Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases
Abstract
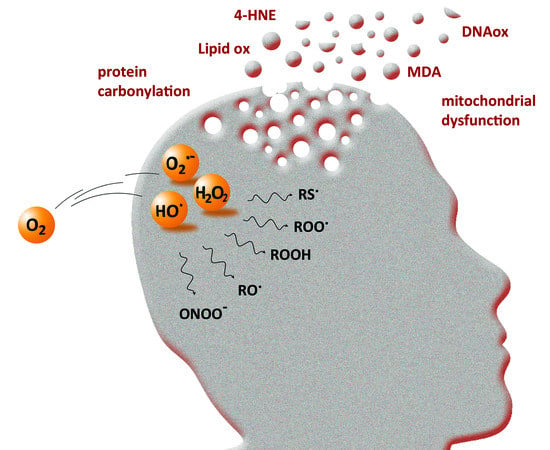
:1. Introduction
2. Production of ROS
2.1. Production of ROS In Vivo, Regulation and Oxidative Stress
2.2. Production of ROS In Vitro
3. Chemical Properties and Reactivity of ROS
3.1. The Superoxide Anion
3.2. The Hydroxyl Radical
3.3. Hydrogen Peroxide
3.4. Peroxyl Radicals, Hydroperoxides and Alkoxyl Radicals
4. The Implication of ROS in Neurodegenerative Diseases
5. Concluding Remarks
Funding
Conflicts of Interest
References
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Oxford science Publications: Oxford, UK, 1999. [Google Scholar]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate constants for reactions of peroxyl radicals in fluid solutions. J. Phys. Chem. Ref. Data 1990, 19, 413–513. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Generation and propagation of radical reactions on proteins. Biochim. Et Biophys. Acta 2001, 1504, 196–219. [Google Scholar] [CrossRef] [Green Version]
- Haber, F.; Weiss, J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. Lond. 1934, 147, 332–351. [Google Scholar]
- Rhee, S.G. Redox signaling: Hydrogen peroxide as intracellular messenger. Exp. Mol. Med. 1999, 31, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhuang, Z.; Wang, W.; He, L.; Wu, H.; Cao, Y.; Pan, F.; Zhao, J.; Hu, Z.; Sekhar, C.; et al. OGG1 is essential in oxidative stress induced DNA demethylation. Cell Signal. 2016, 28, 1163–1171. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Vignais, P.V. The superoxide-generating NADPH oxidase: Structural aspects and activation mechanism. Cell. Mol. Life Sci. 2002, 59, 1428–1459. [Google Scholar] [CrossRef]
- Finkel, T. Redox-dependent signal transduction. FEBS Lett. 2000, 476, 52–54. [Google Scholar] [CrossRef] [Green Version]
- Souza, H.P.; Laurindo, F.R.; Ziegelstein, R.C.; Berlowitz, C.O.; Zweier, J.L. Vascular NAD(P)H oxidase is distinct from the phagocytic enzyme and modulates vascular reactivity control. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H658–H667. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Schmidt, H.M.; Kelley, E.E.; Straub, A.C. The impact of xanthine oxidase (XO) on hemolytic diseases. Redox Biol. 2019, 21, 101072. [Google Scholar] [CrossRef]
- Godber, B.L.; Doel, J.J.; Sapkota, G.P.; Blake, D.R.; Stevens, C.R.; Eisenthal, R.; Harrison, R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J. Biol. Chem. 2000, 275, 7757–7763. [Google Scholar] [CrossRef]
- Bonizzi, G.; Piette, J.; Merville, M.P.; Bours, V. Cell type-specific role for reactive oxygen species in nuclear factor-kappaB activation by interleukin-1. Biochem. Pharmacol. 2000, 59, 7–11. [Google Scholar] [CrossRef]
- van der Donk, W.A.; Tsai, A.L.; Kulmacz, R.J. The cyclooxygenase reaction mechanism. Biochemistry 2002, 41, 15451–15458. [Google Scholar] [CrossRef]
- Estabrook, R.W. A passion for P450s (rememberances of the early history of research on cytochrome P450). Drug Metab. Dispos. Biol. Fate Chem. 2003, 31, 1461–1473. [Google Scholar] [CrossRef]
- Coon, M.J.; Ding, X.X.; Pernecky, S.J.; Vaz, A.D. Cytochrome P450: Progress and predictions. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1992, 6, 669–673. [Google Scholar] [CrossRef]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef]
- Hsu, J.L.; Hsieh, Y.; Tu, C.; O’Connor, D.; Nick, H.S.; Silverman, D.N. Catalytic properties of human manganese superoxide dismutase. J. Biol. Chem. 1996, 271, 17687–17691. [Google Scholar] [CrossRef]
- Oury, T.D.; Crapo, J.D.; Valnickova, Z.; Enghild, J.J. Human extracellular superoxide dismutase is a tetramer composed of two disulphide-linked dimers: A simplified, high-yield purification of extracellular superoxide dismutase. Biochem. J. 1996, 317 (Pt 1), 51–57. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Stanley, B.A.; Sivakumaran, V.; Shi, S.; McDonald, I.; Lloyd, D.; Watson, W.H.; Aon, M.A.; Paolocci, N. Thioredoxin reductase-2 is essential for keeping low levels of H(2)O(2) emission from isolated heart mitochondria. J. Biol. Chem. 2011, 286, 33669–33677. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M.; Brigelius-Flohe, R.; Aumann, K.D.; Roveri, A.; Schomburg, D.; Flohe, L. Diversity of glutathione peroxidases. Methods Enzym. 1995, 252, 38–53. [Google Scholar]
- Lee, C.M.; Lee, J.; Nam, M.J.; Choi, Y.S.; Park, S.H. Tomentosin Displays Anti-Carcinogenic Effect in Human Osteosarcoma MG-63 Cells via the Induction of Intracellular Reactive Oxygen Species. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Spinks, J.W.T.; Woods, R.J. Water and inorganic aqueous systems. In Introduction to Radiation Chemistry; John Wiley & Sons: New York, NY, USA, 1990; pp. 243–313. [Google Scholar]
- Bielski, B.H.J. Re-Evaluation of Spectral and Kinetic-Properties of Ho2 and (0--)2 Free-Radicals. Photochem. Photobiol. 1978, 28, 645–649. [Google Scholar] [CrossRef]
- Thomas, J.K. The rate constants for H atom reactions in aqueous solutions 1. J. Phys. Chem. 1963, 67, 2593–2595. [Google Scholar] [CrossRef]
- Matthews, R.W.; Sangster, D.F. Measurement by Benzoate Radiolytic Decarboxylation of Relative Rate Constants for Hydroxyl Radical Reactions. J. Phys. Chem. 1965, 69, 1938–1946. [Google Scholar] [CrossRef]
- Rabani, J.; Matheson, M.S. The Pulse Radiolysis of Aqueous Solutions of Potassium Ferrocyanide1. J. Phys. Chem. 1966, 70, 761–769. [Google Scholar] [CrossRef]
- Janata, E.; Schuler, R.H. Rate constant for scavenging eaq- in nitrous oxide-saturated solutions. J. Phys. Chem. 1982, 86, 2078–2084. [Google Scholar] [CrossRef]
- Asmus, K.D.; Fendler, J.H. Reaction of sulfur hexafluoride with hydrated electrons. J. Phys. Chem. 1968, 72, 4285–4289. [Google Scholar] [CrossRef]
- Hille, R.; Hall, J.; Basu, P. The mononuclear molybdenum enzymes. Chem. Rev. 2014, 114, 3963–4038. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, C.; Xu, H.H.; Liu, Y.N.; Zhou, F. Binding of alpha-synuclein with Fe(III) and with Fe(II) and biological implications of the resultant complexes. J. Inorg. Biochem. 2010, 104, 365–370. [Google Scholar] [CrossRef]
- Miotto, M.C.; Rodriguez, E.E.; Valiente-Gabioud, A.A.; Torres-Monserrat, V.; Binolfi, A.; Quintanar, L.; Zweckstetter, M.; Griesinger, C.; Fernandez, C.O. Site-specific copper-catalyzed oxidation of alpha-synuclein: Tightening the link between metal binding and protein oxidative damage in Parkinson’s disease. Inorg. Chem. 2014, 53, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Faller, P.; Testemale, D.; Hureau, C.; Collin, F. Metal-catalyzed oxidation of Abeta and the resulting reorganization of Cu binding sites promote ROS production. Metallomics 2016, 8, 1081–1089. [Google Scholar] [CrossRef]
- Cassagnes, L.E.; Herve, V.; Nepveu, F.; Hureau, C.; Faller, P.; Collin, F. The catalytically active copper-amyloid-Beta state: Coordination site responsible for reactive oxygen species production. Angew. Chem. Int. Ed. Engl. 2013, 52, 11110–11113. [Google Scholar] [CrossRef]
- Cheignon, C.; Hureau, C.; Collin, F. Real-time evolution of A beta(40) metal-catalyzed oxidation reveals Asp1 as the main target and a dependence on metal binding site. Inorg. Chim. Acta 2018, 472, 111–118. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/O2− Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Wardman, P. Reduction Potentials of One-Electron Couples Involving Free-Radicals in Aqueous-Solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef]
- Ferradini, C.; Foos, J.; Houee, C.; Pucheault, J. Reaction between Superoxide Anion and Hydrogen-Peroxide. Photochem. Photobiol. 1978, 28, 697–700. [Google Scholar] [CrossRef]
- Reybier, K.; Ayala, S.; Alies, B.; Rodrigues, J.V.; Bustos Rodriguez, S.; La Penna, G.; Collin, F.; Gomes, C.M.; Hureau, C.; Faller, P. Free Superoxide is an Intermediate in the Production of H2O2 by Copper(I)-Abeta Peptide and O2. Angew. Chem. Int. Ed. 2016, 55, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Bielski, B.H.J.; Arudi, R.L.; Sutherland, M.W. A Study of the Reactivity of Ho2/O2- with Unsaturated Fatty-Acids. J. Biol. Chem. 1983, 258, 4759–4761. [Google Scholar] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Rigo, A.; Stevanato, R.; Finazzi-Agro, A.; Rotilio, G. An attempt to evaluate the rate of the Haber-Weiss reaction by using OH radical scavengers. FEBS Lett. 1977, 80, 130–132. [Google Scholar] [CrossRef]
- Weinstein, J.; Bielski, B.H.J. Kinetics of the Interaction of Ho2 and O2-Radicals with Hydrogen-Peroxide - Haber-Weiss Reaction. J. Am. Chem. Soc. 1979, 101, 58–62. [Google Scholar] [CrossRef]
- Kissner, R.; Nauser, T.; Bugnon, P.; Lye, P.G.; Koppenol, W.H. Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem. Res. Toxicol. 1997, 10, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, G.L.; Pryor, W.A. The formation of peroxynitrite in vivo from nitric oxide and superoxide. Chem. Biol. Interact. 1995, 96, 203–206. [Google Scholar] [CrossRef]
- Packer, M.A.; Porteous, C.M.; Murphy, M.P. Superoxide production by mitochondria in the presence of nitric oxide forms peroxynitrite. Biochem. Mol. Biol. Int. 1996, 40, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Uchida, K.; Kawakishi, S. Oxidative fragmentation of collagen and prolyl peptide by Cu(II)/H2O2. Conversion of proline residue to 2-pyrrolidone. J. Biol. Chem. 1992, 267, 23646–23651. [Google Scholar] [PubMed]
- Pryor, W.A.; Squadrito, G.L. The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 1995, 268, L699–L722. [Google Scholar] [CrossRef] [PubMed]
- Berlett, B.S.; Friguet, B.; Yim, M.B.; Chock, P.B.; Stadtman, E.R. Peroxynitrite-mediated nitration of tyrosine residues in Escherichia coli glutamine synthetase mimics adenylylation: Relevance to signal transduction. Proc. Natl. Acad. Sci. USA 1996, 93, 1776–1780. [Google Scholar] [CrossRef]
- Munoz-Rugeles, L.; Galano, A.; Alvarez-Idaboy, J.R. The other side of the superoxide radical anion: Its ability to chemically repair DNA oxidized sites. Chem. Commun. 2018, 54, 13710–13713. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical-Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen-Atoms and Hydroxyl Radicals (.OH/.O-) in Aqueous-Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Farhataziz, R.A.; Ross, A. Selected specific rates of reactions of transients from water in aqueous solution. III. Hydroxyl Radic. Perhydroxyl Radic. Radic. Ions Nsrds-Nbs 1977, 59. [Google Scholar]
- Dorfman, L.M.; Adams, G.E. Reactivity of the hydroxyl radical in aqueous solutions; DTIC Document: 1973.
- Von Sonntag, C. The Chemical Basis of Radiation Biology; Taylor and Francis: Abingdon, UK, 1987; p. 515. [Google Scholar]
- Von Sonntag, C. Free-radical reactions involving thiols and disulphides. In Sulfur-Centered Reactive Intermediates in Chemistry and Biology; Chatgilialoglu, C., Asmus, K.-D., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 359–366. [Google Scholar]
- Armstrong, D.A. Application of pulse radiolysis for the study of short-lived sulphur species. In Sulfur-Centered Reactive Intermediates in Chemistry and Biology; Chatgilialoglu, K.-D.A., Ed.; Plenum Press: New York, NY, USA, 1990; pp. 121–134. [Google Scholar]
- Cadet, J.; Delatour, T.; Douki, T.; Gasparutto, D.; Pouget, J.P.; Ravanat, J.L.; Sauvaigo, S. Hydroxyl radicals and DNA base damage. Mutat. Res. 1999, 424, 9–21. [Google Scholar] [CrossRef]
- Schoneich, C. Mechanisms of metal-catalyzed oxidation of histidine to 2-oxo-histidine in peptides and proteins. J. Pharm. Biomed. Anal. 2000, 21, 1093–1097. [Google Scholar] [CrossRef]
- Uchida, K.; Kawakishi, S. 2-Oxo-histidine as a novel biological marker for oxidatively modified proteins. FEBS Lett. 1993, 332, 208–210. [Google Scholar] [CrossRef] [Green Version]
- Berlett, B.S.; Levine, R.L.; Stadtman, E.R. Comparison of the effects of ozone on the modification of amino acid residues in glutamine synthetase and bovine serum albumin. J. Biol. Chem. 1996, 271, 4177–4182. [Google Scholar] [CrossRef]
- Schoneich, C.; Pogocki, D.; Hug, G.L.; Bobrowski, K. Free radical reactions of methionine in peptides: Mechanisms relevant to beta-amyloid oxidation and Alzheimer’s disease. J. Am. Chem. Soc. 2003, 125, 13700–13713. [Google Scholar] [CrossRef]
- Hoshi, T.; Heinemann, S. Regulation of cell function by methionine oxidation and reduction. J. Physiol. 2001, 531, 1–11. [Google Scholar] [CrossRef]
- Hasegawa, K.; Patterson, L.K. Pulse-Radiolysis Studies in Model Lipid Systems: Formation and Behavior of Peroxy Radicals in Fatty-Acids. Photochem. Photobiol. 1978, 28, 817–823. [Google Scholar] [CrossRef]
- Gardner, H.W. Oxygen radical chemistry of polyunsaturated fatty acids. Free Radic. Biol. Med. 1989, 7, 65–86. [Google Scholar] [CrossRef]
- Yin, H.; Havrilla, C.M.; Gao, L.; Morrow, J.D.; Porter, N.A. Mechanisms for the formation of isoprostane endoperoxides from arachidonic acid. “Dioxetane” intermediate versus beta-fragmentation of peroxyl radicals. J. Biol. Chem. 2003, 278, 16720–16725. [Google Scholar] [CrossRef]
- Greco, A.; Minghetti, L.; Levi, G. Isoprostanes, novel markers of oxidative injury, help understanding the pathogenesis of neurodegenerative diseases. Neurochem. Res. 2000, 25, 1357–1364. [Google Scholar] [CrossRef]
- Stadtman, E.R. Protein oxidation in aging and age-related diseases. Ann. N. Y. Acad. Sci. 2001, 928, 22–38. [Google Scholar] [CrossRef]
- Davies, K.J.; Delsignore, M.E.; Lin, S.W. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J. Biol. Chem. 1987, 262, 9902–9907. [Google Scholar] [PubMed]
- Breen, A.P.; Murphy, J.A. Reactions of oxyl radicals with DNA. Free Radic. Biol. Med. 1995, 18, 1033–1077. [Google Scholar] [CrossRef]
- Stadtman, E.R. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu. Rev. Biochem. 1993, 62, 797–821. [Google Scholar] [CrossRef] [PubMed]
- Lal, M. Radiation-Induced Oxidation of Sulfhydryl Molecules in Aqueous-Solutions - a Comprehensive Review. Radiat. Phys. Chem. 1994, 43, 595–611. [Google Scholar] [CrossRef]
- Atwood, C.S.; Perry, G.; Zeng, H.; Kato, Y.; Jones, W.D.; Ling, K.Q.; Huang, X.; Moir, R.D.; Wang, D.; Sayre, L.M.; et al. Copper mediates dityrosine cross-linking of Alzheimer’s amyloid-beta. Biochemistry 2004, 43, 560–568. [Google Scholar] [CrossRef]
- Garrison, W.M. Reaction-Mechanisms in the Radiolysis of Peptides, Polypeptides, and Proteins. Chem. Rev. 1987, 87, 381–398. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Metodiewa, D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic. Biol. Med. 1999, 27, 322–328. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Hampton, M.B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008, 45, 549–561. [Google Scholar] [CrossRef]
- Vasquez-Vivar, J.; Denicola, A.; Radi, R.; Augusto, O. Peroxynitrite-mediated decarboxylation of pyruvate to both carbon dioxide and carbon dioxide radical anion. Chem. Res. Toxicol. 1997, 10, 786–794. [Google Scholar] [CrossRef]
- Bakhmutova-Albert, E.V.; Yao, H.; Denevan, D.E.; Richardson, D.E. Kinetics and mechanism of peroxymonocarbonate formation. Inorg. Chem. 2010, 49, 11287–11296. [Google Scholar] [CrossRef] [PubMed]
- Trindade, D.F.; Cerchiaro, G.; Augusto, O. A role for peroxymonocarbonate in the stimulation of biothiol peroxidation by the bicarbonate/carbon dioxide pair. Chem. Res. Toxicol. 2006, 19, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.E.; Yao, H.R.; Frank, K.M.; Bennett, D.A. Equilibria, kinetics, and mechanism in the bicarbonate activation of hydrogen peroxide: Oxidation of sulfides by peroxymonocarbonate. J. Am. Chem. Soc. 2000, 122, 1729–1739. [Google Scholar] [CrossRef]
- Kowalik-Jankowska, T.; Ruta, M.; Wisniewska, K.; Lankiewicz, L.; Dyba, M. Products of Cu(II)-catalyzed oxidation in the presence of hydrogen peroxide of the 1-10, 1-16 fragments of human and mouse beta-amyloid peptide. J. Inorg. Biochem. 2004, 98, 940–950. [Google Scholar] [CrossRef]
- Davies, M.J.; Hawkins, C.L.; Pattison, D.I.; Rees, M.D. Mammalian heme peroxidases: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 1199–1234. [Google Scholar] [CrossRef]
- Davies, M.J.; Dean, R.T. Radical-Mediated Protein Oxidation: From Chemistry to Medicine; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Chevion, M. A site-specific mechanism for free radical induced biological damage: The essential role of redox-active transition metals. Free Radic. Biol. Med. 1988, 5, 27–37. [Google Scholar] [CrossRef]
- Stadtman, E.R. Metal ion-catalyzed oxidation of proteins: Biochemical mechanism and biological consequences. Free Radic. Biol. Med. 1990, 9, 315–325. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.J.; Fu, S.; Dean, R.T. Protein hydroperoxides can give rise to reactive free radicals. Biochem. J. 1995, 305, 643–649. [Google Scholar] [CrossRef] [Green Version]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic metals, ascorbate and free radicals: Combinations to avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef]
- Von Sonntag, C. Peroxyl Radicals in Aqueous Media. In Oxygen Radicals in Biology and Medicine; Simic, M.G., Taylor, K.A., Ward, J.F., von Sonntag, C., Eds.; Springer: Berlin/Heidelberg, Germany, 1988; Volume 49. [Google Scholar]
- Von Sonntag, C.; Schuchmann, H.-P. Peroxyl radicals in aqueous solution. In Peroxyl Radicals; Alfassi, Z.B., Ed.; John Wiley and Sons: Chichester, UK, 1997; pp. 173–234. [Google Scholar]
- Bothe, E.; Schuchmann, M.N.; Schultefrohlinde, D.; Vonsonntag, C. Ho2 Elimination from Alpha-Hydroxyalkylperoxyl Radicals in Aqueous-Solution. Photochem. Photobiol. 1978, 28, 639–644. [Google Scholar] [CrossRef]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef]
- El-Agamey, A.; McGarvey, D.J. Peroxyl radical reactions with carotenoids in microemulsions: Influence of microemulsion composition and the nature of peroxyl radical precursor. Free Radic. Biol. Med. 2016, 90, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, B.C.; Holmes, R.G.G.; Laue, H.A.H.; Norman, R.O.C. Electron-Spin Resonance Studies. 50. Reactions of Alkoxyl Radicals Generated from Alkyl Hydroperoxides and Titanium(Iii) Ion in Aqueous-Solution. J. Chem. Soc. Perk. Trans. 1976, 2, 1047–1052. [Google Scholar] [CrossRef]
- Gilbert, B.C.; David, P.; Marshall, R.; Norman, R.O.C.; Pineda, N.; Williams, P.S. Electron-Spin Resonance Studies. 61. The Generation and Reactions of the Tert-Butoxyl Radical in Aqueous-Solution. J. Chem. Soc. Perk. Trans. 1981, 2, 1392–1400. [Google Scholar] [CrossRef]
- Headlam, H.A.; Davies, M.J. Beta-scission of side-chain alkoxyl radicals on peptides and proteins results in the loss of side-chains as aldehydes and ketones. Free Radic. Biol. Med. 2002, 32, 1171–1184. [Google Scholar] [CrossRef]
- Bors, W.; Tait, D.; Michel, C.; Saran, M.; Erbenruss, M. Reactions of Alkoxy Radicals in Aqueous-Solutions. Isr. J. Chem. 1984, 24, 17–24. [Google Scholar] [CrossRef]
- Erbenruss, M.; Michel, C.; Bors, W.; Saran, M. Absolute Rate Constants of Alkoxyl Radical Reactions in Aqueous-Solution. J. Phys. Chem. 1987, 91, 2362–2365. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Hyde, J.S. Concentration of oxygen in lipid bilayers using a spin-label method. Biophys. J. 1983, 41, 283–286. [Google Scholar] [CrossRef]
- Beal, M.F.; Ferrante, R.J.; Browne, S.E.; Matthews, R.T.; Kowall, N.W.; Brown, R.H., Jr. Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann. Neurol. 1997, 42, 644–654. [Google Scholar] [CrossRef]
- Ferrante, R.J.; Browne, S.E.; Shinobu, L.A.; Bowling, A.C.; Baik, M.J.; MacGarvey, U.; Kowall, N.W.; Brown, R.H., Jr.; Beal, M.F. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J. Neurochem. 1997, 69, 2064–2074. [Google Scholar] [CrossRef]
- Giasson, B.I.; Duda, J.E.; Murray, I.V.; Chen, Q.; Souza, J.M.; Hurtig, H.I.; Ischiropoulos, H.; Trojanowski, J.Q.; Lee, V.M. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 2000, 290, 985–989. [Google Scholar] [CrossRef]
- Hensley, K.; Maidt, M.L.; Yu, Z.; Sang, H.; Markesbery, W.R.; Floyd, R.A. Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation. J. Neurosci. 1998, 18, 8126–8132. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Reed, T.; Newman, S.F.; Sultana, R. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment. Free Radic. Biol. Med. 2007, 43, 658–677. [Google Scholar] [CrossRef]
- Selfridge, J.E.; E., L.; Lu, J.; Swerdlow, R.H. Role of mitochondrial homeostasis and dynamics in Alzheimer’s disease. Neurobiol. Dis. 2013, 51, 3–12. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef]
- Hands, S.; Sajjad, M.U.; Newton, M.J.; Wyttenbach, A. In vitro and in vivo aggregation of a fragment of huntingtin protein directly causes free radical production. J. Biol. Chem. 2011, 286, 44512–44520. [Google Scholar] [CrossRef]
- Markesbery, W.R. Oxidative Stress Hypothesis in Alzheimer’s Disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid β-peptide. Trends Mol. Med. 2001, 7, 548–554. [Google Scholar] [CrossRef]
- Naslund, J.; Schierhorn, A.; Hellman, U.; Lannfelt, L.; Roses, A.D.; Tjernberg, L.O.; Silberring, J.; Gandy, S.E.; Winblad, B.; Greengard, P.; et al. Relative abundance of Alzheimer A beta amyloid peptide variants in Alzheimer disease and normal aging. Proc. Natl. Acad. Sci. USA 1994, 91, 8378–8382. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Lauderback, C.M. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: Potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic. Biol. Med. 2002, 32, 1050–1060. [Google Scholar] [CrossRef]
- Sultana, R.; Boyd-Kimball, D.; Poon, H.F.; Cai, J.; Pierce, W.M.; Klein, J.B.; Merchant, M.; Markesbery, W.R.; Butterfield, D.A. Redox proteomics identification of oxidized proteins in Alzheimer’s disease hippocampus and cerebellum: An approach to understand pathological and biochemical alterations in AD. Neurobiol. Aging 2006, 27, 1564–1576. [Google Scholar] [CrossRef]
- Hensley, K.; Hall, N.; Subramaniam, R.; Cole, P.; Harris, M.; Aksenov, M.; Aksenova, M.; Gabbita, S.P.; Wu, J.F.; Carney, J.M.; et al. Brain regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J. Neurochem. 1995, 65, 2146–2156. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Yatin, S.M.; Varadarajan, S.; Koppal, T. Amyloid beta-peptide-associated free radical oxidative stress, neurotoxicity, and Alzheimer’s disease. Methods Enzym. 1999, 309, 746–768. [Google Scholar]
- Nitsch, R.M.; Blusztajn, J.K.; Pittas, A.G.; Slack, B.E.; Growdon, J.H.; Wurtman, R.J. Evidence for a membrane defect in Alzheimer disease brain. Proc. Natl. Acad. Sci. USA 1992, 89, 1671–1675. [Google Scholar] [CrossRef]
- Svennerholm, L.; Gottfries, C.G. Membrane lipids, selectively diminished in Alzheimer brains, suggest synapse loss as a primary event in early-onset form (type I) and demyelination in late-onset form (type II). J. Neurochem. 1994, 62, 1039–1047. [Google Scholar] [CrossRef]
- Markesbery, W.R.; Lovell, M.A. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol. Aging 1998, 19, 33–36. [Google Scholar] [CrossRef]
- Neely, M.D.; Sidell, K.R.; Graham, D.G.; Montine, T.J. The lipid peroxidation product 4-hydroxynonenal inhibits neurite outgrowth, disrupts neuronal microtubules, and modifies cellular tubulin. J. Neurochem. 1999, 72, 2323–2333. [Google Scholar] [CrossRef]
- Keller, J.N.; Pang, Z.; Geddes, J.W.; Begley, J.G.; Germeyer, A.; Waeg, G.; Mattson, M.P. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: Role of the lipid peroxidation product 4-hydroxynonenal. J. Neurochem. 1997, 69, 273–284. [Google Scholar] [CrossRef]
- Hardas, S.S.; Sultana, R.; Clark, A.M.; Beckett, T.L.; Szweda, L.I.; Murphy, M.P.; Butterfield, D.A. Oxidative modification of lipoic acid by HNE in Alzheimer disease brain. Redox Biol. 2013, 1, 80–85. [Google Scholar] [CrossRef]
- Gegotek, A.; Skrzydlewska, E. Biological effect of protein modifications by lipid peroxidation products. Chem. Phys. Lipids 2019, 221, 46–52. [Google Scholar] [CrossRef]
- Sayre, L.M.; Zelasko, D.A.; Harris, P.L.; Perry, G.; Salomon, R.G.; Smith, M.A. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J. Neurochem. 1997, 68, 2092–2097. [Google Scholar] [CrossRef]
- Liu, Q.; Smith, M.A.; Avila, J.; DeBernardis, J.; Kansal, M.; Takeda, A.; Zhu, X.; Nunomura, A.; Honda, K.; Moreira, P.I.; et al. Alzheimer-specific epitopes of tau represent lipid peroxidation-induced conformations. Free Radic. Biol. Med. 2005, 38, 746–754. [Google Scholar] [CrossRef]
- Pryor, W.A.; Porter, N.A. Suggested mechanisms for the production of 4-hydroxy-2-nonenal from the autoxidation of polyunsaturated fatty acids. Free Radic. Biol. Med. 1990, 8, 541–543. [Google Scholar] [CrossRef]
- Montine, T.J.; Markesbery, W.R.; Morrow, J.D.; Roberts, L.J., 2nd. Cerebrospinal fluid F2-isoprostane levels are increased in Alzheimer’s disease. Ann. Neurol. 1998, 44, 410–413. [Google Scholar] [CrossRef]
- Nourooz-Zadeh, J.; Liu, E.H.; Yhlen, B.; Anggard, E.E.; Halliwell, B. F4-isoprostanes as specific marker of docosahexaenoic acid peroxidation in Alzheimer’s disease. J. Neurochem. 1999, 72, 734–740. [Google Scholar] [CrossRef]
- Reich, E.E.; Markesbery, W.R.; Roberts, L.J., 2nd; Swift, L.L.; Morrow, J.D.; Montine, T.J. Brain regional quantification of F-ring and D-/E-ring isoprostanes and neuroprostanes in Alzheimer’s disease. Am. J. Pathol. 2001, 158, 293–297. [Google Scholar] [CrossRef]
- Smith, M.A.; Perry, G.; Richey, P.L.; Sayre, L.M.; Anderson, V.E.; Beal, M.F.; Kowall, N. Oxidative damage in Alzheimer’s. Nature 1996, 382, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Tramutola, A.; Lanzillotta, C.; Perluigi, M.; Butterfield, D.A. Oxidative stress, protein modification and Alzheimer disease. Brain Res. Bull. 2016. [Google Scholar] [CrossRef] [PubMed]
- Terni, B.; Boada, J.; Portero-Otin, M.; Pamplona, R.; Ferrer, I. Mitochondrial ATP-synthase in the entorhinal cortex is a target of oxidative stress at stages I/II of Alzheimer’s disease pathology. Brain Pathol. 2010, 20, 222–233. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. Redox proteomics and amyloid beta-peptide: Insights into Alzheimer disease. J. Neurochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; MacGarvey, U.; Beal, M.F. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann. Neurol. 1994, 36, 747–751. [Google Scholar] [CrossRef]
- Gabbita, S.P.; Lovell, M.A.; Markesbery, W.R. Increased nuclear DNA oxidation in the brain in Alzheimer’s disease. J. Neurochem. 1998, 71, 2034–2040. [Google Scholar] [CrossRef]
- Lovell, M.A.; Soman, S.; Bradley, M.A. Oxidatively modified nucleic acids in preclinical Alzheimer’s disease (PCAD) brain. Mech. Ageing Dev. 2011, 132, 443–448. [Google Scholar] [CrossRef]
- Nunomura, A.; Perry, G.; Pappolla, M.A.; Wade, R.; Hirai, K.; Chiba, S.; Smith, M.A. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J. Neurosci. 1999, 19, 1959–1964. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53 (Suppl. 3), S26–S38. [Google Scholar] [CrossRef]
- Bosco, D.A.; Fowler, D.M.; Zhang, Q.; Nieva, J.; Powers, E.T.; Wentworth, P., Jr.; Lerner, R.A.; Kelly, J.W. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat. Chem. Biol. 2006, 2, 249–253. [Google Scholar] [CrossRef]
- Alam, Z.I.; Daniel, S.E.; Lees, A.J.; Marsden, D.C.; Jenner, P.; Halliwell, B. A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J. Neurochem. 1997, 69, 1326–1329. [Google Scholar] [CrossRef] [PubMed]
- Nakabeppu, Y.; Tsuchimoto, D.; Yamaguchi, H.; Sakumi, K. Oxidative damage in nucleic acids and Parkinson’s disease. J. Neurosci. Res. 2007, 85, 919–934. [Google Scholar] [CrossRef]
- Selley, M.L. (E)-4-hydroxy-2-nonenal may be involved in the pathogenesis of Parkinson’s disease. Free Radic. Biol. Med. 1998, 25, 169–174. [Google Scholar] [CrossRef]
- Halliwell, B. The wanderings of a free radical. Free Radic. Biol. Med. 2009, 46, 531–542. [Google Scholar] [CrossRef]
- Jellinger, K.; Kienzl, E.; Rumpelmair, G.; Riederer, P.; Stachelberger, H.; Ben-Shachar, D.; Youdim, M.B. Iron-melanin complex in substantia nigra of parkinsonian brains: An x-ray microanalysis. J. Neurochem. 1992, 59, 1168–1171. [Google Scholar] [CrossRef]
- Fahn, S.; Cohen, G. The oxidant stress hypothesis in Parkinson’s disease: Evidence supporting it. Ann. Neurol. 1992, 32, 804–812. [Google Scholar] [CrossRef]
- Surgucheva, I.; Sharov, V.S.; Surguchov, A. gamma-Synuclein: Seeding of alpha-synuclein aggregation and transmission between cells. Biochemistry 2012, 51, 4743–4754. [Google Scholar] [CrossRef]
- Mendez, E.F.; Sattler, R. Biomarker development for C9orf72 repeat expansion in ALS. Brain Res. 2015, 1607, 26–35. [Google Scholar] [CrossRef]
- Smith, R.G.; Henry, Y.K.; Mattson, M.P.; Appel, S.H. Presence of 4-hydroxynonenal in cerebrospinal fluid of patients with sporadic amyotrophic lateral sclerosis. Ann. Neurol. 1998, 44, 696–699. [Google Scholar] [CrossRef]
- Bogdanov, M.; Brown, R.H.; Matson, W.; Smart, R.; Hayden, D.; O’Donnell, H.; Flint Beal, M.; Cudkowicz, M. Increased oxidative damage to DNA in ALS patients. Free Radic. Biol. Med. 2000, 29, 652–658. [Google Scholar] [CrossRef]
- Miller, E.; Morel, A.; Saso, L.; Saluk, J. Isoprostanes and neuroprostanes as biomarkers of oxidative stress in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2014, 2014, 572491. [Google Scholar] [CrossRef]
- Mitsumoto, H.; Santella, R.M.; Liu, X.; Bogdanov, M.; Zipprich, J.; Wu, H.C.; Mahata, J.; Kilty, M.; Bednarz, K.; Bell, D.; et al. Oxidative stress biomarkers in sporadic ALS. Amyotroph. Lateral Scler. 2008, 9, 177–183. [Google Scholar] [CrossRef]
- Bozzo, F.; Mirra, A.; Carri, M.T. Oxidative stress and mitochondrial damage in the pathogenesis of ALS: New perspectives. Neurosci. Lett. 2017, 636, 3–8. [Google Scholar] [CrossRef]
- Blokhuis, A.M.; Groen, E.J.; Koppers, M.; van den Berg, L.H.; Pasterkamp, R.J. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013, 125, 777–794. [Google Scholar] [CrossRef] [Green Version]
- Lovejoy, D.B.; Guillemin, G.J. The potential for transition metal-mediated neurodegeneration in amyotrophic lateral sclerosis. Front. Aging Neurosci. 2014, 6, 173. [Google Scholar] [CrossRef]
- Rosas, H.D.; Chen, Y.I.; Doros, G.; Salat, D.H.; Chen, N.K.; Kwong, K.K.; Bush, A.; Fox, J.; Hersch, S.M. Alterations in brain transition metals in Huntington disease: An evolving and intricate story. Arch. Neurol. 2012, 69, 887–893. [Google Scholar] [CrossRef]
- Hersch, S.M.; Gevorkian, S.; Marder, K.; Moskowitz, C.; Feigin, A.; Cox, M.; Como, P.; Zimmerman, C.; Lin, M.; Zhang, L.; et al. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2’dG. Neurology 2006, 66, 250–252. [Google Scholar] [CrossRef]
- Chen, C.M.; Wu, Y.R.; Cheng, M.L.; Liu, J.L.; Lee, Y.M.; Lee, P.W.; Soong, B.W.; Chiu, D.T. Increased oxidative damage and mitochondrial abnormalities in the peripheral blood of Huntington’s disease patients. Biochem. Biophys. Res. Commun. 2007, 359, 335–340. [Google Scholar] [CrossRef]
- Stack, E.C.; Matson, W.R.; Ferrante, R.J. Evidence of oxidant damage in Huntington’s disease: Translational strategies using antioxidants. Ann. N. Y. Acad. Sci. 2008, 1147, 79–92. [Google Scholar] [CrossRef]
- Dragunow, M.; Faull, R.L.; Lawlor, P.; Beilharz, E.J.; Singleton, K.; Walker, E.B.; Mee, E. In situ evidence for DNA fragmentation in Huntington’s disease striatum and Alzheimer’s disease temporal lobes. Neuroreport 1995, 6, 1053–1057. [Google Scholar] [CrossRef]
- Browne, S.E.; Ferrante, R.J.; Beal, M.F. Oxidative stress in Huntington’s disease. Brain Pathol. 1999, 9, 147–163. [Google Scholar] [CrossRef]
- Tunez, I.; Sanchez-Lopez, F.; Aguera, E.; Fernandez-Bolanos, R.; Sanchez, F.M.; Tasset-Cuevas, I. Important role of oxidative stress biomarkers in Huntington’s disease. J. Med. Chem. 2011, 54, 5602–5606. [Google Scholar] [CrossRef]
- LeBars, P.L.; Katz, M.M.; Berman, N.; Itil, T.M.; Freedman, A.M.; Schatzberg, A.F. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. J. Am. Med. Assoc. 1997, 278, 1327–1332. [Google Scholar]
- Purushothuman, S.; Nandasena, C.; Peoples, C.L.; El Massri, N.; Johnstone, D.M.; Mitrofanis, J.; Stone, J. Saffron Pre-Treatment Offers Neuroprotection to Nigral and Retinal Dopaminergic Cells of MPTP-Treated mice. J. Parkinson Dis. 2013, 3, 77–83. [Google Scholar]
- Barkats, M.; Millecamps, S.; Abrioux, P.; Geoffroy, M.C.; Mallet, J. Overexpression of glutathione peroxidase increases the resistance of neuronal cells to Abeta-mediated neurotoxicity. J. Neurochem. 2000, 75, 1438–1446. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]





© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. https://doi.org/10.3390/ijms20102407
Collin F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. International Journal of Molecular Sciences. 2019; 20(10):2407. https://doi.org/10.3390/ijms20102407
Chicago/Turabian StyleCollin, Fabrice. 2019. "Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases" International Journal of Molecular Sciences 20, no. 10: 2407. https://doi.org/10.3390/ijms20102407
APA StyleCollin, F. (2019). Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. International Journal of Molecular Sciences, 20(10), 2407. https://doi.org/10.3390/ijms20102407