is imperative to reassess the neuroprotective efficacy of TSPO ligands employing transgenic animal models, as it would validate the functional role of TSPO as a therapeutic target in various neuropathological conditions.
9. Conclusions
TSPO is an evolutionarily conserved protein with enigmatic functions. Apart from the identification of TSPO as a biomarker of glial activation future studies are warranted characterizing the precise role of TSPO in mitochondrial functioning as well as in cellular inflammatory and oxidative responses and it would also validate the therapeutic potential of TSPO in various pathological conditions.
10. Ethics Statement
Animal studies were reviewed and approved by the Committee on Biosafety and Animal Care and Use at Augusta University (protocol #2012-0459; 5 May 2015), in compliance with NIH and USDA guidelines.
Author Contributions
F.B. carried out the immunohistochemical studies. S.S-R. designed the experiments, conducted the animal surgeries, and wrote the manuscript.
Acknowledgments
This work was supported by a grant from the American Heart Association (14SDG18730034) and Augusta University start-up funds to S.S-R. None of the funding bodies had a role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript. We also would like to acknowledge Carlos Isales, MD, Department of Neuroscience and Regenerative Medicine, Augusta University for providing us with the aged mice.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| TSPO | 18 kDa translocator protein |
| GABA | gamma-aminobutyric acid |
| HUGO | human genome organization |
| CNS | central nervous system |
| ROS | reactive oxygen species |
| ALA | 5-amino-laevulinic acid |
| CRISPR/Cas9 | clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9 |
| Iba1 | ionized calcium binding adaptor molecule 1 |
| GFAP | glial fibrillary acidic protein |
| DPA-713 | N,N-diethyl-2-[2-(4-[methoxyphenyl)-5,7-dimethyl-pyrazolo-[1,5-α]pyrimidin-3-yl]-acetamide |
| GABAA receptors | gamma-aminobutyric acid type A receptors |
References
- Braestrup, C.; Squires, R.F. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc. Natl. Acad. Sci. USA 1977, 74, 3805–3809. [Google Scholar] [CrossRef] [PubMed]
- Tallman, J.F.; Thomas, J.W.; Gallager, D.W. GABAergic modulation of benzodiazepine binding site sensitivity. Nature 1978, 274, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Tallman, J.F.; Paul, S.M.; Skolnick, P.; Gallager, D.W. Receptors for the age of anxiety: Pharmacology of the benzodiazepines. Science 1980, 207, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapere, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.G.; Mohler, H. Benzodiazepine receptors. Neuropharmacology 1984, 23, 233–242. [Google Scholar] [CrossRef]
- Woods, M.J.; Williams, D.C. Multiple forms and locations for the peripheral-type benzodiazepine receptor. Biochem. Pharmacol. 1996, 52, 1805–1814. [Google Scholar] [CrossRef]
- Gavish, M.; Bachman, I.; Shoukrun, R.; Katz, Y.; Veenman, L.; Weisinger, G.; Weizman, A. Enigma of the peripheral benzodiazepine receptor. Pharmacol. Rev. 1999, 51, 629–650. [Google Scholar] [PubMed]
- Surinkaew, S.; Chattipakorn, S.; Chattipakorn, N. Roles of mitochondrial benzodiazepine receptor in the heart. Can. J. Cardiol. 2011, 27, 262.e3–262.e13. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiao, J.; Liang, D.; Zhang, H.; Zhang, G.; Liu, Y.; Zhang, Y.; Liu, Y.; Yu, Z.; Yan, B.; et al. Inhibition of mitochondrial translocator protein prevents atrial fibrillation. Eur. J. Pharmacol. 2010, 632, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Gavish, M. The peripheral-type benzodiazepine receptor and the cardiovascular system. Implications for drug development. Pharmacol. Ther. 2006, 110, 503–524. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Lindemann, P.; Feuilloley, M.G.; Papadopoulos, V. Structural and functional evolution of the translocator protein (18 kDa). Curr. Mol. Med. 2012, 12, 369–386. [Google Scholar] [PubMed]
- Galiegue, S.; Casellas, P.; Kramar, A.; Tinel, N.; Simony-Lafontaine, J. Immunohistochemical assessment of the peripheral benzodiazepine receptor in breast cancer and its relationship with survival. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 2058–2064. [Google Scholar] [CrossRef]
- Batarseh, A.; Papadopoulos, V. Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol. Cell. Endocrinol. 2010, 327, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yeliseev, A.A.; Krueger, K.E.; Kaplan, S. A mammalian mitochondrial drug receptor functions as a bacterial “oxygen” sensor. Proc. Natl. Acad. Sci. USA 1997, 94, 5101–5106. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; McCabe, R.T.; Rennert, H.; Budarf, M.L.; Sayegh, R.; Emanuel, B.S.; Skolnick, P.; Strauss, J.F., 3rd. The human “peripheral-type” benzodiazepine receptor: Regional mapping of the gene and characterization of the receptor expressed from cDNA. DNA Cell Biol. 1992, 11, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Riond, J.; Mattei, M.G.; Kaghad, M.; Dumont, X.; Guillemot, J.C.; Le Fur, G.; Caput, D.; Ferrara, P. Molecular cloning and chromosomal localization of a human peripheral-type benzodiazepine receptor. Eur. J. Biochem./FEBS 1991, 195, 305–311. [Google Scholar] [CrossRef]
- Bucan, M.; Gatalica, B.; Nolan, P.; Chung, A.; Leroux, A.; Grossman, M.H.; Nadeau, J.H.; Emanuel, B.S.; Budarf, M. Comparative mapping of 9 human chromosome 22q loci in the laboratory mouse. Hum. Mol. Genet. 1993, 2, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.K.; Guilarte, T.R. Translocator protein 18 kDa (TSPO): Molecular sensor of brain injury and repair. Pharmacol. Ther. 2008, 118, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cosenza-Nashat, M.; Zhao, M.L.; Suh, H.S.; Morgan, J.; Natividad, R.; Morgello, S.; Lee, S.C. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol. Appl. Neurobiol. 2009, 35, 306–328. [Google Scholar] [CrossRef] [PubMed]
- Anholt, R.R.; Pedersen, P.L.; De Souza, E.B.; Snyder, S.H. The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J. Biol. Chem. 1986, 261, 576–583. [Google Scholar] [PubMed]
- Mukhin, A.G.; Papadopoulos, V.; Costa, E.; Krueger, K.E. Mitochondrial benzodiazepine receptors regulate steroid biosynthesis. Proc. Natl. Acad. Sci. USA 1989, 86, 9813–9816. [Google Scholar] [CrossRef] [PubMed]
- Krueger, K.E.; Papadopoulos, V. Peripheral-type benzodiazepine receptors mediate translocation of cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. J. Biol. Chem. 1990, 265, 15015–15022. [Google Scholar] [PubMed]
- Rupprecht, R.; Papadopoulos, V.; Rammes, G.; Baghai, T.C.; Fan, J.; Akula, N.; Groyer, G.; Adams, D.; Schumacher, M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010, 9, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Yasin, N.; Veenman, L.; Singh, S.; Azrad, M.; Bode, J.; Vainshtein, A.; Caballero, B.; Marek, I.; Gavish, M. Classical and Novel TSPO Ligands for the Mitochondrial TSPO Can Modulate Nuclear Gene Expression: Implications for Mitochondrial Retrograde Signaling. Int. J. Mol. Sci. 2017, 18, 786. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, X.; Zhao, L.; Ma, W.; Rodriguez, I.R.; Fariss, R.N.; Wong, W.T. Macroglia-microglia interactions via TSPO signaling regulates microglial activation in the mouse retina. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 3793–3806. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, A.C.; Guilarte, T.R. Cellular and subcellular localization of peripheral benzodiazepine receptors after trimethyltin neurotoxicity. J. Neurochem. 2000, 74, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Bonsack, F.; Alleyne, C.H., Jr.; Sukumari-Ramesh, S. Augmented expression of TSPO after intracerebral hemorrhage: A role in inflammation? J. Neuroinflamm. 2016, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Auta, J.; Guidotti, A.; Korneyev, A.; Romeo, E. The pharmacology of neurosteroidogenesis. J. Steroid Biochem. Mol. Biol. 1994, 49, 385–389. [Google Scholar] [CrossRef]
- Lacapere, J.J.; Papadopoulos, V. Peripheral-type benzodiazepine receptor: Structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids 2003, 68, 569–585. [Google Scholar] [CrossRef]
- Romeo, E.; Cavallaro, S.; Korneyev, A.; Kozikowski, A.P.; Ma, D.; Polo, A.; Costa, E.; Guidotti, A. Stimulation of brain steroidogenesis by 2-aryl-indole-3-acetamide derivatives acting at the mitochondrial diazepam-binding inhibitor receptor complex. J. Pharmacol. Exp. Ther. 1993, 267, 462–471. [Google Scholar] [PubMed]
- Rone, M.B.; Fan, J.; Papadopoulos, V. Cholesterol transport in steroid biosynthesis: Role of protein-protein interactions and implications in disease states. Biochim. Biophys. Acta 2009, 1791, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Mukhin, A.G.; Costa, E.; Krueger, K.E. The peripheral-type benzodiazepine receptor is functionally linked to Leydig cell steroidogenesis. J. Biol. Chem. 1990, 265, 3772–3779. [Google Scholar] [PubMed]
- Chung, J.Y.; Chen, H.; Midzak, A.; Burnett, A.L.; Papadopoulos, V.; Zirkin, B.R. Drug ligand-induced activation of translocator protein (TSPO) stimulates steroid production by aged brown Norway rat Leydig cells. Endocrinology 2013, 154, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Lecanu, L.; Brown, R.C.; Han, Z.; Yao, Z.X. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience 2006, 138, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Cavallini, C.; Da Pozzo, E.; Taliani, S.; Da Settimo, F.; Martini, C. The Anxiolytic Etifoxine Binds to TSPO Ro5-4864 Binding Site with Long Residence Time Showing a High Neurosteroidogenic Activity. ACS Chem. Neurosci. 2017, 8, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Da Pozzo, E.; Giacomelli, C.; Barresi, E.; Taliani, S.; Da Settimo, F.; Martini, C. TSPO ligand residence time: A new parameter to predict compound neurosteroidogenic efficacy. Sci. Rep. 2016, 6, 18164. [Google Scholar] [CrossRef] [PubMed]
- Farges, R.; Joseph-Liauzun, E.; Shire, D.; Caput, D.; Le Fur, G.; Ferrara, P. Site-directed mutagenesis of the peripheral benzodiazepine receptor: Identification of amino acids implicated in the binding site of Ro5-4864. Mol. Pharmacol. 1994, 46, 1160–1167. [Google Scholar] [PubMed]
- Jaremko, L.; Jaremko, M.; Giller, K.; Becker, S.; Zweckstetter, M. Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science 2014, 343, 1363–1366. [Google Scholar] [CrossRef] [PubMed]
- Le Fur, G.; Vaucher, N.; Perrier, M.L.; Flamier, A.; Benavides, J.; Renault, C.; Dubroeucq, M.C.; Gueremy, C.; Uzan, A. Differentiation between two ligands for peripheral benzodiazepine binding sites, [3H]RO5-4864 and [3H]PK 11195, by thermodynamic studies. Life Sci. 1983, 33, 449–457. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Amri, H.; Boujrad, N.; Cascio, C.; Culty, M.; Garnier, M.; Hardwick, M.; Li, H.; Vidic, B.; Brown, A.S.; et al. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids 1997, 62, 21–28. [Google Scholar] [CrossRef]
- Wang, H.; Zhai, K.; Xue, Y.; Yang, J.; Yang, Q.; Fu, Y.; Hu, Y.; Liu, F.; Wang, W.; Cui, L.; et al. Global Deletion of TSPO Does Not Affect the Viability and Gene Expression Profile. PLoS ONE 2016, 11, e0167307. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.N.; Morohaku, K.; Manna, P.R.; Pelton, S.H.; Butler, W.R.; Stocco, D.M.; Selvaraj, V. Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J. Biol. Chem. 2014, 289, 27444–27454. [Google Scholar] [CrossRef] [PubMed]
- Banati, R.B.; Middleton, R.J.; Chan, R.; Hatty, C.R.; Kam, W.W.; Quin, C.; Graeber, M.B.; Parmar, A.; Zahra, D.; Callaghan, P.; et al. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat. Commun. 2014, 5, 5452. [Google Scholar] [CrossRef] [PubMed]
- Morohaku, K.; Pelton, S.H.; Daugherty, D.J.; Butler, W.R.; Deng, W.; Selvaraj, V. Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinology 2014, 155, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.R.; Fan, J.; Campioli, E.; Venugopal, S.; Midzak, A.; Daly, E.; Harlay, A.; Issop, L.; Libri, V.; Kalogiannopoulou, D.; et al. TSPO mutations in rats and a human polymorphism impair the rate of steroid synthesis. Biochem. J. 2017, 474, 3985–3999. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.M.; Ji, B.; Kito, S.; Suhara, T.; Higuchi, M. Steroidogenic abnormalities in translocator protein knockout mice and significance in the aging male. Biochem. J. 2018, 475, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Berkovich, A.; Krueger, K.E.; Costa, E.; Guidotti, A. Diazepam binding inhibitor and its processing products stimulate mitochondrial steroid biosynthesis via an interaction with mitochondrial benzodiazepine receptors. Endocrinology 1991, 129, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.N.; Zhao, A.H.; Stocco, D.M.; Selvaraj, V. PK11195 effect on steroidogenesis is not mediated through the translocator protein (TSPO). Endocrinology 2015, 156, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.H.; Tu, L.N.; Mukai, C.; Sirivelu, M.P.; Pillai, V.V.; Morohaku, K.; Cohen, R.; Selvaraj, V. Mitochondrial Translocator Protein (TSPO) Function Is Not Essential for Heme Biosynthesis. J. Biol. Chem. 2016, 291, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Chelli, B.; Falleni, A.; Salvetti, F.; Gremigni, V.; Lucacchini, A.; Martini, C. Peripheral-type benzodiazepine receptor ligands: Mitochondrial permeability transition induction in rat cardiac tissue. Biochem. Pharmacol. 2001, 61, 695–705. [Google Scholar] [CrossRef]
- Sileikyte, J.; Blachly-Dyson, E.; Sewell, R.; Carpi, A.; Menabo, R.; Di Lisa, F.; Ricchelli, F.; Bernardi, P.; Forte, M. Regulation of the mitochondrial permeability transition pore by the outer membrane does not involve the peripheral benzodiazepine receptor (Translocator Protein of 18 kDa (TSPO)). J. Biol. Chem. 2014, 289, 13769–13781. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.N.; Zhao, A.H.; Hussein, M.; Stocco, D.M.; Selvaraj, V. Translocator Protein (TSPO) Affects Mitochondrial Fatty Acid Oxidation in Steroidogenic Cells. Endocrinology 2016, 157, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Lacapere, J.J.; Delavoie, F.; Li, H.; Peranzi, G.; Maccario, J.; Papadopoulos, V.; Vidic, B. Structural and functional study of reconstituted peripheral benzodiazepine receptor. Biochem. Biophys. Res. Commun. 2001, 284, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, A.; Forchetti, C.M.; Corda, M.G.; Konkel, D.; Bennett, C.D.; Costa, E. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc. Natl. Acad. Sci. USA 1983, 80, 3531–3535. [Google Scholar] [CrossRef] [PubMed]
- Besman, M.J.; Yanagibashi, K.; Lee, T.D.; Kawamura, M.; Hall, P.F.; Shively, J.E. Identification of des-(Gly-Ile)-endozepine as an effector of corticotropin-dependent adrenal steroidogenesis: Stimulation of cholesterol delivery is mediated by the peripheral benzodiazepine receptor. Proc. Natl. Acad. Sci. USA 1989, 86, 4897–4901. [Google Scholar] [CrossRef] [PubMed]
- Bovolin, P.; Schlichting, J.; Miyata, M.; Ferrarese, C.; Guidotti, A.; Alho, H. Distribution and characterization of diazepam binding inhibitor (DBI) in peripheral tissues of rat. Regul. Pept. 1990, 29, 267–281. [Google Scholar] [CrossRef]
- Alho, H.; Harjuntausta, T.; Schultz, R.; Pelto-Huikko, M.; Bovolin, P. Immunohistochemistry of diazepam binding inhibitor (DBI) in the central nervous system and peripheral organs: Its possible role as an endogenous regulator of different types of benzodiazepine receptors. Neuropharmacology 1991, 30, 1381–1386. [Google Scholar] [CrossRef]
- Alho, H.; Varga, V.; Krueger, K.E. Expression of mitochondrial benzodiazepine receptor and its putative endogenous ligand diazepam binding inhibitor in cultured primary astrocytes and C-6 cells: Relation to cell growth. Cell Growth Differ. 1994, 5, 1005–1014. [Google Scholar] [PubMed]
- Lihrmann, I.; Plaquevent, J.C.; Tostivint, H.; Raijmakers, R.; Tonon, M.C.; Conlon, J.M.; Vaudry, H. Frog diazepam-binding inhibitor: Peptide sequence, cDNA cloning, and expression in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 6899–6903. [Google Scholar] [CrossRef] [PubMed]
- Malagon, M.; Vaudry, H.; Van Strien, F.; Pelletier, G.; Gracia-Navarro, F.; Tonon, M.C. Ontogeny of diazepam-binding inhibitor-related peptides (endozepines) in the rat brain. Neuroscience 1993, 57, 777–786. [Google Scholar] [CrossRef]
- Wendler, G.; Lindemann, P.; Lacapere, J.J.; Papadopoulos, V. Protoporphyrin IX binding and transport by recombinant mouse PBR. Biochem. Biophys. Res. Commun. 2003, 311, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Nye, J.S.; Snyder, S.H. Porphyrins are endogenous ligands for the mitochondrial (peripheral-type) benzodiazepine receptor. Proc. Natl. Acad. Sci. USA 1987, 84, 2256–2260. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xia, Y.; Meiler, J.; Ferguson-Miller, S. Characterization and modeling of the oligomeric state and ligand binding behavior of purified translocator protein 18 kDa from Rhodobacter sphaeroides. Biochemistry 2013, 52, 5884–5899. [Google Scholar] [CrossRef] [PubMed]
- Vanhee, C.; Zapotoczny, G.; Masquelier, D.; Ghislain, M.; Batoko, H. The Arabidopsis multistress regulator TSPO is a heme binding membrane protein and a potential scavenger of porphyrins via an autophagy-dependent degradation mechanism. Plant Cell 2011, 23, 785–805. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.H.; Verma, A.; Trifiletti, R.R. The peripheral-type benzodiazepine receptor: A protein of mitochondrial outer membranes utilizing porphyrins as endogenous ligands. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1987, 1, 282–288. [Google Scholar] [CrossRef]
- Verma, A.; Snyder, S.H. Peripheral type benzodiazepine receptors. Annu. Rev. Pharmacol. Toxicol. 1989, 29, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Vainshtein, A.; Yasin, N.; Azrad, M.; Gavish, M. Tetrapyrroles as Endogenous TSPO Ligands in Eukaryotes and Prokaryotes: Comparisons with Synthetic Ligands. Int. J. Mol. Sci. 2016, 17, 880. [Google Scholar] [CrossRef] [PubMed]
- Taketani, S.; Kohno, H.; Furukawa, T.; Tokunaga, R. Involvement of peripheral-type benzodiazepine receptors in the intracellular transport of heme and porphyrins. J. Biochem. 1995, 117, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Korkhov, V.M.; Sachse, C.; Short, J.M.; Tate, C.G. Three-dimensional structure of TSPO by electron cryomicroscopy of helical crystals. Structure 2010, 18, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Zoghbi, S.S.; Hong, J.; Verma, A.; Pike, V.W.; Innis, R.B.; Fujita, M. In vivo binding of protoporphyrin IX to rat translocator protein imaged with positron emission tomography. Synapse 2010, 64, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Zeno, S.; Veenman, L.; Katz, Y.; Bode, J.; Gavish, M.; Zaaroor, M. The 18 kDa mitochondrial translocator protein (TSPO) prevents accumulation of protoporphyrin IX. Involvement of reactive oxygen species (ROS). Curr. Mol. Med. 2012, 12, 494–501. [Google Scholar] [PubMed]
- Gemelli, C.; Dongmo, B.M.; Ferrarini, F.; Grande, A.; Corsi, L. Cytotoxic effect of hemin in colonic epithelial cell line: Involvement of 18 kDa translocator protein (TSPO). Life Sci. 2014, 107, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Ginter, C.; Kiburu, I.; Boudker, O. Chemical catalysis by the translocator protein (18 kDa). Biochemistry 2013, 52, 3609–3611. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kalathur, R.C.; Liu, Q.; Kloss, B.; Bruni, R.; Ginter, C.; Kloppmann, E.; Rost, B.; Hendrickson, W.A. Protein structure. Structure and activity of tryptophan-rich TSPO proteins. Science 2015, 347, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, S.L.; Matthews, E.K. Modification of the photodynamic action of delta-aminolaevulinic acid (ALA) on rat pancreatoma cells by mitochondrial benzodiazepine receptor ligands. Br. J. Cancer 1995, 71, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.D.; Ryan, P.J. Tranquillizers can block mitogenesis in 3T3 cells and induce differentiation in Friend cells. Nature 1980, 287, 160–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.K.; Morgan, J.I.; Spector, S. Differentiation of Friend erythroleukemia cells induced by benzodiazepines. Proc. Natl. Acad. Sci. USA 1984, 81, 3770–3772. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Papadopoulos, V.; Gavish, M. Channel-like functions of the 18kDa translocator protein (TSPO): Regulation of apoptosis and steroidogenesis as part of the host-defense response. Curr. Pharm. Des. 2007, 13, 2385–2405. [Google Scholar] [CrossRef] [PubMed]
- Zeno, S.; Zaaroor, M.; Leschiner, S.; Veenman, L.; Gavish, M. CoCl2 induces apoptosis via the 18 kDa translocator protein in U118MG human glioblastoma cells. Biochemistry 2009, 48, 4652–4661. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Ifuku, M.; Noda, M.; Guilarte, T.R. Translocator protein (18 kDa)/peripheral benzodiazepine receptor specific ligands induce microglia functions consistent with an activated state. Glia 2011, 59, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Lenaz, G. Role of mitochondria in oxidative stress and ageing. Biochim. Biophys. Acta 1998, 1366, 53–67. [Google Scholar] [CrossRef]
- Larcher, J.C.; Vayssiere, J.L.; Le Marquer, F.J.; Cordeau, L.R.; Keane, P.E.; Bachy, A.; Gros, F.; Croizat, B.P. Effects of peripheral benzodiazepines upon the O2 consumption of neuroblastoma cells. Eur. J. Pharmacol. 1989, 161, 197–202. [Google Scholar] [CrossRef]
- Fischer, R.; Schmitt, M.; Bode, J.G.; Haussinger, D. Expression of the peripheral-type benzodiazepine receptor and apoptosis induction in hepatic stellate cells. Gastroenterology 2001, 120, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Carayon, P.; Portier, M.; Dussossoy, D.; Bord, A.; Petitpretre, G.; Canat, X.; Le Fur, G.; Casellas, P. Involvement of peripheral benzodiazepine receptors in the protection of hematopoietic cells against oxygen radical damage. Blood 1996, 87, 3170–3178. [Google Scholar] [PubMed]
- Delavoie, F.; Li, H.; Hardwick, M.; Robert, J.C.; Giatzakis, C.; Peranzi, G.; Yao, Z.X.; Maccario, J.; Lacapere, J.J.; Papadopoulos, V. In vivo and in vitro peripheral-type benzodiazepine receptor polymerization: Functional significance in drug ligand and cholesterol binding. Biochemistry 2003, 42, 4506–4519. [Google Scholar] [CrossRef] [PubMed]
- Banati, R.B. Visualising microglial activation in vivo. Glia 2002, 40, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Scarf, A.M.; Ittner, L.M.; Kassiou, M. The translocator protein (18 kDa): Central nervous system disease and drug design. J. Med. Chem. 2009, 52, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.B.; Khoo, C.; Ryu, J.K.; van Breemen, E.; Kim, S.U.; McLarnon, J.G. Inhibition of lipopolysaccharide-induced cyclooxygenase-2, tumor necrosis factor-α and [Ca2+]i responses in human microglia by the peripheral benzodiazepine receptor ligand PK11195. J. Neurochem. 2002, 83, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Wilms, H.; Claasen, J.; Rohl, C.; Sievers, J.; Deuschl, G.; Lucius, R. Involvement of benzodiazepine receptors in neuroinflammatory and neurodegenerative diseases: Evidence from activated microglial cells in vitro. Neurobiol. Dis. 2003, 14, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Karlstetter, M.; Nothdurfter, C.; Aslanidis, A.; Moeller, K.; Horn, F.; Scholz, R.; Neumann, H.; Weber, B.H.; Rupprecht, R.; Langmann, T. Translocator protein (18 kDa) (TSPO) is expressed in reactive retinal microglia and modulates microglial inflammation and phagocytosis. J. Neuroinflamm. 2014, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Maeda, J.; Higuchi, M.; Inaji, M.; Ji, B.; Haneda, E.; Okauchi, T.; Zhang, M.R.; Suzuki, K.; Suhara, T. Phase-dependent roles of reactive microglia and astrocytes in nervous system injury as delineated by imaging of peripheral benzodiazepine receptor. Brain Res. 2007, 1157, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.K.; Guilarte, T.R. Imaging the peripheral benzodiazepine receptor response in central nervous system demyelination and remyelination. Toxicol. Sci. 2006, 91, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Venneti, S.; Lopresti, B.J.; Wang, G.; Hamilton, R.L.; Mathis, C.A.; Klunk, W.E.; Apte, U.M.; Wiley, C.A. PK11195 labels activated microglia in Alzheimer’s disease and in vivo in a mouse model using PET. Neurobiol. Aging 2009, 30, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Banati, R.B.; Goerres, G.W.; Myers, R.; Gunn, R.N.; Turkheimer, F.E.; Kreutzberg, G.W.; Brooks, D.J.; Jones, T.; Duncan, J.S. [11C](R)-PK11195 positron emission tomography imaging of activated microglia in vivo in Rasmussen’s encephalitis. Neurology 1999, 53, 2199–2203. [Google Scholar] [CrossRef] [PubMed]
- Petit-Taboue, M.C.; Baron, J.C.; Barre, L.; Travere, J.M.; Speckel, D.; Camsonne, R.; MacKenzie, E.T. Brain kinetics and specific binding of [11C]PK11195 to ω 3 sites in baboons: Positron emission tomography study. Eur. J. Pharmacol. 1991, 200, 347–351. [Google Scholar] [CrossRef]
- Boutin, H.; Chauveau, F.; Thominiaux, C.; Kuhnast, B.; Gregoire, M.C.; Jan, S.; Trebossen, R.; Dolle, F.; Tavitian, B.; Mattner, F.; et al. In vivo imaging of brain lesions with [11C]CLINME, a new PET radioligand of peripheral benzodiazepine receptors. Glia 2007, 55, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, F.; Boutin, H.; Van Camp, N.; Thominiaux, C.; Hantraye, P.; Rivron, L.; Marguet, F.; Castel, M.N.; Rooney, T.; Benavides, J.; et al. In vivo imaging of neuroinflammation in the rodent brain with [11C]SSR180575, a novel indoleacetamide radioligand of the translocator protein (18 kDa). Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Da Pozzo, E.; Giacomelli, C.; Barresi, E.; Costa, B.; Taliani, S.; Passetti Fda, S.; Martini, C. Targeting the 18-kDa translocator protein: Recent perspectives for neuroprotection. Biochem. Soc. Trans. 2015, 43, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Lecanu, L. Translocator protein (18 kDa) TSPO: An emerging therapeutic target in neurotrauma. Exp. Neurol. 2009, 219, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Girard, C.; Liu, S.; Cadepond, F.; Adams, D.; Lacroix, C.; Verleye, M.; Gillardin, J.M.; Baulieu, E.E.; Schumacher, M.; Schweizer-Groyer, G. Etifoxine improves peripheral nerve regeneration and functional recovery. Proc. Natl. Acad. Sci. USA 2008, 105, 20505–20510. [Google Scholar] [CrossRef] [PubMed]
- Leaver, K.R.; Reynolds, A.; Bodard, S.; Guilloteau, D.; Chalon, S.; Kassiou, M. Effects of translocator protein (18 kDa) ligands on microglial activation and neuronal death in the quinolinic-acid-injected rat striatum. ACS Chem. Neurosci. 2012, 3, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.M.; Garcia-Segura, L.M.; Caruso, D.; Jayaraman, A.; Lee, J.W.; Melcangi, R.C.; Pike, C.J. Ligand for translocator protein reverses pathology in a mouse model of Alzheimer’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 8891–8897. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, D.J.; Chechneva, O.; Mayrhofer, F.; Deng, W. The hGFAP-driven conditional TSPO knockout is protective in a mouse model of multiple sclerosis. Sci. Rep. 2016, 6, 22556. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).