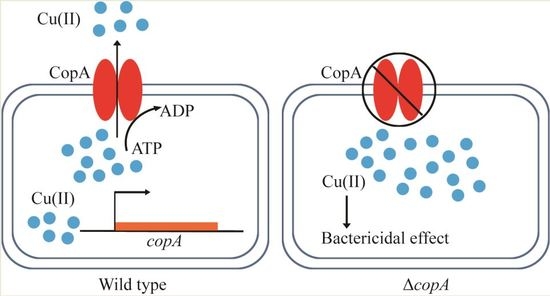
CopA Protects Streptococcus suis against Copper Toxicity
Abstract
1. Introduction
2. Results
2.1. S. suis CopA Is a Homologue of the Copper Efflux System
2.2. S. suis Up-regulates copA Expression in Response to Copper
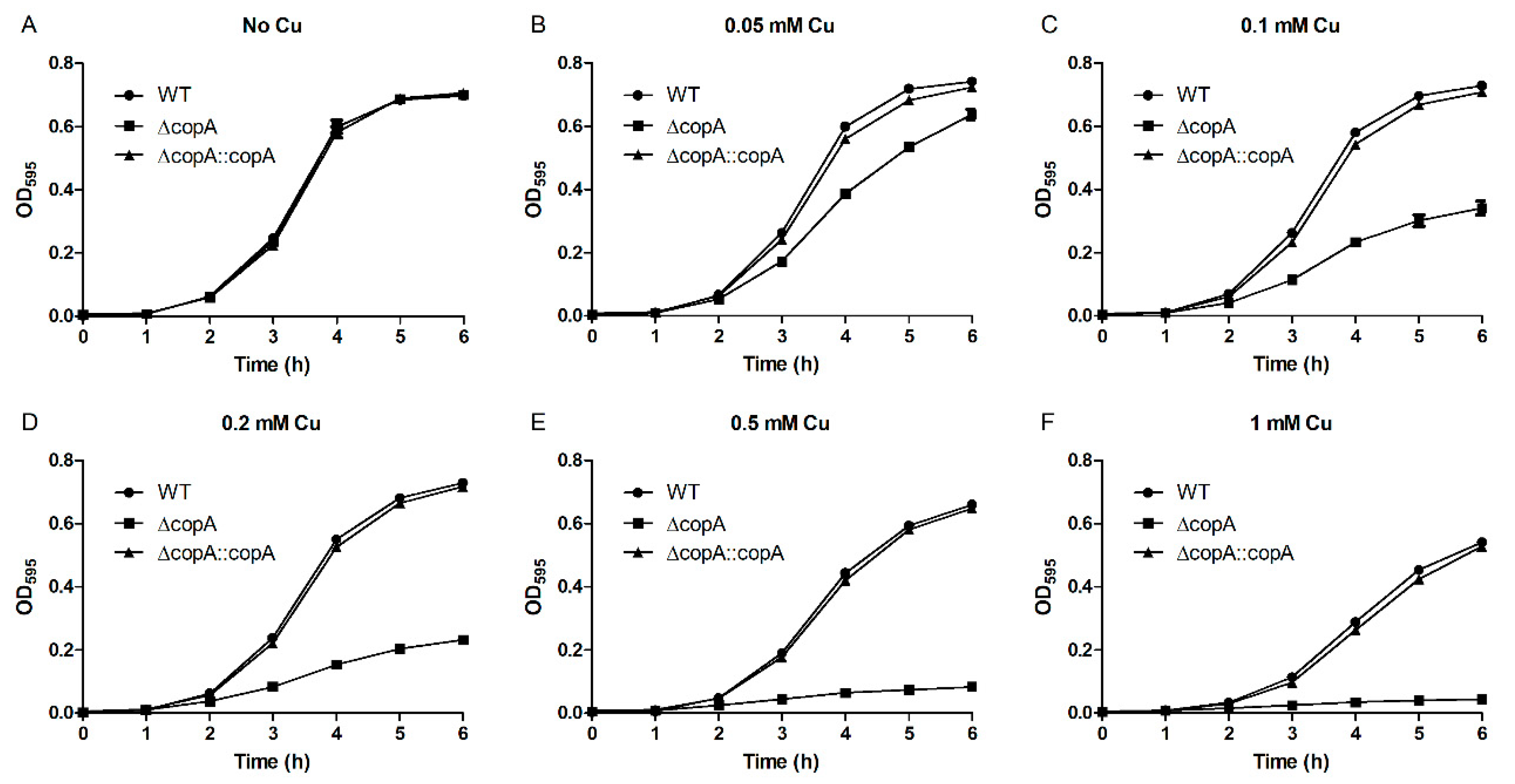
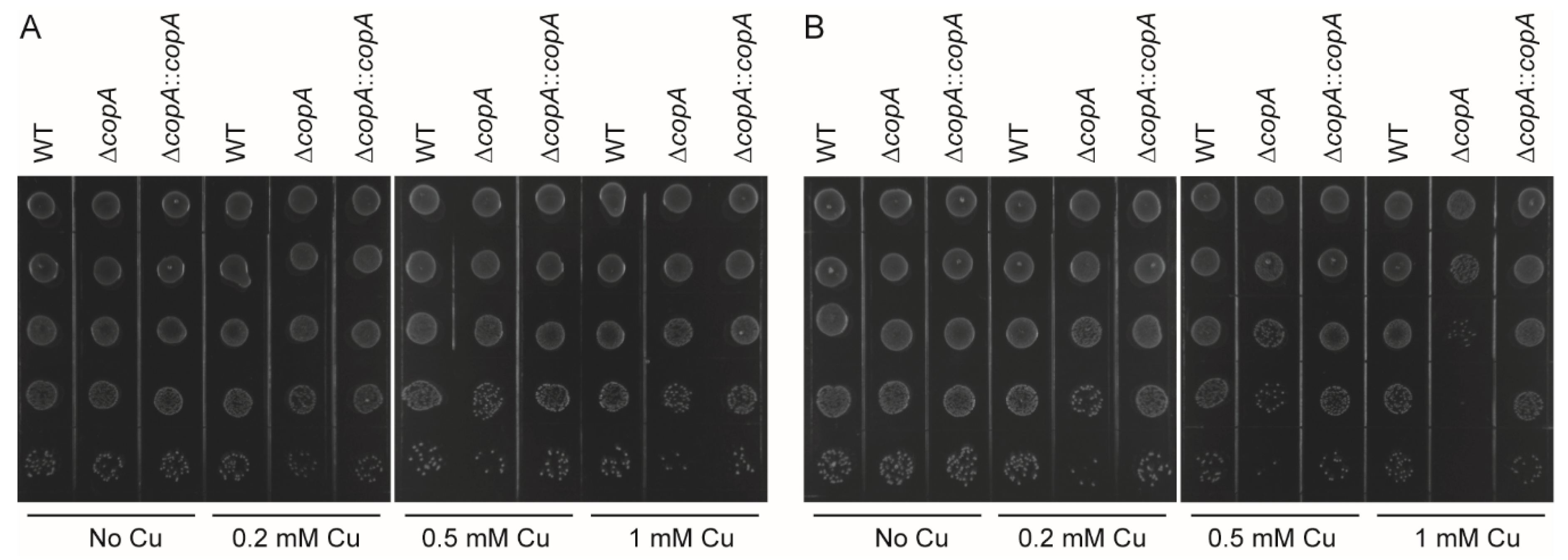
2.3. CopA Is Required for Copper Resistance in S. suis
2.4. copA Deletion Leads to Increased Intracellular Accumulation of Copper
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, and Growth Conditions
4.2. Bioinformatic Analysis
4.3. copA Expression Analysis
4.4. Construction of the Complementation Strain
4.5. Growth Curve Analyses
4.6. Spot Dilution Assays
4.7. Intracellular Copper Content Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| WT | wild-type |
| ICP-OES | inductively coupled plasma-optical emission spectroscopy |
| TSBS | Tryptic Soy Broth supplemented with 10% newborn bovine serum |
| TSAS | Tryptic Soy Agar supplemented with 10% newborn bovine serum |
| PBS | phosphate buffered saline |
References
- Lun, Z.R.; Wang, Q.P.; Chen, X.G.; Li, A.X.; Zhu, X.Q. Streptococcus suis: An emerging zoonotic pathogen. Lancet. Infect. Dis. 2007, 7, 201–209. [Google Scholar] [CrossRef]
- Wertheim, H.F.; Nghia, H.D.; Taylor, W.; Schultsz, C. Streptococcus suis: An emerging human pathogen. Clin. Infect. Dis. 2009, 48, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.; Zheng, H.; de Greeff, A.; Gao, G.F.; Grenier, D.; Jiang, Y.; Lu, C.; Maskell, D.; Oishi, K.; Okura, M.; et al. Latest developments on Streptococcus suis: An emerging zoonotic pathogen: Part 1. Future Microbiol. 2014, 9, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.; Gottschalk, M.; Boudreau, M.; Lebrun, A.; Henrichsen, J. Description of six new capsular types (29–34) of Streptococcus suis. J. Vet. Diagn Invest. 1995, 7, 405–406. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.E.; Gottschalk, M.; Brousseau, R.; Harel, J.; Hemmingsen, S.M.; Goh, S.H. Biochemical analysis, cpn60 and 16S rDNA sequence data indicate that Streptococcus suis serotypes 32 and 34, isolated from pigs, are Streptococcus orisratti. Vet. Microbiol. 2005, 107, 63–69. [Google Scholar] [CrossRef]
- Le, H.T.T.; Nishibori, T.; Nishitani, Y.; Nomoto, R.; Osawa, R. Reappraisal of the taxonomy of Streptococcus suis serotypes 20, 22, 26, and 33 based on DNA-DNA homology and sodA and recN phylogenies. Vet. Microbiol. 2013, 162, 842–849. [Google Scholar]
- Nomoto, R.; Maruyama, F.; Ishida, S.; Tohya, M.; Sekizaki, T.; Osawa, R. Reappraisal of the taxonomy of Streptococcus suis serotypes 20, 22 and 26: Streptococcus parasuis sp nov. Int. J. Syst. Evol. Micr. 2015, 65, 438–443. [Google Scholar] [CrossRef]
- Tohya, M.; Arai, S.; Tomida, J.; Watanabe, T.; Kawamura, Y.; Katsumi, M.; Ushimizu, M.; Ishida-Kuroki, K.; Yoshizumi, M.; Uzawa, Y.; et al. Defining the taxonomic status of Streptococcus suis serotype 33: The proposal for Streptococcus ruminantium sp nov. Int. J. Syst. Evol. Micr. 2017, 67, 3660–3665. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, H.; Wu, Z.; Wang, S.; Cao, M.; Hu, D.; Wang, C. Streptococcus suis infection: An emerging/reemerging challenge of bacterial infectious diseases? Virulence 2014, 5, 477–497. [Google Scholar] [CrossRef]
- Goyette-Desjardins, G.; Auger, J.P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 2014, 3, e45. [Google Scholar] [CrossRef]
- Samanovic, M.I.; Ding, C.; Thiele, D.J.; Darwin, K.H. Copper in Microbial Pathogenesis: Meddling with the Metal. Cell Host Microbe 2012, 11, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, V.; Petris, M.J. Copper Homeostasis at the Host-Pathogen Interface. J. Biol. Chem. 2012, 287, 13549–13555. [Google Scholar] [CrossRef] [PubMed]
- Ladomersky, E.; Petris, M.J. Copper tolerance and virulence in bacteria. Metallomics 2015, 7, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Wolschendorf, F.; Ackart, D.; Shrestha, T.B.; Hascall-Dove, L.; Nolan, S.; Lamichhane, G.; Wang, Y.; Bossmann, S.H.; Basaraba, R.J.; Niederweis, M. Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci USA 2011, 108, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Begg, S.L. The role of metal ions in the virulence and viability of bacterial pathogens. Biochem. Soc. Trans. 2019, 47, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Solioz, M.; Abicht, H.K.; Mermod, M.; Mancini, S. Response of gram-positive bacteria to copper stress. J. Biol. Inorg. Chem. 2010, 15, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Vats, N.; Lee, S.F. Characterization of a copper-transport operon, copYAZ, from Streptococcus mutans. Microbiology 2001, 147, 653–662. [Google Scholar] [CrossRef][Green Version]
- Singh, K.; Senadheera, D.B.; Levesque, C.M.; Cvitkovitch, D.G. The copYAZ Operon Functions in Copper Efflux, Biofilm Formation, Genetic Transformation, and Stress Tolerance in Streptococcus mutans. J. Bacteriol. 2015, 197, 2545–2557. [Google Scholar] [CrossRef]
- Mitrakul, K.; Loo, C.Y.; Hughes, C.V.; Ganeshkumar, N. Role of a Streptococcus gordonii copper-transport operon, copYAZ, in biofilm detachment. Oral Microbiol. Immunol. 2004, 19, 395–402. [Google Scholar] [CrossRef]
- Shafeeq, S.; Yesilkaya, H.; Kloosterman, T.G.; Narayanan, G.; Wandel, M.; Andrew, P.W.; Kuipers, O.P.; Morrissey, J.A. The cop operon is required for copper homeostasis and contributes to virulence in Streptococcus pneumoniae. Mol. Microbiol. 2011, 81, 1255–1270. [Google Scholar] [CrossRef]
- Young, C.A.; Gordon, L.D.; Fang, Z.; Holder, R.C.; Reid, S.D. Copper Tolerance and Characterization of a Copper-Responsive Operon, copYAZ, in an M1T1 Clinical Strain of Streptococcus pyogenes. J. Bacteriol. 2015, 197, 2580–2592. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Xu, J.; Li, J.; Hu, L.; Xia, J.; Fan, J.; Guo, W.; Chen, H.; Bei, W. Two Spx regulators modulate stress tolerance and virulence in Streptococcus suis serotype 2. PLoS ONE 2014, 9, e108197. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Ren, S.; Xu, J.; Zhao, X.; Shi, G.; Wu, J.; Li, J.; Chen, H.; Bei, W. Contribution of NADH oxidase to oxidative stress tolerance and virulence of Streptococcus suis serotype 2. Virulence 2017, 8, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Solioz, M.; Stoyanov, J.V. Copper homeostasis in Enterococcus hirae. FEMS Microbiol Rev. 2003, 27, 183–195. [Google Scholar] [CrossRef]
- Djoko, K.Y.; Franiek, J.A.; Edwards, J.L.; Falsetta, M.L.; Kidd, S.P.; Potter, A.J.; Chen, N.H.; Apicella, M.A.; Jennings, M.P.; McEwan, A.G. Phenotypic characterization of a copA mutant of Neisseria gonorrhoeae identifies a link between copper and nitrosative stress. Infect. Immun. 2012, 80, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Alquethamy, S.F.; Khorvash, M.; Pederick, V.G.; Whittall, J.J.; Paton, J.C.; Paulsen, I.T.; Hassan, K.A.; McDevitt, C.A.; Eijkelkamp, B.A. The Role of the CopA Copper Efflux System in Acinetobacter baumannii Virulence. Int. J. Mol. Sci 2019, 20, 575. [Google Scholar] [CrossRef] [PubMed]
- Vollmecke, C.; Drees, S.L.; Reimann, J.; Albers, S.V.; Lubben, M. The ATPases CopA and CopB both contribute to copper resistance of the thermoacidophilic archaeon Sulfolobus solfataricus. Microbiology 2012, 158, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.K.; Hoye, E.A.; Talaat, A.M. The global responses of Mycobacterium tuberculosis to physiological levels of copper. J. Bacteriol. 2008, 190, 2939–2946. [Google Scholar] [CrossRef]
- Lim, S.Y.; Joe, M.H.; Song, S.S.; Lee, M.H.; Foster, J.W.; Park, Y.K.; Choi, S.Y.; Lee, I.S. cuiD is a crucial gene for survival at high copper environment in Salmonella enterica serovar typhimurium. Mol. Cells 2002, 14, 177–184. [Google Scholar]
- Zhang, T.F.; Ding, Y.; Li, T.T.; Wan, Y.; Li, W.; Chen, H.C.; Zhou, R. A Fur-like protein PerR regulates two oxidative stress response related operons dpr and metQIN in Streptococcus suis. BMC Microbiol. 2012, 12, 85. [Google Scholar] [CrossRef]
- Hu, Y.L.; Hu, Q.; Wei, R.; Li, R.C.; Zhao, D.; Ge, M.; Yao, Q.; Yu, X.L. The XRE Family Transcriptional Regulator SrtR in Streptococcus suis Is Involved in Oxidant Tolerance and Virulence. Front. Cell Infect. Microbiol 2019, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Zhang, X.Y.; Wu, W.; Lu, Z.Y.; Fang, W.H. Inactivation of the sodA gene of Streptococcus suis type 2 encoding superoxide dismutase leads to reduced virulence to mice. Vet. Microbiol. 2012, 158, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.H.; Shen, H.X.; Tang, Y.L.; Fang, W.H. Superoxide dismutase of Streptococcus suis serotype 2 plays a role in anti-autophagic response by scavenging reactive oxygen species in infected macrophages. Vet. Microbiol. 2015, 176, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Marrero, K.; Sanchez, A.; Gonzalez, L.J.; Ledon, T.; Rodriguez-Ulloa, A.; Castellanos-Serra, L.; Perez, C.; Fando, R. Periplasmic proteins encoded by VCA0261-0260 and VC2216 genes together with copA and cueR products are required for copper tolerance but not for virulence in Vibrio cholerae. Microbiology 2012, 158, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Arenas, J.; Bossers-de Vries, R.; Harders-Westerveen, J.; Buys, H.; Ruuls-van Stalle, L.M.F.; Stockhofe-Zurwieden, N.; Zaccaria, E.; Tommassen, J.; Wells, J.M.; Smith, H.E.; et al. In vivo transcriptomes of Streptococcus suis reveal genes required for niche-specific adaptation and pathogenesis. Virulence 2019, 10, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Dong, X.; Zhou, Y.; Li, Z.; Deng, L.; Chen, H.; Wang, X.; Li, J. Draft Genome Sequence of Hypervirulent and Vaccine Candidate Streptococcus suis Strain SC19. Genome Announc. 2017, 5, e01484-16. [Google Scholar] [CrossRef]
- Takamatsu, D.; Osaki, M.; Sekizaki, T. Thermosensitive suicide vectors for gene replacement in Streptococcus suis. Plasmid 2001, 46, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]






| S. suis Strains | Locus Tag | Gene Sequence Identity (%) 1 |
|---|---|---|
| LSM102 | A9494_06425 | 100 |
| SC19 | B9H01_06680 | 100 |
| SS2-1 | BVD85_06510 | 100 |
| ZY05719 | ZY05719_06610 | 100 |
| A7 | SSUA7_1228 | 100 |
| P1/7 | SSU1214 | 100 |
| BM407 | SSUBM407_0575 | 100 |
| SC84 | SSUSC84_1247 | 100 |
| S735 | - | 99 |
| GZ1 | SSGZ1_1230 | 99 |
| SS12 | SSU12_1279 | 99 |
| 05ZYH33 | SSU05_1385 | 99 |
| 98HAH33 | SSU98_1400 | 99 |
| SH0104 | - | 97 |
| HA0609 | CR542_03955 | 97 |
| 90-1330 | AN924_03380 | 97 |
| NSUI060 | APQ97_02765 | 97 |
| NSUI002 | AA105_03890 | 97 |
| 05HAS68 | HAS68_0686 | 97 |
| YB51 | YB51_2960 | 97 |
| D9 | SSUD9_0599 | 97 |
| ST3 | SSUST3_0597 | 97 |
| CS100322 | CR541_06915 | 97 |
| T15 | T15_0568 | 97 |
| SC070731 | NJAUSS_1288 | 97 |
| JS14 | SSUJS14_1360 | 97 |
| ST1 | SSUST1_0574 | 96 |
| ISU2812 | A7J09_03980 | 96 |
| SH1510 | DP111_07130 | 96 |
| GZ0565 | BFP66_02780 | 95 |
| DN13 | A6M16_02880 | 95 |
| 6407 | ID09_03115 | 95 |
| TL13 | TL13_0615 | 95 |
| CZ130302 | CVO91_03355 | 95 |
| HN105 | DF184_07440 | 95 |
| HN136 | CWM22_09360 | 95 |
| SRD478 | A7J08_03040 | 92 |
| 1081 | BKM67_07590 | 93 |
| 0061 | BKM66_07040 | 93 |
| D12 | SSUD12_0568 | 92 |
| HA1003 | DP112_07660 | 92 |
| AH681 | CWI26_08525 | 92 |
| Strain or Plasmid | Relevant Characteristics | Source or Reference |
|---|---|---|
| Strains | ||
| SC19 | Virulent S. suis 2 strain isolated from the brain of a dead pig | [36] |
| ΔcopA | copA deletion mutant of strain SC19 | [23] |
| ΔcopA::copA | Complemented strain of ΔcopA | This study |
| DH5α | Cloning host for recombinant vector | TransGen |
| Plasmids | ||
| pSET4s | Thermosensitive suicide vector; SpcR 1 | [37] |
| pSET4s::CcopA | pSET4s containing copA and its flanking regions | This study |
| Primer | Sequence (5’-3’) 1 | Size (bp) | Target Gene |
|---|---|---|---|
| QcopA1 | AGAGGATAGGGATGAGCAAGATAACT | 148 | an internal region of copA |
| QcopA2 | TTTGTCTGGTCAGCAGCATTTACT | ||
| Q16S1 | TAGTCCACGCCGTAAACGATG | 159 | an internal region of 16S rRNA |
| Q16S2 | TAAACCACATGCTCCACCGC | ||
| L1 | CCCCGTCGACAATGAGGGCCAAAACGTC | 3758 | copA and its flanking regions |
| R2 | CGCCGAATTCACCATCGACCAGCACTGAG | ||
| In1 | TATCACCGAAAGACCACGAC | 629 | an internal region of copA |
| In2 | ATAATGTTTTTGGCGGCAC | ||
| Out1 | GAGGACAAAATCAGGGGCT | 2769/378 | a fragment containing copA |
| Out2 | AGGGAACAGGCTGAAAACC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, C.; Jia, M.; Lu, T.; Gao, M.; Li, L. CopA Protects Streptococcus suis against Copper Toxicity. Int. J. Mol. Sci. 2019, 20, 2969. https://doi.org/10.3390/ijms20122969
Zheng C, Jia M, Lu T, Gao M, Li L. CopA Protects Streptococcus suis against Copper Toxicity. International Journal of Molecular Sciences. 2019; 20(12):2969. https://doi.org/10.3390/ijms20122969
Chicago/Turabian StyleZheng, Chengkun, Mengdie Jia, Tianyu Lu, Miaomiao Gao, and Lingzhi Li. 2019. "CopA Protects Streptococcus suis against Copper Toxicity" International Journal of Molecular Sciences 20, no. 12: 2969. https://doi.org/10.3390/ijms20122969
APA StyleZheng, C., Jia, M., Lu, T., Gao, M., & Li, L. (2019). CopA Protects Streptococcus suis against Copper Toxicity. International Journal of Molecular Sciences, 20(12), 2969. https://doi.org/10.3390/ijms20122969