Characterization of UDP-Activated Purinergic Receptor P2Y6 Involved in Japanese Flounder Paralichthys olivaceus Innate Immunity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sequence Analysis of Japanese Flounder P2Y6 Receptor
2.2. Expression of P2Y6 Receptor mRNA Transcripts in Japanese Flounder Tissues
2.3. The Effects of Inflammatory Challenge on P2Y6 Receptor Expression in Japanese Flounder Immune Cells
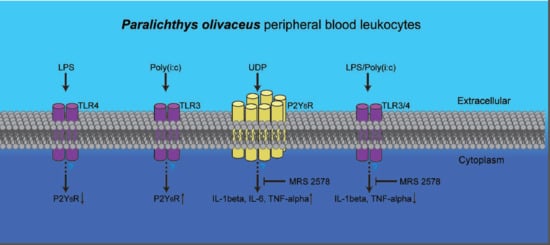
2.4. P2Y6 Receptor-Mediated Innate Immune Response in Japanese Flounder PBL Cells
3. Material and Methods
3.1. Ethics Statement
3.2. Fish Maintenance
3.3. RNA Purification, cDNA Synthesis and Gene Cloning
3.4. Sequence Alignment and Phylogenetic Analysis
3.5. Analysis of P2Y6 Receptor Gene Expression in Japanese Flounder Tissues
3.6. Isolation, Cell Culture and Inflammatory Stimulation of Japanese Flounder Head Kidney Macrophages and Peripheral Blood Leukocytes
3.7. Pharmacological Treatment
3.8. Gene Expression Analysis
3.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Elliott, M.; Chekeni, F.; Trampont, P.; Lazarowski, E.; Kadl, A.; Walk, S.; Park, D.; Woodson, R.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Zhang, G.; Zhang, X.; Tan, B.; Lv, Z.; Liu, M.; Ren, H.; Qian, M.; Du, B. TLR-Activated Gap Junction Channels Protect Mice against Bacterial Infection through Extracellular UDP Release. J. Immunol. 2016, 196, 1790–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chekeni, F.B.; Elliott, M.R.; Sandilos, J.K.; Walk, S.F.; Kinchen, J.M.; Lazarowski, E.R.; Armstrong, A.J.; Penuela, S.; Laird, D.W.; Salvesen, G.S.; et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 2010, 467, 863–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 2014, 509, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purine and pyrimidine receptors. Cell. Mol. Life. Sci. 2007, 64, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.P.; Müller, T.; Grimm, M.; von Gernler, V.; Vetter, B.; Dürk, T.; Cicko, S.; Ayata, C.K.; Sorichter, S.; Robaye, B.; et al. Purinergic Receptor Type 6 Contributes to Airway Inflammation and Remodeling in Experimental Allergic Airway Inflammation. Am. J. Respir. Crit. Care Med. 2011, 184, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, S.; Shigemoto-Mogami, Y.; Nasu-Tada, K.; Shinozaki, Y.; Ohsawa, K.; Tsuda, M.; Joshi, B.V.; Jacobson, K.A.; Kohsaka, S.; Inoue, K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 2007, 446, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Warny, M.; Aboudola, S.; Robson, S.C.; Sévigny, J.; Communi, D.; Soltoff, S.P.; Kelly, C.P. P2Y6 Nucleotide Receptor Mediates Monocyte Interleukin-8 Production in Response to UDP or Lipopolysaccharide. J. Biochem. Physiol. 2001, 276, 26051–26056. [Google Scholar]
- Cox, M.A.; Gomes, B.; Palmer, K.; Du, K.; Wiekowski, M.; Wilburn, B.; Petro, M.; Chou, C.-C.; Desquitado, C.; Schwarz, M.; et al. The pyrimidinergic P2Y6 receptor mediates a novel release of proinflammatory cytokines and chemokines in monocytic cells stimulated with UDP. Biochem. Biophys. Res. Commun. 2005, 330, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Korcok, J.; Raimundo, L.N.; Du, X.; Sims, S.M.; Dixon, S.J. P2Y6 Nucleotide Receptors Activate NF-κB and Increase Survival of Osteoclasts. J. Biol. Chem. 2005, 280, 16909–16915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Z.; Ren, H.; Yue, M.; Huang, K.; Gu, H.; Liu, M.; Du, B.; Qian, M. P2Y6 Agonist Uridine 5′-Diphosphate Promotes Host Defense against Bacterial Infection via Monocyte Chemoattractant Protein-1–Mediated Monocytes/Macrophages Recruitment. J. Immunol. 2011, 186, 5376–5387. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hao, G.; Xu, Y.; Wang, N.; Li, J.; Geng, X.; Sun, J. Functional characterization of purinergic P2Y2 and P2Y12 receptors involved in Japanese flounder (Paralichthys olivaceus) innate immune responses. Fish Shellfish Immunol. 2018, 75, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.; Paoletta, S.; Katritch, V.; Wu, B.; Gao, Z.; Zhao, Q.; Stevens, R.; Kiselev, E. Nucleotides Acting at P2Y Receptors: Connecting Structure and Function. Mol. Pharmacol. 2015, 88, 220–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinson, A.E.; Harden, T.K. Differential Regulation of the Uridine Nucleotide-activated P2Y4 and P2Y6 Receptors: SER-333 AND SER-334 IN THE CARBOXYL TERMINUS ARE INVOLVED IN AGONIST-DEPENDENT PHOSPHORYLATION DESENSITIZATION AND INTERNALIZATION OF THE P2Y4 RECEPTOR. J. Biol. Chem. 2001, 276, 11939–11948. [Google Scholar] [CrossRef] [PubMed]
- Abbracchio, M.P.; Burnstock, G.; Boeynaems, J.-M.; Barnard, E.A.; Boyer, J.L.; Kennedy, C.; Knight, G.E.; Fumagalli, M.; Gachet, C.; Jacobson, K.A.; et al. International Union of Pharmacology LVIII: Update on the P2Y G Protein-Coupled Nucleotide Receptors: From Molecular Mechanisms and Pathophysiology to Therapy. Pharmacol. Rev. 2006, 58, 281–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, D.; Chambers, J.; Wahlin, J.; Tan, K.; Moore, G.; Jenkins, O.; Emson, P.; Murdock, P. Expression pattern of human P2Y receptor subtypes: A quantitative reverse transcription-polymerase chain reaction study. Biochim. Biophys. Acta. 2001, 1521, 107–119. [Google Scholar] [CrossRef]
- Chang, K.; Hanaoka, K.; Kumada, M.; Takuwa, Y. Molecular Cloning and Functional Analysis of a Novel P2 Nucleotide Receptor. J. Biol. Chem. 1995, 270, 26152–26158. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, X.; Hao, G.; Geng, X.; Zhan, W.; Sun, J. Identification and characterization of ATP-gated P2X2 receptor gene dominantly expressed in the Japanese flounder (Paralichthys olivaceus) head kidney macrophages. Fish Shellfish Immunol. 2016, 54, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Coddou, C.; Geng, X.; Wei, J.; Sun, J. Molecular Characterization and Expression Analysis of ATP-Gated P2X7 Receptor Involved in Japanese Flounder (Paralichthys olivaceus) Innate Immune Response. PLoS ONE 2014, 9, e96625. [Google Scholar] [CrossRef] [PubMed]
- Glass, A.M.; Snyder, E.G.; Taffet, S.M. Connexins and pannexins in the immune system and lymphatic organs. Cell. Mol. Life Sci. 2015, 72, 2899–2910. [Google Scholar] [CrossRef] [PubMed]
- Grbic, D.M.; Degagné, É.; Langlois, C.; Dupuis, A.-A.; Gendron, F.-P. Intestinal Inflammation Increases the Expression of the P2Y6 Receptor on Epithelial Cells and the Release of CXC Chemokine Ligand 8 by UDP. J. Immunol. 2008, 180, 2659–2668. [Google Scholar] [CrossRef] [PubMed]
- Somers, G.R.; Hammet, F.M.; Trute, L.; Southey, M.C.; Venter, D.J. Expression of the P2Y6 purinergic receptor in human T cells infiltrating inflammatory bowel disease. Lab. Investig. 1998, 78, 1375–1383. [Google Scholar] [PubMed]
- Riegel, A.-K.; Faigle, M.; Zug, S.; Rosenberger, P.; Robaye, B.; Boeynaems, J.-M.; Idzko, M.; Eltzschig, H.K. Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation. Blood 2011, 117, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Mamedova, L.K.; Joshi, B.V.; Gao, Z.-G.; von Kügelgen, I.; Jacobson, K.A. Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem. Pharmacol. 2004, 67, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Yebdri, F.B.; Kukulski, F.; Tremblay, A.; Sévigny, J. Concomitant activation of P2Y2 and P2Y6 receptors on monocytes is required for TLR1/2-induced neutrophil migration by regulating IL-8 secretion. Eur. J. Immunol. 2009, 39, 2885–2894. [Google Scholar] [CrossRef] [PubMed]
- Kukulski, F.; Yebdri, F.B.; Lefebvre, J.; Warny, M.; Tessier, P.A.; Sévigny, J. Extracellular nucleotides mediate LPS-induced neutrophil migration in vitro and in vivo. J. Leukocyte Biol. 2007, 81, 1269–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar, I.; Guns, P.-J.; Metallo, J.; Cammarata, D.; Wilkin, F.; Boeynams, J.-M.; Bult, H.; Robaye, B. Knockout Mice Reveal a Role for P2Y6 Receptor in Macrophages, Endothelial Cells, and Vascular Smooth Muscle Cells. Mol. Pharmacol. 2008, 74, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.A.; Yan, M.; Search, D.; Zhang, R.; Carson, N.L.; Ryan, C.S.; Smith-Monroy, C.; Zheng, J.; Chen, J.; Kong, Y.; et al. P2Y6 Receptor Potentiates Pro-Inflammatory Responses in Macrophages and Exhibits Differential Roles in Atherosclerotic Lesion Development. PLoS ONE 2014, 9, e111385. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jia, Z.; Li, X.; Geng, X.; Sun, J. Identification and expression analysis of lipopolysaccharide-induced TNF-α factor gene in Chinese mitten crab Eriocheir sinensis. Fish Shellfish Immunol. 2014, 38, 190–195. [Google Scholar] [CrossRef] [PubMed]






| Primer Name | Sequences (5′→3′) | Applications |
|---|---|---|
| P2Y6-f | ATGAAGATGCCACATTCT | Gene cloning |
| P2Y6-r qP2Y6-f qP2Y6-r | TTGTTCGCTCACACACTC AGTGCGGAGATGCGGGAC GGTATCGTGGCTGTGTGAAGTA | quantitative real-time RT-PCR |
| IL-1β-f IL-1β-r IL-6-f IL-6-r | CCTGTCGTTCTGGGCATCAA CACCCCGCTGTCCTGCTT CAGCTGCTGCAAGACATGGA GATGTTGTGCGCCGTCATC | |
| TNF-α-f | CCGACTGGATGTGTAAGGTG | |
| TNF-α-r | GTTGTGGGGTTCTGTTTTCTC | |
| β-actin-f β-actin-r | AGGTTCCGTTGTCCCG TGGTTCCTCCAGATAGCAC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, J.; Wang, N.; Hao, G.; Sun, J. Characterization of UDP-Activated Purinergic Receptor P2Y6 Involved in Japanese Flounder Paralichthys olivaceus Innate Immunity. Int. J. Mol. Sci. 2018, 19, 2095. https://doi.org/10.3390/ijms19072095
Li S, Li J, Wang N, Hao G, Sun J. Characterization of UDP-Activated Purinergic Receptor P2Y6 Involved in Japanese Flounder Paralichthys olivaceus Innate Immunity. International Journal of Molecular Sciences. 2018; 19(7):2095. https://doi.org/10.3390/ijms19072095
Chicago/Turabian StyleLi, Shuo, Jiafang Li, Nan Wang, Gaixiang Hao, and Jinsheng Sun. 2018. "Characterization of UDP-Activated Purinergic Receptor P2Y6 Involved in Japanese Flounder Paralichthys olivaceus Innate Immunity" International Journal of Molecular Sciences 19, no. 7: 2095. https://doi.org/10.3390/ijms19072095
APA StyleLi, S., Li, J., Wang, N., Hao, G., & Sun, J. (2018). Characterization of UDP-Activated Purinergic Receptor P2Y6 Involved in Japanese Flounder Paralichthys olivaceus Innate Immunity. International Journal of Molecular Sciences, 19(7), 2095. https://doi.org/10.3390/ijms19072095