AMPK Alters Detrusor Contractility During Emptying in Normal Bladder and Hypertrophied Bladder with Partial Bladder Outlet Obstruction via CaMKKβ
Abstract
1. Introduction
2. Results
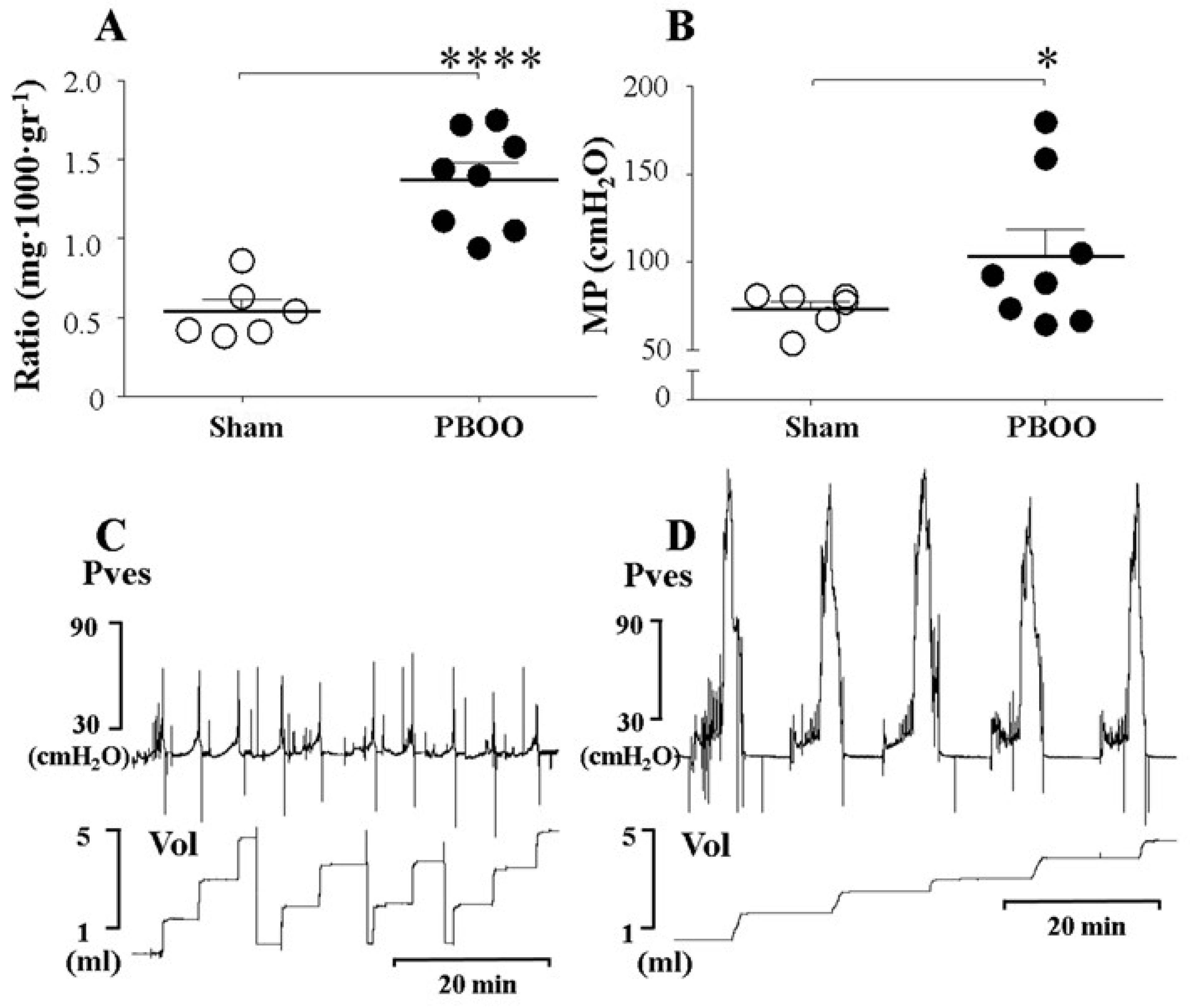
2.1. Structural Changes of Bladders with PBOO
2.2. Functional Changes of Bladders with PBOO
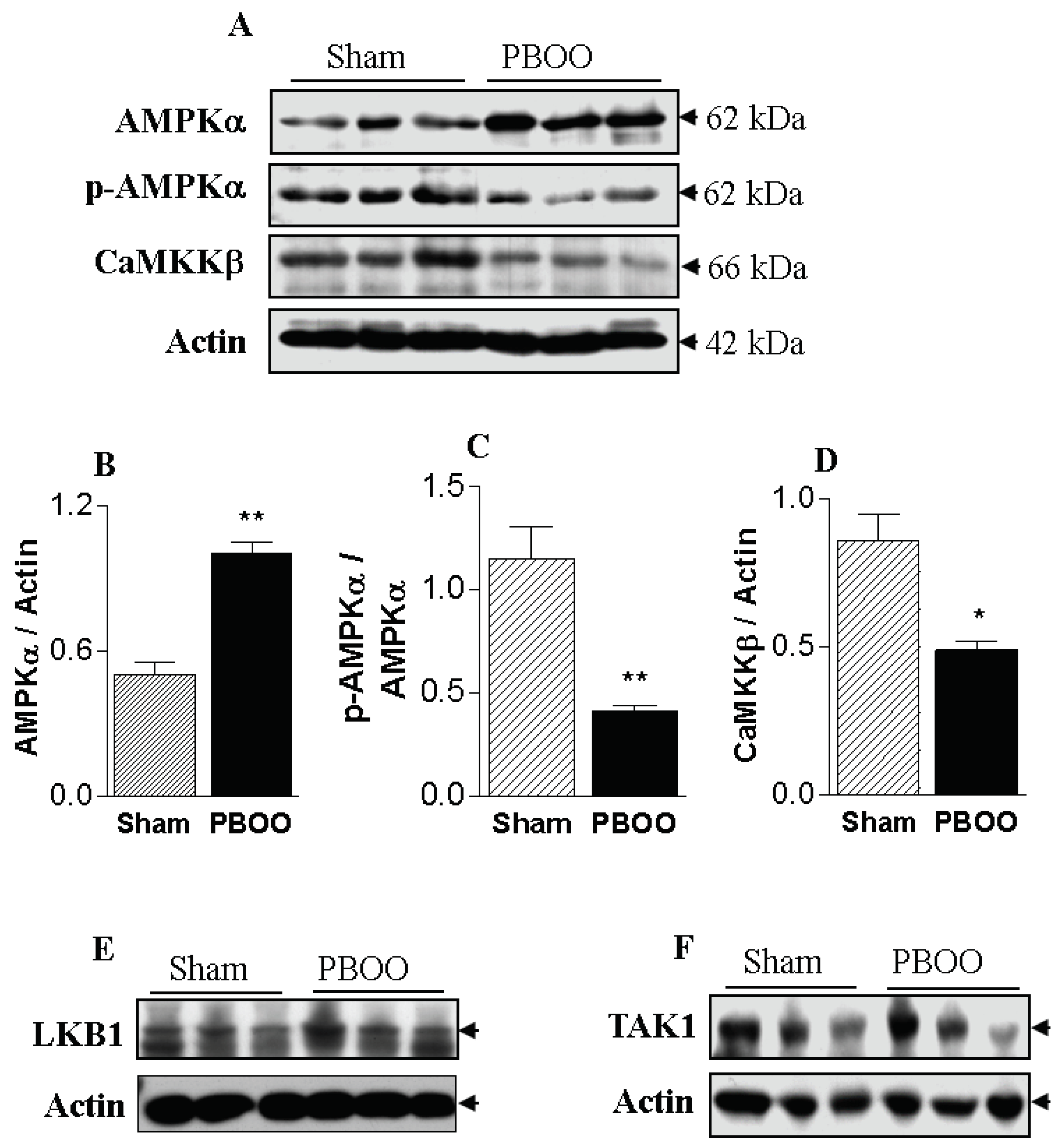
2.3. Immunoblot Characteristics of Bladders with PBOO
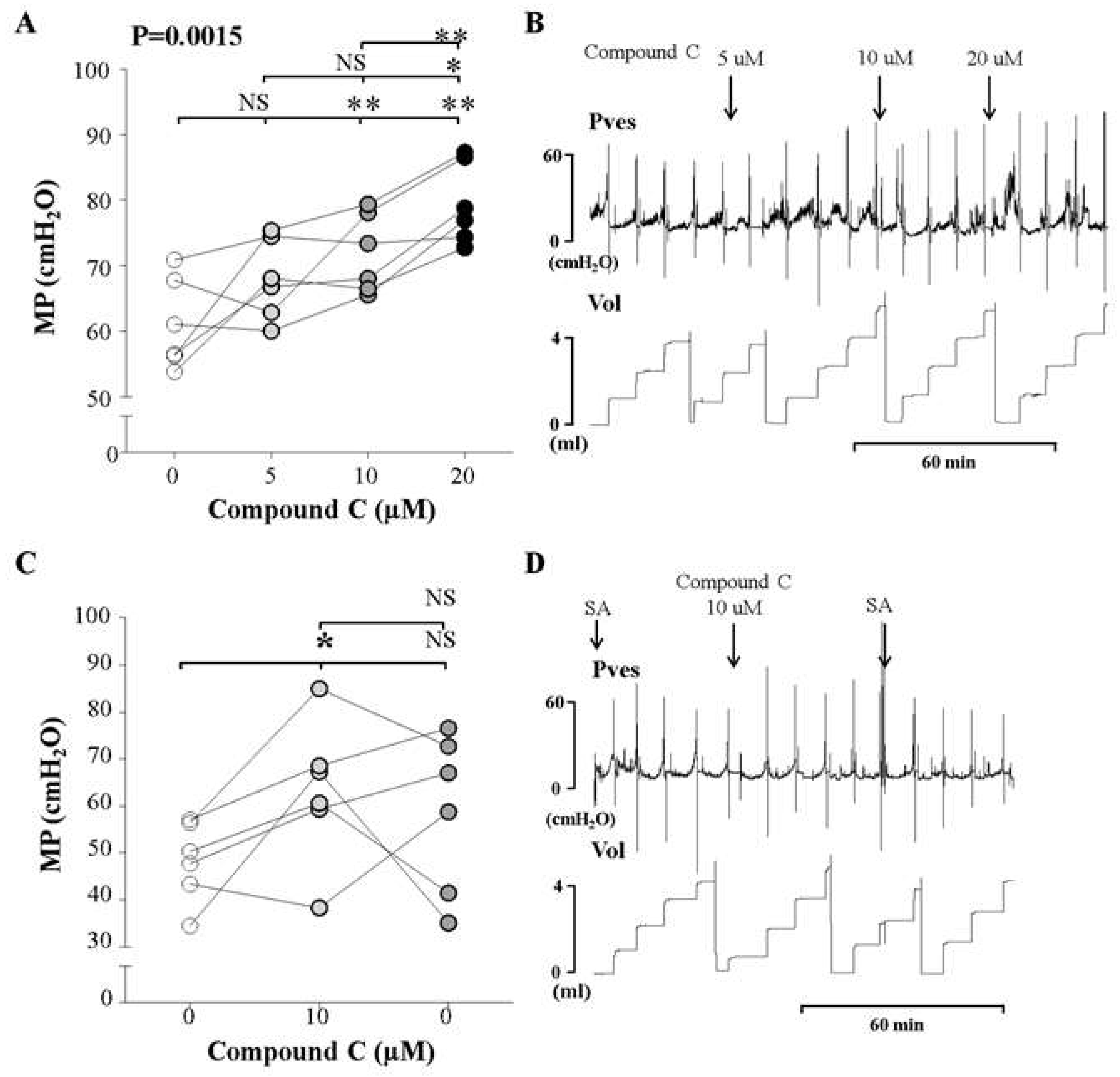
2.4. In Vivo Investigation Using An Inhibitor of AMPK
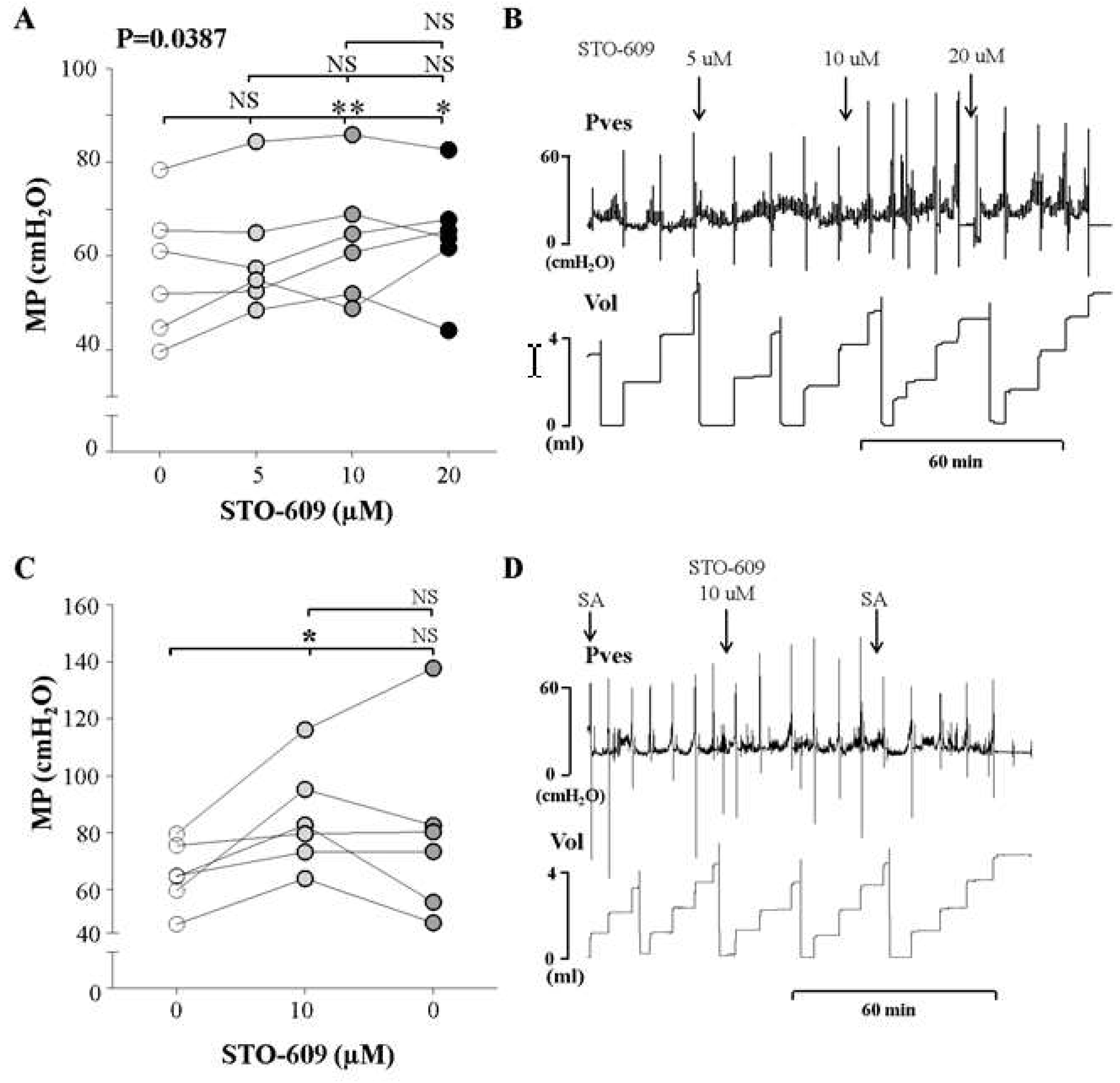
2.5. In Vivo Investigation Using An Inhibitor of CaMKKβ, An Upstream Kinase of AMPK
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Surgical Procedures
4.2. Reagents
4.3. Cystometric Investigations
4.4. Immunoblot Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMPK-activated protein kinase |
| PBOO | Partial bladder outlet obstruction |
| AMPKKs | AMPK kinases |
| CaMKKβ | Ca2+/calmodulin-dependent protein kinase kinase |
| LKB1 | Liver kinase B1 |
| TAK1 | TGFβ-Activated Kinase 1 |
| ATP | Adenosine triphosphate |
| BPH | Benign prostatic hyperplasia |
| MP | Maximum bladder pressure related to the micturition |
| BaP | Basal pressure |
| TP | Threshold pressure |
| MV | Micturition volume |
| RV | Residual volume |
| BC | Bladder capacity |
| MI | Micturition interval |
References
- Holubarsch, C.; Ludemann, J.; Wiessner, S.; Ruf, T.; Schulte-Baukloh, H.; Schmidt-Schweda, S.; Pieske, B.; Posival, H.; Just, H. Shortening versus isometric contractions in isolated human failing and non-failing left ventricular myocardium: Dependency of external work and force on muscle length, heart rate and inotropic stimulation. Cardiovasc. Res. 1998, 37, 46–57. [Google Scholar] [CrossRef]
- Levin, R.M.; Monson, F.C.; Haugaard, N.; Buttyan, R.; Hudson, A.; Roelofs, M.; Sartore, S.; Wein, A.J. Genetic and cellular characteristics of bladder outlet obstruction. Urol. Clin. North Am. 1995, 22, 263–283. [Google Scholar]
- Kitta, T.; Kanno, Y.; Chiba, H.; Higuchi, M.; Ouchi, M.; Togo, M.; Moriya, K.; Shinohara, N. Benefits and limitations of animal models in partial bladder outlet obstruction for translational research. Int. J. Urol. 2018, 25, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.M.; O’Connor, L.J.; Leggett, R.E.; Whitbeck, C.; Chichester, P. Focal hypoxia of the obstructed rabbit bladder wall correlates with intermediate decompensation. Neurourol. Urodyn. 2003, 22, 156–163. [Google Scholar] [CrossRef]
- Hypolite, J.A.; Longhurst, P.A.; Haugaard, N.; Levin, R.M. Effect of partial outlet obstruction on 14C-adenine incorporation in the rabbit urinary bladder. Neurourol. Urodyn. 1997, 16, 201–208. [Google Scholar] [CrossRef]
- Kang, Y.J.; Jin, L.H.; Park, C.S.; Shin, H.Y.; Yoon, S.M.; Lee, T. Early sequential changes in bladder function after partial bladder outlet obstruction in awake sprague-dawley rats: Focus on the decompensated bladder. Korean J. Urol. 2011, 52, 835–841. [Google Scholar] [CrossRef]
- Lim, K.B. Epidemiology of clinical benign prostatic hyperplasia. Asian J. Urol. 2017, 4, 148–151. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated protein kinase-an energy sensor that regulates all aspects of cell function. Gene. Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef]
- Arad, M.; Seidman, C.E.; Seidman, J.G. AMP-activated protein kinase in the heart: Role during health and disease. Circ. Res. 2007, 100, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Davison, M.; Woods, A.; Davies, S.P.; Beri, R.K.; Carling, D.; Hardie, D.G. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 1996, 271, 27879–27887. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D. Is TAK1 a Direct Upstream Kinase of AMPK? Int. J. Mol. Sci. 2018, 19, 2412. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Chhipa, R.R. Evolving Lessons on the Complex Role of AMPK in Normal Physiology and Cancer. Trends Pharmacol. Sci. 2016, 37, 192–206. [Google Scholar] [CrossRef]
- Carvajal, K.; Zarrinpashneh, E.; Szarszoi, O.; Joubert, F.; Athea, Y.; Mateo, P.; Gillet, B.; Vaulont, S.; Viollet, B.; Bigard, X.; et al. Dual cardiac contractile effects of the alpha2-AMPK deletion in low-flow ischemia and reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H3136–H3147. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.Y.; Jin, F.S.; Yao, C.; Zhang, T.; Zhang, G.H.; Ai, X. Ursolic acid-induced AMP-activated protein kinase (AMPK) activation contributes to growth inhibition and apoptosis in human bladder cancer T24 cells. Biochem. Biophys. Res. Commun. 2012, 419, 741–747. [Google Scholar] [CrossRef]
- Choi, B.H.; Jin, L.H.; Kim, K.H.; Kang, S.A.; Kang, J.H.; Yoon, S.M.; Park, C.S.; Lee, T. Cystometric parameters and the activity of signaling proteins in association with the compensation or decompensation of bladder function in an animal experimental model of partial bladder outlet obstruction. Int. J. Mol. Med. 2013, 32, 1435–1441. [Google Scholar] [CrossRef]
- Jin, L.H.; Andersson, K.E.; Han, J.U.; Kwon, Y.H.; Park, C.S.; Shin, H.Y.; Yoon, S.M.; Lee, T. Persistent detrusor overactivity in rats after relief of partial urethral obstruction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R896–R904. [Google Scholar] [CrossRef]
- Anger, J.T.; Goldman, H.B.; Luo, X.; Carlsson, M.O.; Chapman, D.; Zou, K.H.; Russell, D.; Ntanios, F.; Esinduy, C.B.; Clemens, J.Q. Patterns of medical management of overactive bladder (OAB) and benign prostatic hyperplasia (BPH) in the United States. Neurourol. Urodyn. 2018, 37, 213–222. [Google Scholar] [CrossRef]
- Gammie, A.; Kaper, M.; Dorrepaal, C.; Kos, T.; Abrams, P. Signs and Symptoms of Detrusor Underactivity: An Analysis of Clinical Presentation and Urodynamic Tests From a Large Group of Patients Undergoing Pressure Flow Studies. Eur. Urol. 2016, 69, 361–369. [Google Scholar] [CrossRef]
- Bratslavsky, G.; Whitbeck, C.; Horan, P.; Levin, R.M. Effects of in vivo ischemia on contractile responses of rabbit bladder to field stimulation, carbachol, ATP and KCl. Pharmacology 1999, 59, 221–226. [Google Scholar] [CrossRef]
- Andersson, K.E.; Boedtkjer, D.B.; Forman, A. The link between vascular dysfunction, bladder ischemia, and aging bladder dysfunction. Ther. Adv. Urol. 2017, 9, 11–27. [Google Scholar] [CrossRef]
- Hellstrand, P.; Vogel, H.J. Phosphagens and intracellular pH in intact rabbit smooth muscle studied by 31P-NMR. Am. J. Physiol. 1985, 248 Pt 1, C320–C329. [Google Scholar] [CrossRef]
- Levin, R.M.; Ruggieri, M.R.; Gill, H.S.; Haugaard, N.; Wein, A.J. Effect of bethanechol on glycolysis and high energy phosphate metabolism of the rabbit urinary bladder. J. Urol. 1988, 139, 646–649. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.Y.; Soltys, C.L.; Young, M.E.; Proud, C.G.; Dyck, J.R. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J. Biol. Chem. 2004, 279, 32771–32779. [Google Scholar] [CrossRef]
- Goirand, F.; Solar, M.; Athea, Y.; Viollet, B.; Mateo, P.; Fortin, D.; Leclerc, J.; Hoerter, J.; Ventura-Clapier, R.; Garnier, A. Activation of AMP kinase alpha1 subunit induces aortic vasorelaxation in mice. J. Physiol. 2007, 581 Pt 3, 1163–1171. [Google Scholar] [CrossRef]
- Koeck, I.; Burkhard, F.C.; Monastyrskaya, K. Activation of common signaling pathways during remodeling of the heart and the bladder. Biochem. Pharmacol. 2016, 102, 7–19. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, J.; Gao, K.; Chi, Y.; Mitsui, T.; Ihara, T.; Sawada, N.; Kamiyama, M.; Fan, J.; Takeda, M. AMPK Suppresses Connexin43 Expression in the Bladder and Ameliorates Voiding Dysfunction in Cyclophosphamide-induced Mouse Cystitis. Sci. Rep. 2016, 6, 19708. [Google Scholar] [CrossRef]
- Jin, L.H.; Park, C.S.; Kim, D.; Choi, B.H.; Park, S.H.; Yoon, S.M.; Lee, T. Flow Starting Point and Voiding Mechanisms Measured by Simultaneous Registrations of Intravesical, Intra-abdominal, and Intraurethral Pressures in Awake Rats. Int. Neurourol. J. 2014, 18, 68–76. [Google Scholar] [CrossRef] [PubMed]
| Sham (n = 6) | PBOO (n = 8) | |
|---|---|---|
| Ratio (mg·1000·gr−1) | 0.54 ± 0.08 | 1.37 ± 0.11 *** |
| BaP (cmH2O) | 12.07 ± 0.55 | 15.90 ± 1.61 * |
| TP (cmH2O) | 29.47 ± 3.23 | 46.06 ± 6.41 |
| MP (cmH2O) | 73.18 ± 4.43 | 108.8 ± 15.16 * |
| BC (mL) | 1.16 ± 0.13 | 2.57 ± 0.36 ** |
| MV (mL) | 1.15 ± 0.13 | 2.09 ± 0.34 * |
| RV (mL) | 0.01 ± 0.01 | 0.49 ± 0.28 |
| MI (min) | 3.59 ± 0.42 | 7.70 ± 0.93 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, B.-H.; Jin, L.-H.; Chung, D.Y.; Cho, T.J.; Kang, J.-H.; Lee, T.; Park, C.-S. AMPK Alters Detrusor Contractility During Emptying in Normal Bladder and Hypertrophied Bladder with Partial Bladder Outlet Obstruction via CaMKKβ. Int. J. Mol. Sci. 2019, 20, 2650. https://doi.org/10.3390/ijms20112650
Choi B-H, Jin L-H, Chung DY, Cho TJ, Kang J-H, Lee T, Park C-S. AMPK Alters Detrusor Contractility During Emptying in Normal Bladder and Hypertrophied Bladder with Partial Bladder Outlet Obstruction via CaMKKβ. International Journal of Molecular Sciences. 2019; 20(11):2650. https://doi.org/10.3390/ijms20112650
Chicago/Turabian StyleChoi, Bo-Hwa, Long-Hu Jin, Doo Yong Chung, Tae Jin Cho, Ju-Hee Kang, Tack Lee, and Chang-Shin Park. 2019. "AMPK Alters Detrusor Contractility During Emptying in Normal Bladder and Hypertrophied Bladder with Partial Bladder Outlet Obstruction via CaMKKβ" International Journal of Molecular Sciences 20, no. 11: 2650. https://doi.org/10.3390/ijms20112650
APA StyleChoi, B.-H., Jin, L.-H., Chung, D. Y., Cho, T. J., Kang, J.-H., Lee, T., & Park, C.-S. (2019). AMPK Alters Detrusor Contractility During Emptying in Normal Bladder and Hypertrophied Bladder with Partial Bladder Outlet Obstruction via CaMKKβ. International Journal of Molecular Sciences, 20(11), 2650. https://doi.org/10.3390/ijms20112650





