Direct Enantiomeric Resolution of Seventeen Racemic 1,4-Dihydropyridine-Based Hexahydroquinoline Derivatives by HPLC
Abstract
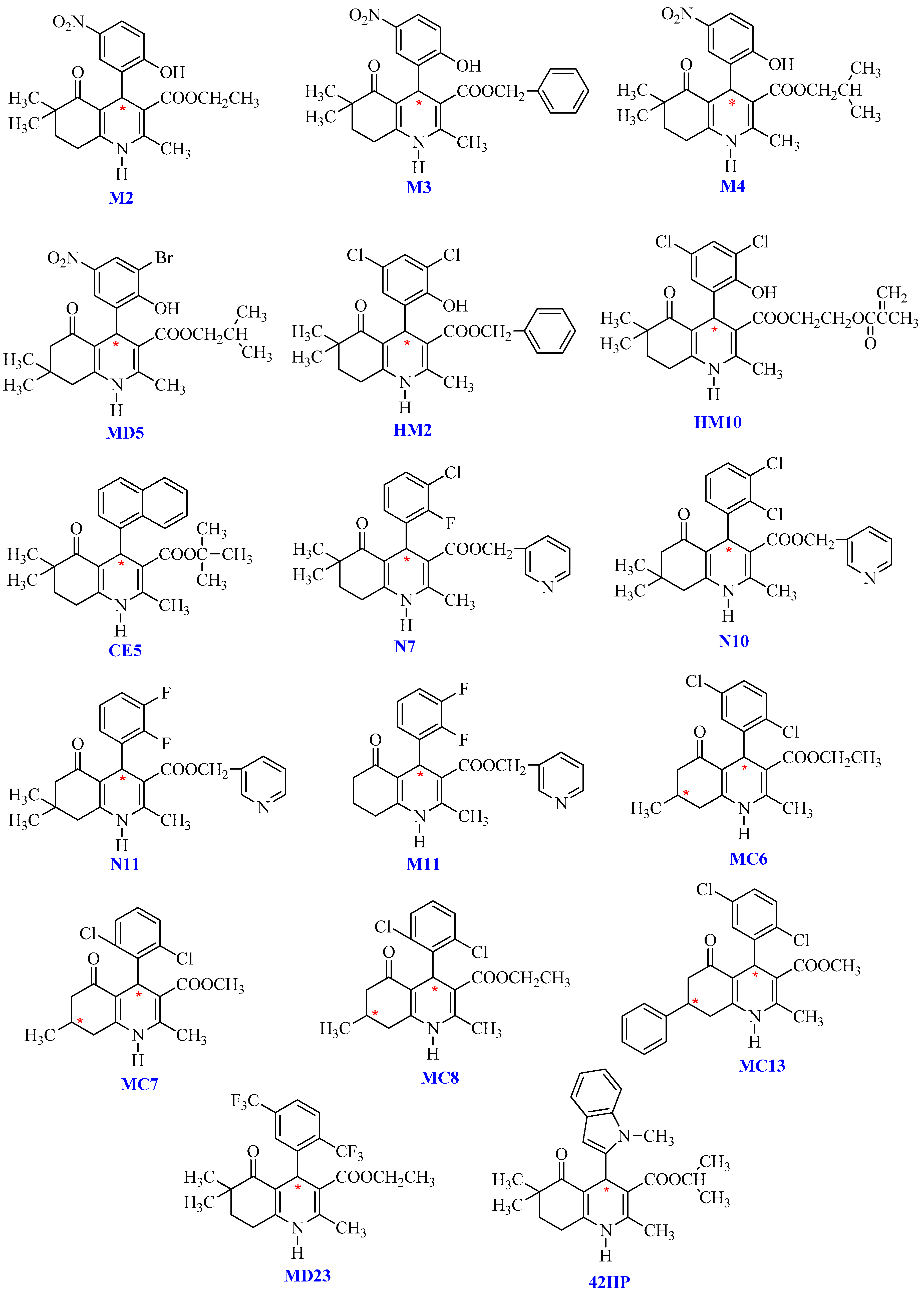
1. Introduction
2. Results and Discussion
2.1. Enantioseparation under Normal-Phase Elution Mode
2.1.1. Under Standard Normal-Phase Elution Mode
2.1.2. Under Nonstandard Elution Mode
2.1.3. Influence of the Alcohol Modifiers on Enantioseparation
2.1.4. Effects of the Additive
2.2. Enantioseparation under Reversed-Phase Elution Mode
2.3. Effects of Temperature and Thermodynamic Parameters
2.4. Application
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Equipment
3.3. Chromatography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DHP | Dihydropyridine |
| CE | Capillary electrophoresis |
| SFC | Supercritical fluid chromatography |
| CEC | Capillary electrochromatography |
| GC | Gas chromatography |
| HPLC | High-performance liquid chromatography |
| CSP | Chiral stationary phase |
| N-hex | N-Hexane |
| IPA | Isopropanol |
| NPA | N-propanol |
| NBA | N-butanol |
| EtOH | Ethanol |
| MeOH | Methanol |
| ACN | Acetonitrile |
References
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, S.; Edraki, N.; Firuzi, O.; Khoshneviszadeh, M.; Miri, R. 5-Oxo-hexahydroquinoline: An attractive scaffold with diverse biological activities. Mol. Divers. 2018. [Google Scholar] [CrossRef]
- Schaller, D.; Gündüz, M.G.; Zhang, F.X.; Zamponi, G.W.; Wolber, G. Binding mechanism investigations guiding the synthesis of novel condensed 1,4-dihydropyridine derivatives with L-/T-type calcium channel blocking activity. Eur. J. Med. Chem. 2018, 155, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bladen, C.; Gündüz, M.G.; Şimşek, R.; Şafak, C.; Zamponi, G.W. Synthesis and Evaluation of 1,4-Dihydropyridine Derivatives with Calcium Channel Blocking Activity. Pflügers Arch. Eur. J. Physiol. 2014, 466, 1355–1363. [Google Scholar] [CrossRef]
- Bladen, C.; Gadotti, V.M.; Gündüz, M.G.; Berger, N.D.; Şimşek, R.; Şafak, C.; Zamponi, G.W. 1,4-Dihydropyridine derivatives with T-type calcium channel blocking activity attenuate inflammatory and neuropathic pain. Pflügers Arch. Eur. J. Physiol. 2015, 467, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Tokuma, Y.; Noguchi, H. Stereoselective pharmacokinetics of dihydropyridine calcium antagonists. J. Chromatogr. A 1995, 694, 181–193. [Google Scholar] [CrossRef]
- Christians, T.; Holzgrabe, U. Enantioseparation of dihydropyridine derivatives by means of neutral and negatively charged β-cyclodextrin derivatives using capillary electrophoresis. Electrophoresis 2000, 21, 3609–3617. [Google Scholar] [CrossRef]
- Kong, D.Z.; Yang, W.; Duan, K.F.; Cui, Y.; Xing, S.S.; Zhang, X.W.; Sheng, X.N.; Zhang, L.T. Capillary electrophoretic enantioseparation of m-nisoldipine using two different β-cyclodextrins. J. Sep. Sci. 2009, 32, 3178–3183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Song, Z.R.; Dong, Y.Q.; Wang, Y.F.; Li, X.G.; Long, H.; Xu, K.; Deng, C.; Meng, M.; Yin, Y.M.; et al. Enantiomeric separation of 1,4-dihydropyridines by liquid-phase microextraction with supercritical fluid chromatography. J. Supercrit. Fluids 2016, 107, 129–136. [Google Scholar] [CrossRef]
- Auditore, R.; Santagati, N.A.; Aturki, Z.; Fanali, S. Enantiomeric separation of amlodipine and its two chiral impurities by nano-liquid chromatography and capillary electrochromatography using a chiral stationary phase based on cellulose tris(4-chloro-3-methylphenylcarbamate). Electrophoresis 2013, 34, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Scharpf, F.; Riedel, K.D.; Laufen, H.; Leitold, M. Enantioselective gas chromatographic assay with electron-capture detection for amlodipine in biological samples. J. Chromatogr. B 1994, 655, 225–233. [Google Scholar] [CrossRef]
- Kawabata, K.; Samata, N.; Urasaki, Y.; Fukazawa, I.; Uchida, N.; Uchida, E.; Yasuhara, H. Enantioselective determination of azelnidipine in human plasma using liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2007, 852, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Lee, D.-J.; Liu, K.-H.; Sunwoo, Y.E.; Kwon, K.-I.; Cha, I.-J.; Shin, J.-G. Analysis of benidipine enantiomers in human plasma by liquid chromatography–mass spectrometry using a macrocyclic antibiotic (Vancomycin) chiral stationary phase column. J. Chromatogr. B 2005, 814, 75–81. [Google Scholar] [CrossRef]
- Okamoto, Y.; Aburatani, R.; Hatada, K.; Honda, M.; Inotsume, N.; Nakano, M. Optical resolution of dihydropyridine enantiomers by high- performance liquid chromatography using phenylcarbamates of polysaccharides as a chiral stationary phase. J. Chromatogr. 1990, 513, 375–378. [Google Scholar] [CrossRef]
- Jibuti, G.; Mskhiladze, A.; Takaishvili, N.; Karchkhadze, M.; Chankvetadze, L.; Farkas, T.; Chankvetadze, B. HPLC separation of dihydropyridine derivatives enantiomers with emphasis on elution order using polysaccharide-based chiral columns. J. Sep. Sci. 2012, 35, 2529–2537. [Google Scholar] [CrossRef]
- Scott Sharp, V.; Gokey, M.A.; Wolfe, C.N.; Rener, G.A.; Cooper, M.R. High performance liquid chromatographic enantioseparation development and analytical method characterization of the carboxylate ester of evacetrapib using an immobilized chiral stationary phase with a non-conventional eluent system. J. Chromatogr. A 2015, 1416, 83–93. [Google Scholar] [CrossRef]
- Yu, J.; Tang, J.; Yuan, X.W.; Guo, X.J.; Zhao, L.S. Evaluation of the chiral recognition properties and the column performances of three chiral stationary phases based on cellulose for the enantioseparation of six dihydropyridines by high-performance liquid chromatography. Chirality 2017, 29, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Nguyen, D.; Franco, P. Reversed-phase screening strategies for liquid chromatography on polysaccharide-derived chiral stationary phases. J. Chromatogr. A 2010, 1217, 1048–1055. [Google Scholar] [CrossRef]
- Wang, T.; Wenslow, R.M., Jr. Effects of alcohol mobile-phase modifiers on the structure and chiral selectivity of amylose tris(3,5-dimethylphenylcarbamate) chiral stationary phase. J. Chromatogr. A 2003, 1015, 99–110. [Google Scholar] [CrossRef]
- Ye, Y.K.; Lord, B.; Stringham, R.W. Memory effect of mobile phase additives in chiral separations on a Chiralpak AD column. J. Chromatogr. A 2002, 945, 139–146. [Google Scholar] [CrossRef]
- Weng, W.; Guo, H.X.; Zhan, F.P.; Fang, H.L.; Wang, Q.X.; Yao, B.X.; Li, S.X. Chromatographic enantioseparations of binaphthyl compounds on an immobilized polysaccharide-based chiral stationary phase. J. Chromatogr. A 2008, 1210, 178–184. [Google Scholar] [CrossRef]
- Kiisters, E.; Loux, V.; Schmid, E.; Floersheim, P. Enantiomeric separation of chiral sulphoxides: Screening of cellulose-based sorbents with particular reference to cellulose tribenzoate. J. Chromatogr. A 1994, 646, 421–432. [Google Scholar] [CrossRef]
- Özer, E.K.; Gündüz, M.G.; El-Khouly, A.; Sara, M.Y.; Şimşek, R.; İskit, A.B.; Şafak, O.C. Microwave-assisted synthesis of condensed 1,4-dihydropyridines as potential calcium channel modulators. Turk. J. Chem. 2015, 39, 886–896. [Google Scholar] [CrossRef]
- Gündüz, M.; Albayrak, E.; İşli, F.; Fincan, G.; Yildirim, Ş.; Şimşek, R.; Şafak, C.; Sarioğlu, Y.; Yidirim, S.; Butcher, R. Synthesis, structural characterization and myorelaxant activity of 4-naphthylhexahydroquinoline derivatives containing different ester groups. J. Serb. Chem. Soc. 2016, 81, 729–738. [Google Scholar] [CrossRef]
- Şafak, C.; Gündüz, M.G.; İlhan, S.Ö.; Şimşek, R.; İşli, F.; Yıldırım, Ş.; Fincan, G.S.Ö.; Sarıoğlu, Y.; Linden, A. Synthesis and Myorelaxant Activity of Fused 1,4-Dihydropyridines on Isolated Rabbit Gastric Fundus. Drug Dev. Res. 2012, 73, 332–342. [Google Scholar] [CrossRef]
- Gündüz, M.G.; Sevim Öztürk, G.; Vural, İ.M.; Şimşek, R.; Sarıoğlu, Y.; Şafak, C. Evaluation of myorelaxant activity of 7-substituted hexahydroquinoline derivatives in isolated rabbit gastric fundus. Eur. J. Med. Chem. 2008, 43, 562–568. [Google Scholar] [CrossRef]








| Analyte | 20 °C | 25 °C | 30 °C | 35 °C | 40 °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α | α | α | α | α | |||||||||||
| M2 | 4.48 | 3.85 | 1.95 | 4.30 | 3.91 | 1.91 | 4.16 | 3.82 | 1.88 | 3.98 | 3.81 | 1.84 | 3.86 | 3.53 | 1.81 |
| M3 | 5.54 | 0.58 | 1.12 | 5.31 | 0.69 | 1.12 | 5.06 | 0.76 | 1.13 | 4.82 | 0.82 | 1.13 | 4.65 | 0.83 | 1.14 |
| M4 | 2.48 | 2.15 | 1.78 | 2.42 | 2.14 | 1.75 | 2.39 | 2.11 | 1.73 | 2.32 | 2.02 | 1.69 | 2.26 | 1.94 | 1.67 |
| MD5 | 7.56 | 4.43 | 2.07 | 7.01 | 4.48 | 2.09 | 6.54 | 4.57 | 2.12 | 5.95 | 4.66 | 2.13 | 5.60 | 4.68 | 2.14 |
| Analyte | ||||||||
|---|---|---|---|---|---|---|---|---|
| M2 | −5.74 | −0.85 | 0.9972 | −8.54 | −1.34 | 0.9974 | −2.80 | −4.02 |
| M3 | −6.81 | −1.08 | 0.9975 | −6.19 | −0.71 | 0.9977 | 0.64 | 3.11 |
| M4 | −3.50 | −0.52 | 0.9873 | −6.06 | −0.99 | 0.9933 | −2.56 | −3.91 |
| MD5 | −11.67 | −2.76 | 0.9970 | −10.39 | −1.51 | 0.9952 | 1.29 | 10.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Gözde Gündüz, M.; Zhang, J.; Yu, J.; Guo, X. Direct Enantiomeric Resolution of Seventeen Racemic 1,4-Dihydropyridine-Based Hexahydroquinoline Derivatives by HPLC. Int. J. Mol. Sci. 2019, 20, 2513. https://doi.org/10.3390/ijms20102513
Sun J, Gözde Gündüz M, Zhang J, Yu J, Guo X. Direct Enantiomeric Resolution of Seventeen Racemic 1,4-Dihydropyridine-Based Hexahydroquinoline Derivatives by HPLC. International Journal of Molecular Sciences. 2019; 20(10):2513. https://doi.org/10.3390/ijms20102513
Chicago/Turabian StyleSun, Jiayi, Miyase Gözde Gündüz, Junyuan Zhang, Jia Yu, and Xingjie Guo. 2019. "Direct Enantiomeric Resolution of Seventeen Racemic 1,4-Dihydropyridine-Based Hexahydroquinoline Derivatives by HPLC" International Journal of Molecular Sciences 20, no. 10: 2513. https://doi.org/10.3390/ijms20102513
APA StyleSun, J., Gözde Gündüz, M., Zhang, J., Yu, J., & Guo, X. (2019). Direct Enantiomeric Resolution of Seventeen Racemic 1,4-Dihydropyridine-Based Hexahydroquinoline Derivatives by HPLC. International Journal of Molecular Sciences, 20(10), 2513. https://doi.org/10.3390/ijms20102513






