Influence of Acute Exercise on DNA Repair and PARP Activity before and after Irradiation in Lymphocytes from Trained and Untrained Individuals
Abstract
:1. Introduction
1.1. Radioprotective Effect of Exercise
1.2. ROS as Potential Mediator of Exercise-Induced DNA Damage
1.3. Effects of Exercise on DNA Repair
2. Results
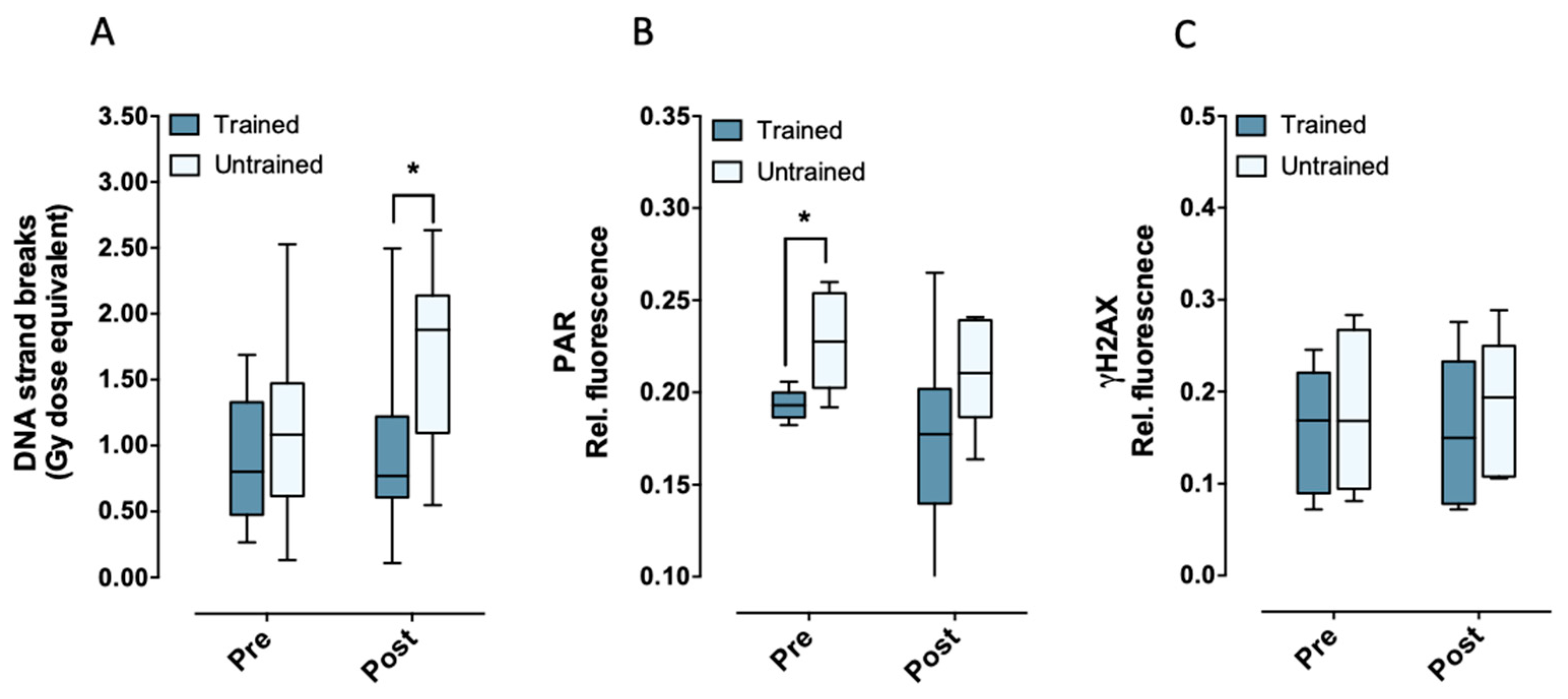
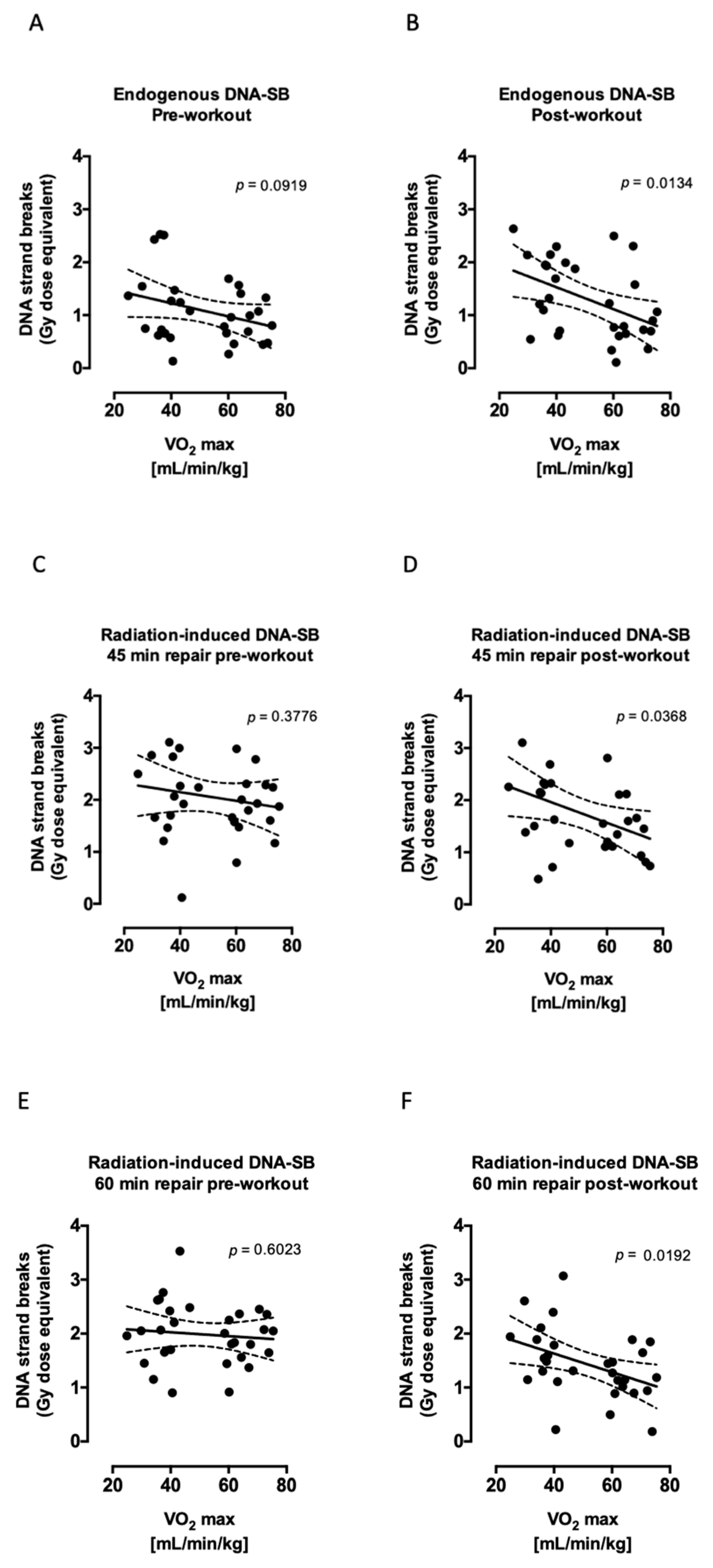
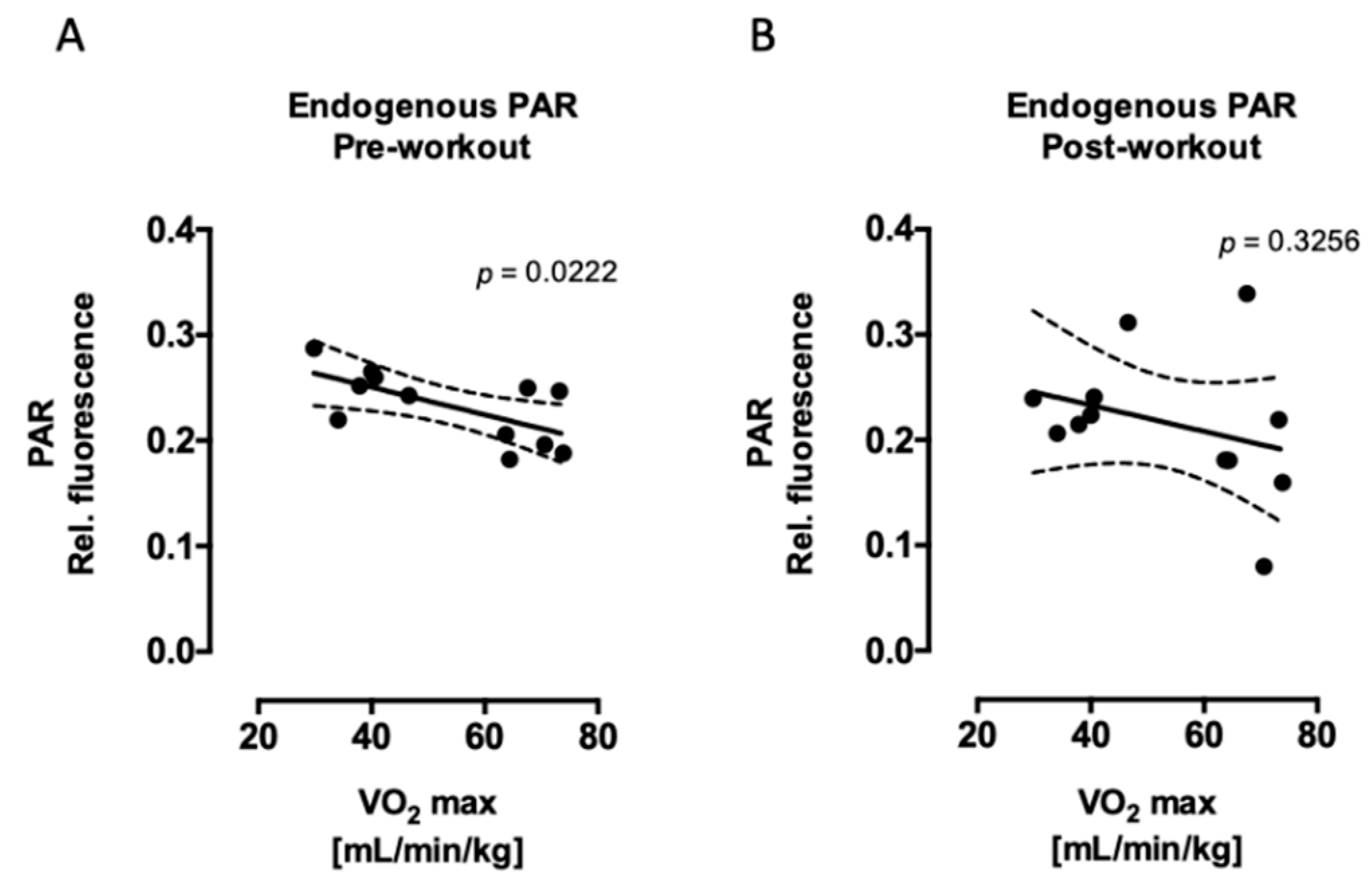
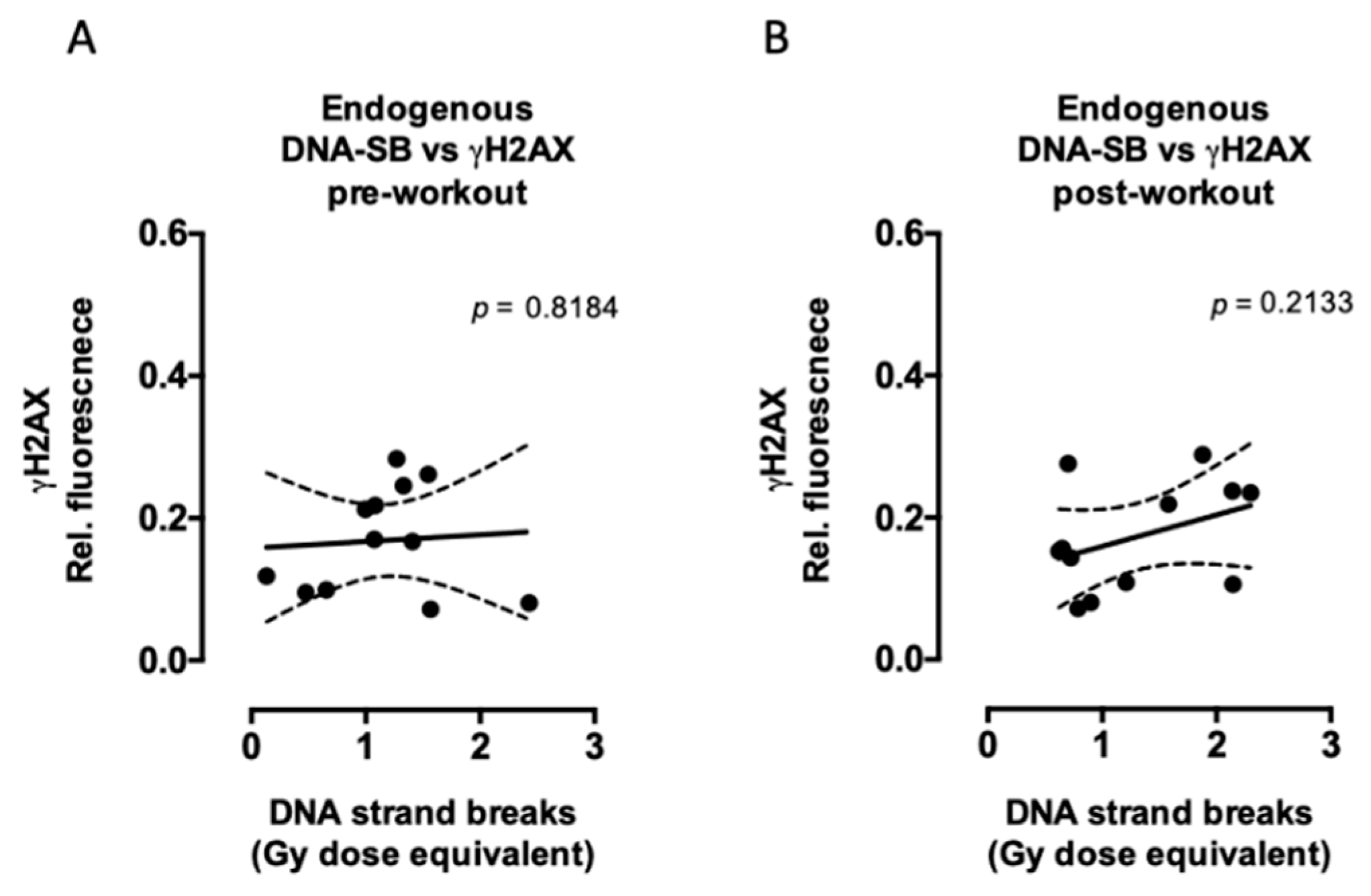
2.1. Aerobic Fitness Maintains Lower Endogenous PAR Levels and Protects Against Acute Exercise-Induced Endogenous DNA Strand Break Formation
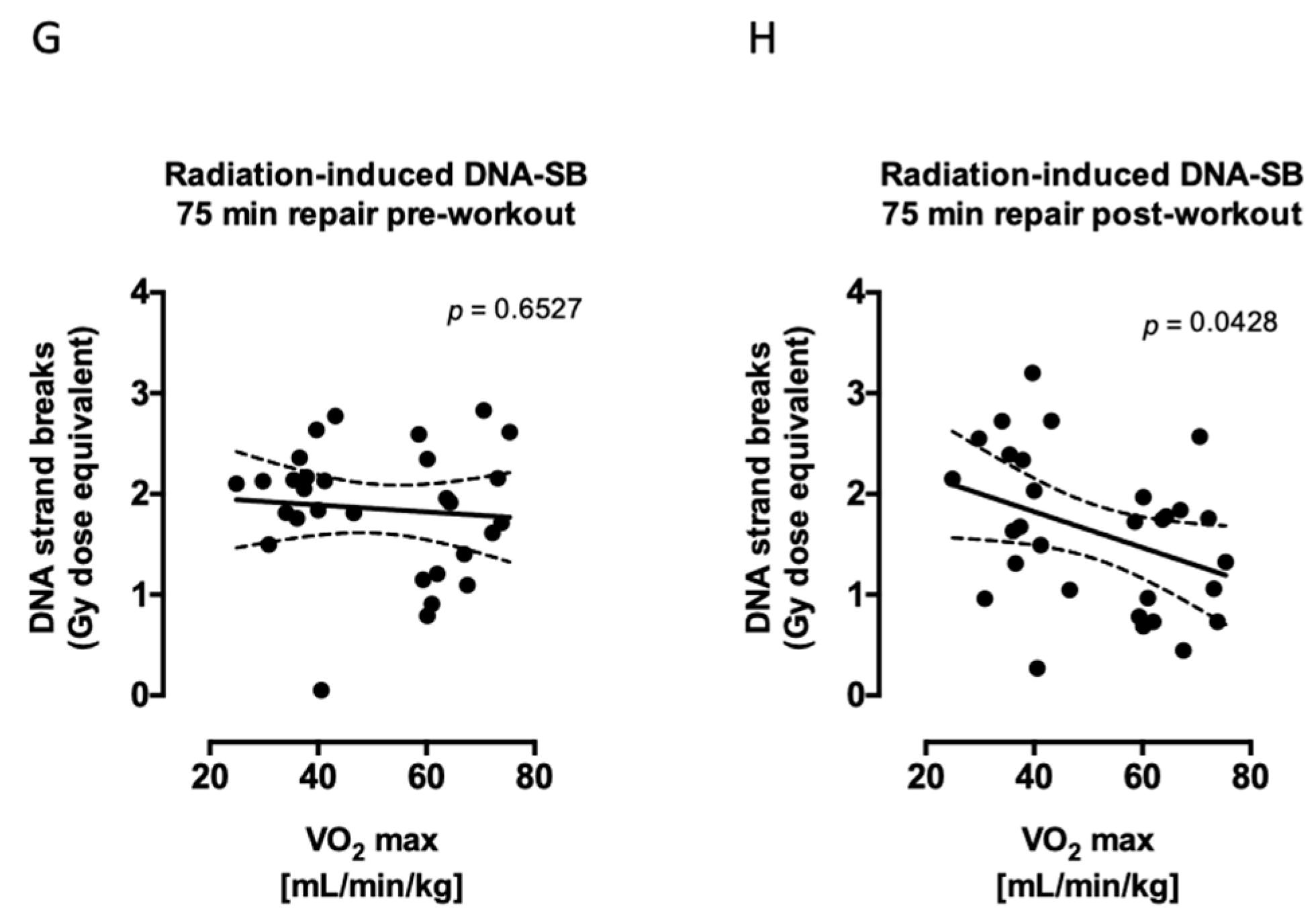
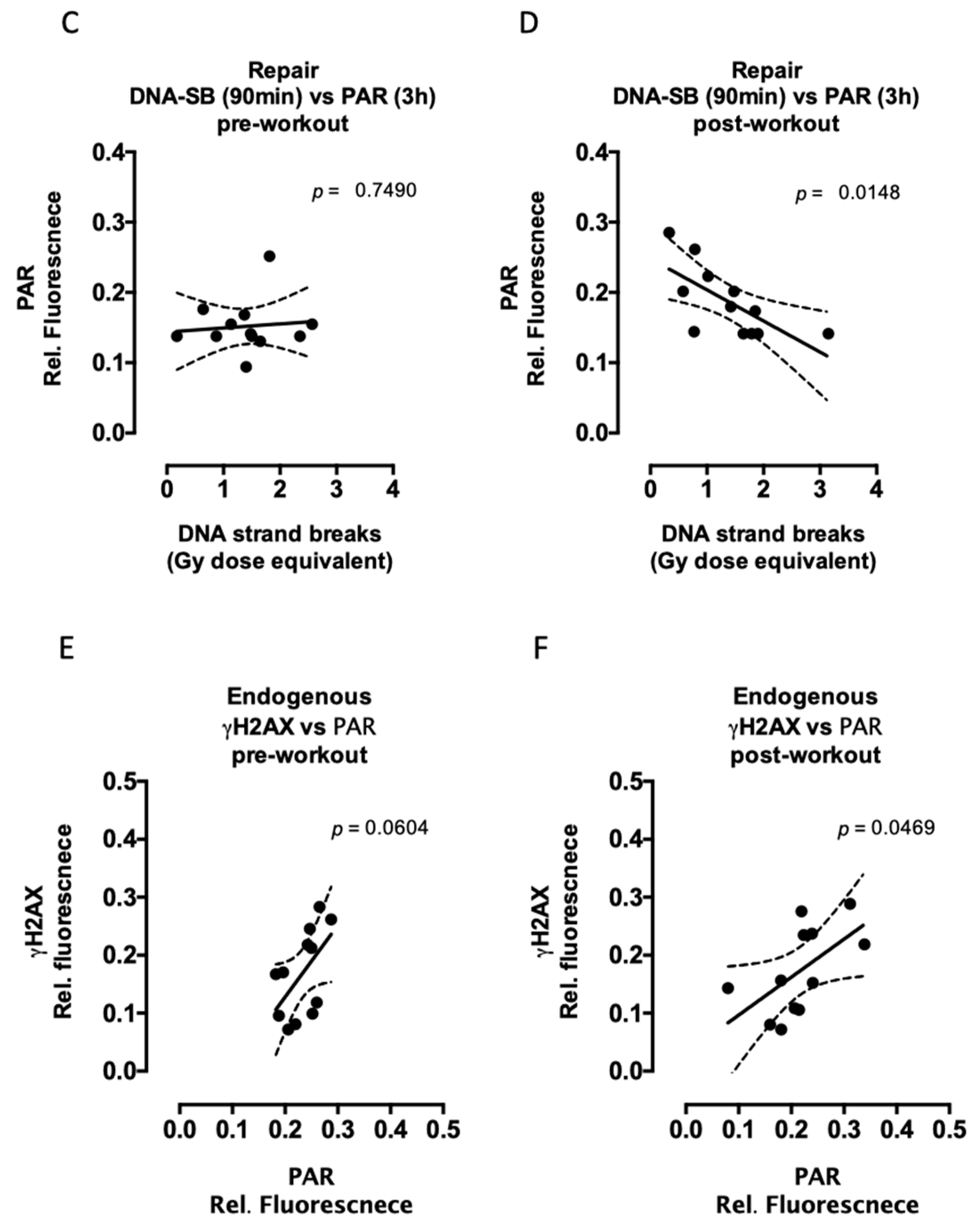
2.2. Aerobic Fitness Enhances DNA Repair and Maintains PAR Levels after Radiation
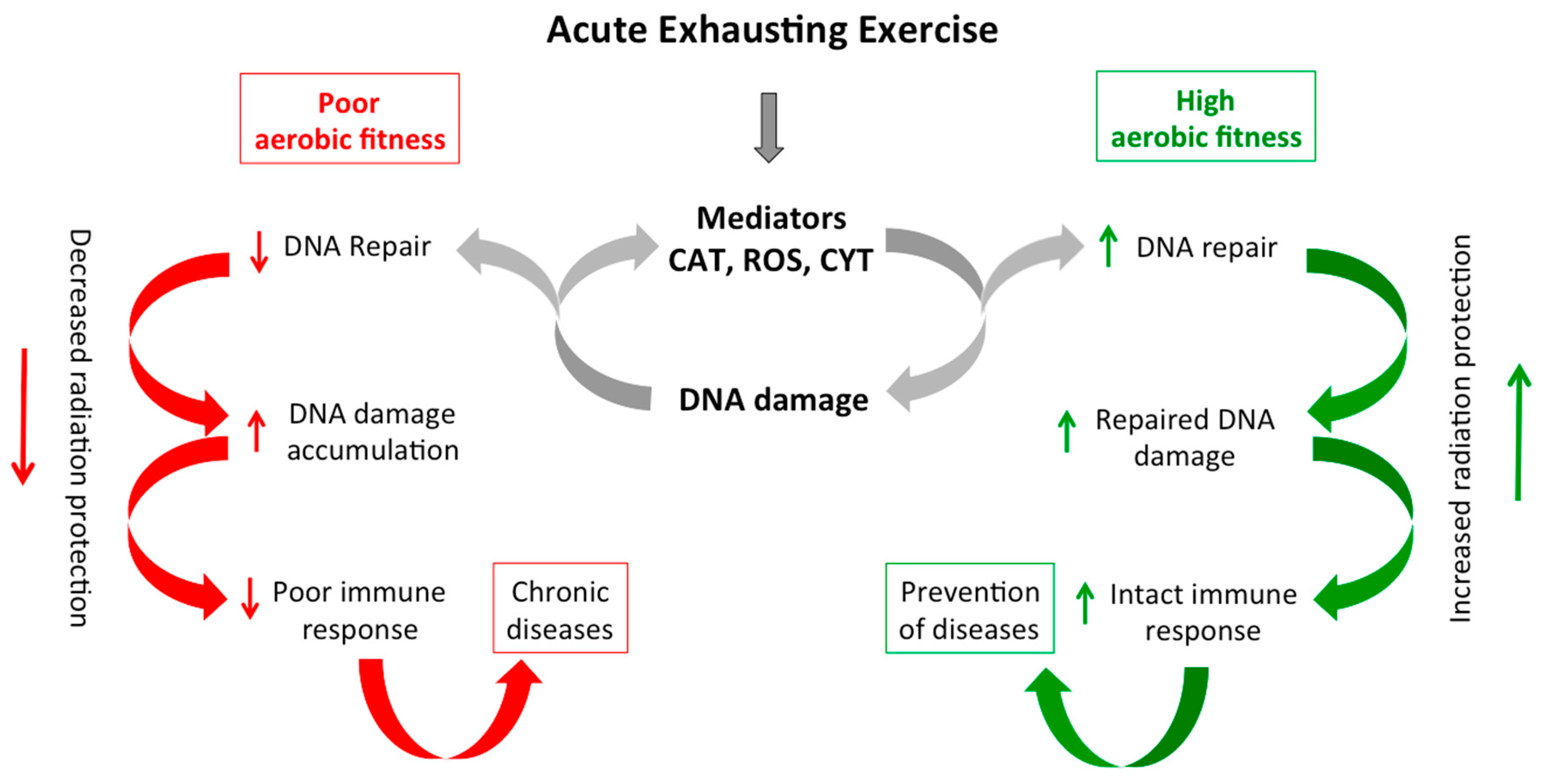
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Blood Draw
4.3. DNA Single-Strand Breaks Detection
4.4. PAR and γH2AX Detection
4.5. Exhaustive Bout of Exercise, Assessment of V’O2max
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blair, S.N.; Cheng, Y.; Holder, J.S. Is physical activity or physical fitness more important in defining health benefits? Med. Sci. Sports Exerc. 2001, 33, S379–S399, discussion S419–S420. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, Z.; Sporis, G.; Weston, M. Effectiveness of high-intensity interval training (hit) and continuous endurance training for VO2max improvements: A systematic review and meta-analysis of controlled trials. Sports Med. 2015, 45, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Which type of exercise keeps you young? Curr. Opin. Clin. Nutr. Metab. Care. 2019, 22, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.D.; Macneil, L.G.; Tarnopolsky, M.A. Long-term aerobic exercise is associated with greater muscle strength throughout the life span. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Stroth, S.; Hille, K.; Spitzer, M.; Reinhardt, R. Aerobic endurance exercise benefits memory and affect in young adults. Neuropsychol. Rehabil. 2009, 19, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.; Jaekel, P.; Rosenberger, A.; Weber, T.; Scott, J.; Castrucci, F.; Lambrecht, G.; Ploutz-Snyder, L.; Damann, V.; Kozlovskaya, I.; et al. Exercise in space: The european space agency approach to in-flight exercise countermeasures for long-duration missions on iss. Extrem. Physiol. Med. 2016, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [PubMed]
- Danese, E.; Lippi, G.; Sanchis-Gomar, F.; Brocco, G.; Rizzo, M.; Banach, M.; Montagnana, M. Physical exercise and DNA injury: Good or evil? Adv. Clin. Chem. 2017, 81, 193–230. [Google Scholar]
- Schmidt, R.H.; Nickerson, J.M.; Boatright, J.H. Exercise as gene therapy: BDNF and DNA damage repair. Asia Pac. J. Ophthalmol. 2016, 5, 309–311. [Google Scholar] [CrossRef]
- Cash, S.W.; Beresford, S.A.; Vaughan, T.L.; Heagerty, P.J.; Bernstein, L.; White, E.; Neuhouser, M.L. Recent physical activity in relation to DNA damage and repair using the comet assay. J. Phys. Act. Health 2014, 11, 770–776. [Google Scholar] [CrossRef]
- De Lisio, M.; Phan, N.; Boreham, D.R.; Parise, G. Exercise-induced protection of bone marrow cells following exposure to radiation. Appl. Physiol. Nutr. Metab. 2011, 36, 80–87. [Google Scholar] [CrossRef]
- Umegaki, K.; Higuchi, M.; Inoue, K.; Esashi, T. Influence of one bout of intensive running on lymphocyte micronucleus frequencies in endurance-trained and untrained men. Int. J. Sports Med. 1998, 19, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Caldecott, K.W. Single-strand break repair and genetic disease. Nat. Rev. Genet. 2008, 9, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.M.; Galendo, D.; Wang, Z.Q. Role of DNA break-sensing molecule poly(ADP-ribose) polymerase (PARP) in cellular function and radiation toxicity. Cold Spring Harb. Symp. Quant. Biol. 2000, 65, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, C.J.; Davies, M.I.; Goodwin, P.M.; Halldorsson, H.; Lewis, P.J.; Shall, S.; Zia’ee, A.A. The involvement of poly(ADP-ribose) polymerase in the degradation of NAD caused by gamma-radiation and N-methyl-N-nitrosourea. Eur. J. Biochem. 1979, 101, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Hassa, P.O.; Hottiger, M.O. The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front. Biosci. 2008, 13, 3046–3082. [Google Scholar] [CrossRef] [PubMed]
- Altmeyer, M.; Messner, S.; Hassa, P.O.; Fey, M.; Hottiger, M.O. Molecular mechanism of poly(ADP-ribosyl)ation by PARP1 and identification of lysine residues as ADP-ribose acceptor sites. Nucleic Acids Res. 2009, 37, 3723–3738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvath, E.M.; Zsengeller, Z.K.; Szabo, C. Quantification of PARP activity in human tissues: Ex vivo assays in blood cells and immunohistochemistry in human biopsies. Methods Mol. Biol. 2011, 780, 267–275. [Google Scholar]
- Polo, S.E.; Jackson, S.P. Dynamics of DNA damage response proteins at DNA breaks: A focus on protein modifications. Genes Dev. 2011, 25, 409–433. [Google Scholar] [CrossRef]
- Haince, J.F.; McDonald, D.; Rodrigue, A.; Dery, U.; Masson, J.Y.; Hendzel, M.J.; Poirier, G.G. PARP1-dependent kinetics of recruitment of MRE11 and NBS1 proteins to multiple DNA damage sites. J. Biol. Chem. 2008, 283, 1197–1208. [Google Scholar] [CrossRef]
- Hanzlikova, H.; Gittens, W.; Krejcikova, K.; Zeng, Z.; Caldecott, K.W. Overlapping roles for PARP1 and PARP2 in the recruitment of endogenous XRCC1 and PNKP into oxidized chromatin. Nucleic Acids Res. 2017, 45, 2546–2557. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Sakellariou, G.K.; Murray, S.; Waldron, S.; Gregson, W.; Burniston, J.G.; Morton, J.P.; Iwanejko, L.A.; Close, G.L. Lifelong endurance training attenuates age-related genotoxic stress in human skeletal muscle. Longev. Healthspan 2013, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Achey, P.; Duryea, H. Production of DNA strand breaks by the hydroxyl radical. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1974, 25, 595–601. [Google Scholar] [CrossRef]
- Kawamura, T.; Muraoka, I. Exercise-induced oxidative stress and the effects of antioxidant intake from a physiological viewpoint. Antioxidants 2018, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B. Invited review: Redox modulation of skeletal muscle contraction: What we know and what we don’t. J. Appl. Physiol. 2001, 90, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Margonis, K.; Fatouros, I.G.; Jamurtas, A.Z.; Nikolaidis, M.G.; Douroudos, I.; Chatzinikolaou, A.; Mitrakou, A.; Mastorakos, G.; Papassotiriou, I.; Taxildaris, K.; et al. Oxidative stress biomarkers responses to physical overtraining: Implications for diagnosis. Free Radic. Biol. Med. 2007, 43, 901–910. [Google Scholar] [CrossRef]
- Belviranlı, M.; Gökbel, H. Acute exercise induced oxidative stress and antioxidant changes. Eur. J. Gen. Med. 2006, 3, 126–131. [Google Scholar] [CrossRef]
- Hoffman-Goetz, L.; Pedersen, B.K. Exercise and the immune system: A model of the stress response? Immunol. Today 1994, 15, 382–387. [Google Scholar] [CrossRef]
- Strobel, G.; Friedmann, B.; Siebold, R.; Bartsch, P. Effect of severe exercise on plasma catecholamines in differently trained athletes. Med. Sci. Sports Exerc. 1999, 31, 560–565. [Google Scholar] [CrossRef]
- Crespo, M.E.; Bicho, M.P. Membrane-mediated effects of catecholamines on the DNA of human leukocytes: The role of reactive oxygen species. Biol. Signals 1995, 4, 78–85. [Google Scholar] [CrossRef]
- Allen, J.; Sun, Y.; Woods, J.A. Exercise and the regulation of inflammatory responses. Prog. Mol. Biol. Transl. Sci. 2015, 135, 337–354. [Google Scholar] [PubMed]
- Kidane, D.; Chae, W.J.; Czochor, J.; Eckert, K.A.; Glazer, P.M.; Bothwell, A.L.; Sweasy, J.B. Interplay between DNA repair and inflammation, and the link to cancer. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 116–139. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Kumagai, S.; Nakamoto, H.; Goto, S. 8-oxoguanosine and uracil repair of nuclear and mitochondrial DNA in red and white skeletal muscle of exercise-trained old rats. J. Appl. Physiol. 2007, 102, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Smolka, M.B.; Zoppi, C.C.; Alves, A.A.; Silveira, L.R.; Marangoni, S.; Pereira-Da-Silva, L.; Novello, J.C.; Macedo, D.V. HSP72 as a complementary protection against oxidative stress induced by exercise in the soleus muscle of rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1539–R1545. [Google Scholar] [CrossRef] [PubMed]
- Steinbacher, P.; Eckl, P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, H.; Kaneko, T.; Tahara, S.; Hayashi, E.; Naito, H.; Radak, Z.; Goto, S. Regular exercise reduces 8-oxodg in the nuclear and mitochondrial DNA and modulates the DNA repair activity in the liver of old rats. Exp. Gerontol. 2007, 42, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.A.; King, N.; McFarlane, J.R.; Graham, P.L.; Dieberg, G. Effect of exercise training on liver function in adults who are overweight or exhibit fatty liver disease: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 834–843. [Google Scholar] [CrossRef]
- Di Paolo, T.; Falardeau, P.; Morissette, M. Striatal D-2 dopamine agonist binding sites fluctuate during the rat estrous cycle. Life Sci. 1988, 43, 665–672. [Google Scholar] [CrossRef]
- Devi, S.A.; Kiran, T.R. Regional responses in antioxidant system to exercise training and dietary vitamin e in aging rat brain. Neurobiol. Aging 2004, 25, 501–508. [Google Scholar] [CrossRef]
- Somani, S.M.; Ravi, R.; Rybak, L.P. Effect of exercise training on antioxidant system in brain regions of rat. Pharmacol. Biochem. Behav. 1995, 50, 635–639. [Google Scholar] [CrossRef]
- Somasekar Reddy, N.; Shanmugam, K.R.; Korivi, M.; Sathyavelu Reddy, K. Exercise training modulates the antioxidant enzymes in brain tissue: A study with reference to ageing. J. Ecophysiol. Occup. Health 2009, 9, 5–10. [Google Scholar]
- Turner, J.E.; Bosch, J.A.; Aldred, S. Measurement of exercise-induced oxidative stress in lymphocytes. Biochem. Soc. Trans. 2011, 39, 1299–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spanidis, Y.; Veskoukis, A.S.; Papanikolaou, C.; Stagos, D.; Priftis, A.; Deli, C.K.; Jamurtas, A.Z.; Kouretas, D. Exercise-induced reductive stress is a protective mechanism against oxidative stress in peripheral blood mononuclear cells. Oxid. Med. Cell. Longev. 2018, 2018, 3053704. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Saini, D.; Kumar, P.R.V.; Jaikrishan, G.; Das, B. Efficient repair of DNA double strand breaks in individuals from high level natural radiation areas of Kerala coast, south-west India. Mutat. Res. 2017, 806, 39–50. [Google Scholar] [CrossRef]
- Johansson, P.; Fasth, A.; Ek, T.; Hammarsten, O. Validation of a flow cytometry-based detection of gamma-H2AX, to measure DNA damage for clinical applications. Cytom. B Clin. Cytom. 2017, 92, 534–540. [Google Scholar] [CrossRef]
- Moroni, M.; Maeda, D.; Whitnall, M.H.; Bonner, W.M.; Redon, C.E. Evaluation of the gamma-H2AX assay for radiation biodosimetry in a swine model. Int. J. Mol. Sci. 2013, 14, 14119–14135. [Google Scholar] [CrossRef]
- Sharma, P.M.; Ponnaiya, B.; Taveras, M.; Shuryak, I.; Turner, H.; Brenner, D.J. Correction: High throughput measurement of gammah2AX DSB repair kinetics in a healthy human population. PLoS ONE 2015, 10, e0131620. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef]
- Singh, S.; Vrishni, S.; Singh, B.K.; Rahman, I.; Kakkar, P. Nrf2-are stress response mechanism: A control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radic. Res. 2010, 44, 1267–1288. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Li, J.; Liu, Z.; Chuang, C.C.; Yang, W.; Zuo, L. Redox mechanism of reactive oxygen species in exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Niess, A.M.; Grunert-Fuchs, M.; Poch, B.; Speit, G. Vitamin e prevents exercise-induced DNA damage. Mutat. Res. 1995, 346, 195–202. [Google Scholar] [CrossRef]
- Cobley, J.N.; Margaritelis, N.V.; Morton, J.P.; Close, G.L.; Nikolaidis, M.G.; Malone, J.K. The basic chemistry of exercise-induced DNA oxidation: Oxidative damage, redox signaling, and their interplay. Front. Physiol. 2015, 6, 182. [Google Scholar] [CrossRef]
- Soares, J.P.; Silva, A.M.; Oliveira, M.M.; Peixoto, F.; Gaivao, I.; Mota, M.P. Effects of combined physical exercise training on DNA damage and repair capacity: Role of oxidative stress changes. Age 2015, 37, 9799. [Google Scholar] [CrossRef] [PubMed]
- Bryant, P.E.; Warring, R.; Ahnstrom, G. DNA repair kinetics after low doses of X-rays. A comparison of results obtained by the unwinding and nucleoid sedimentation methods. Mutat. Res. 1984, 131, 19–26. [Google Scholar]
- Taleei, R.; Weinfeld, M.; Nikjoo, H. A kinetic model of single-strand annealing for the repair of DNA double-strand breaks. Radiat. Prot. Dosim. 2011, 143, 191–195. [Google Scholar] [CrossRef]
- Van Loon, A.A.; Sonneveld, E.; Hoogerbrugge, J.; van der Schans, G.P.; Grootegoed, J.A.; Lohman, P.H.; Baan, R.A. Induction and repair of DNA single-strand breaks and DNA base damage at different cellular stages of spermatogenesis of the hamster upon in vitro exposure to ionizing radiation. Mutat. Res. 1993, 294, 139–148. [Google Scholar] [CrossRef]
- Leontjeva, G.A.; Mantzighin, Y.A.; Gaziev, A.I. The ultra-fast repair of single-strand breaks in DNA of gamma-irradiated chinese hamster cells. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1976, 30, 577–580. [Google Scholar] [CrossRef]
- Sharma, P.M.; Ponnaiya, B.; Taveras, M.; Shuryak, I.; Turner, H.; Brenner, D.J. High throughput measurement of gammah2AX DSB repair kinetics in a healthy human population. PLoS ONE 2015, 10, e0121083. [Google Scholar] [CrossRef]
- MacRae, S.L.; Croken, M.M.; Calder, R.B.; Aliper, A.; Milholland, B.; White, R.R.; Zhavoronkov, A.; Gladyshev, V.N.; Seluanov, A.; Gorbunova, V.; et al. DNA repair in species with extreme lifespan differences. Aging 2015, 7, 1171–1184. [Google Scholar] [CrossRef]
- Prendergast, A.M.; Cruet-Hennequart, S.; Shaw, G.; Barry, F.P.; Carty, M.P. Activation of DNA damage response pathways in human mesenchymal stem cells exposed to cisplatin or gamma-irradiation. Cell Cycle 2011, 10, 3768–3777. [Google Scholar] [CrossRef] [PubMed]
- Stern, N.; Hochman, A.; Zemach, N.; Weizman, N.; Hammel, I.; Shiloh, Y.; Rotman, G.; Barzilai, A. Accumulation of DNA damage and reduced levels of nicotine adenine dinucleotide in the brains of atm-deficient mice. J. Biol. Chem. 2002, 277, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Quesada, R.; Munoz-Gamez, J.A.; Martin-Oliva, D.; Peralta, A.; Valenzuela, M.T.; Matinez-Romero, R.; Quiles-Perez, R.; Menissier-de Murcia, J.; de Murcia, G.; Ruiz de Almodovar, M.; et al. Interaction between ATM and PARP-1 in response to DNA damage and sensitization of ATM deficient cells through PARP inhibition. BMC Mol. Biol. 2007, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, V.J.; Rouleau, M.; Poirier, G.G. PARP-1, a determinant of cell survival in response to DNA damage. Exp. Hematol. 2003, 31, 446–454. [Google Scholar] [CrossRef]
- Schreiber, V.; Dantzer, F.; Ame, J.C.; de Murcia, G. Poly(adp-ribose): Novel functions for an old molecule. Nat. Rev. Mol. Cell. Biol. 2006, 7, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Dai, X. The relationship between DNA single-stranded damage response and double-stranded damage response. Cell Cycle 2018, 17, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Lin, Y.; Yan, S. Single-strand break end resection in genome integrity: Mechanism and regulation by APE2. Int. J. Mol. Sci. 2018, 19, 2389. [Google Scholar] [CrossRef]
- Higo, T.; Naito, A.T.; Sumida, T.; Shibamoto, M.; Okada, K.; Nomura, S.; Nakagawa, A.; Yamaguchi, T.; Sakai, T.; Hashimoto, A.; et al. DNA single-strand break-induced DNA damage response causes heart failure. Nat. Commun. 2017, 8, 15104. [Google Scholar] [CrossRef]
- Nassour, J.; Martien, S.; Martin, N.; Deruy, E.; Tomellini, E.; Malaquin, N.; Bouali, F.; Sabatier, L.; Wernert, N.; Pinte, S.; et al. Defective DNA single-strand break repair is responsible for senescence and neoplastic escape of epithelial cells. Nat. Commun. 2016, 7, 10399. [Google Scholar] [CrossRef]
- Moreno-Villanueva, M.; Eltze, T.; Dressler, D.; Bernhardt, J.; Hirsch, C.; Wick, P.; von Scheven, G.; Lex, K.; Burkle, A. The automated fadu-assay, a potential high-throughput in vitro method for early screening of DNA breakage. ALTEX 2011, 28, 295–303. [Google Scholar] [CrossRef]
- Moreno-Villanueva, M.; Pfeiffer, R.; Sindlinger, T.; Leake, A.; Muller, M.; Kirkwood, T.B.; Burkle, A. A modified and automated version of the ‘fluorimetric detection of alkaline DNA unwinding’ method to quantify formation and repair of DNA strand breaks. BMC Biotechnol. 2009, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Villanueva, M.; Feiveson, A.H.; Krieger, S.; Kay Brinda, A.; von Scheven, G.; Burkle, A.; Crucian, B.; Wu, H. Synergistic effects of weightlessness, isoproterenol, and radiation on DNA damage response and cytokine production in immune cells. Int. J. Mol. Sci. 2018, 19, 3689. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Villanueva, M.; von Scheven, G.; Feiveson, A.; Burkle, A.; Wu, H.; Goel, N. The degree of radiation-induced DNA strand breaks is altered by acute sleep deprivation and psychological stress and is associated with cognitive performance in humans. Sleep 2018, 41, zsy067. [Google Scholar] [CrossRef]
- Thomas, M.; Palombo, P.; Schuhmacher, T.; von Scheven, G.; Bazylianska, V.; Salzwedel, J.; Schafer, N.; Burkle, A.; Moreno-Villanueva, M. Impaired parp activity in response to the beta-adrenergic receptor agonist isoproterenol. Toxicol. In Vitro 2018, 50, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.; Hejl, A.M.; Dinh, T.S.; Keijzers, G.; Hansen, A.M.; Desler, C.; Moreno-Villanueva, M.; Burkle, A.; Rasmussen, L.J.; Waldemar, G.; et al. Defective mitochondrial respiration, altered dntp pools and reduced ap endonuclease 1 activity in peripheral blood mononuclear cells of alzheimer’s disease patients. Aging 2015, 7, 793–815. [Google Scholar] [CrossRef]
- Palombo, P.; Moreno-Villanueva, M.; Mangerich, A. Day and night variations in the repair of ionizing-radiation-induced DNA damage in mouse splenocytes. DNA Repair 2015, 28, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Kalfalah, F.; Seggewiss, S.; Walter, R.; Tigges, J.; Moreno-Villanueva, M.; Burkle, A.; Ohse, S.; Busch, H.; Boerries, M.; Hildebrandt, B.; et al. Structural chromosome abnormalities, increased DNA strand breaks and DNA strand break repair deficiency in dermal fibroblasts from old female human donors. Aging 2015, 7, 110–122. [Google Scholar] [CrossRef] [Green Version]
- Alster, O.; Bielak-Zmijewska, A.; Mosieniak, G.; Moreno-Villanueva, M.; Dudka-Ruszkowska, W.; Wojtala, A.; Kusio-Kobialka, M.; Korwek, Z.; Burkle, A.; Piwocka, K.; et al. The role of nibrin in doxorubicin-induced apoptosis and cell senescence in nijmegen breakage syndrome patients lymphocytes. PLoS ONE 2014, 9, e104964. [Google Scholar] [CrossRef]
- Morath, J.; Moreno-Villanueva, M.; Hamuni, G.; Kolassa, S.; Ruf-Leuschner, M.; Schauer, M.; Elbert, T.; Burkle, A.; Kolassa, I.T. Effects of psychotherapy on DNA strand break accumulation originating from traumatic stress. Psychother. Psychosom. 2014, 83, 289–297. [Google Scholar] [CrossRef]
- Maynard, S.; Keijzers, G.; Hansen, A.M.; Osler, M.; Molbo, D.; Bendix, L.; Moller, P.; Loft, S.; Moreno-Villanueva, M.; Burkle, A.; et al. Associations of subjective vitality with DNA damage, cardiovascular risk factors and physical performance. Acta Physiol. 2015, 213, 156–170. [Google Scholar] [CrossRef]
- Garm, C.; Moreno-Villanueva, M.; Burkle, A.; Larsen, L.A.; Bohr, V.A.; Christensen, K.; Stevnsner, T. Genetic and environmental influence on DNA strand break repair: A twin study. Environ. Mol. Mutagen. 2013, 54, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Korwek, Z.; Bielak-Zmijewska, A.; Mosieniak, G.; Alster, O.; Moreno-Villanueva, M.; Burkle, A.; Sikora, E. DNA damage-independent apoptosis induced by curcumin in normal resting human t cells and leukaemic jurkat cells. Mutagenesis 2013, 28, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Garm, C.; Moreno-Villanueva, M.; Burkle, A.; Petersen, I.; Bohr, V.A.; Christensen, K.; Stevnsner, T. Age and gender effects on DNA strand break repair in peripheral blood mononuclear cells. Aging Cell 2013, 12, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Korwek, Z.; Sewastianik, T.; Bielak-Zmijewska, A.; Mosieniak, G.; Alster, O.; Moreno-Villanueva, M.; Burkle, A.; Sikora, E. Inhibition of atm blocks the etoposide-induced DNA damage response and apoptosis of resting human t cells. DNA Repair 2012, 11, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Brunner, S.; Herndler-Brandstetter, D.; Arnold, C.R.; Wiegers, G.J.; Villunger, A.; Hackl, M.; Grillari, J.; Moreno-Villanueva, M.; Burkle, A.; Grubeck-Loebenstein, B. Upregulation of mir-24 is associated with a decreased DNA damage response upon etoposide treatment in highly differentiated cd8(+) t cells sensitizing them to apoptotic cell death. Aging Cell 2012, 11, 579–587. [Google Scholar] [CrossRef]
- Junk, M.; Salzwedel, J.; Sindlinger, T.; Burkle, A.; Moreno-Villanueva, M. Mathematical modelling of the automated FADU assay for the quantification of DNA strand breaks and their repair in human peripheral mononuclear blood cells. BMC Biophys. 2014, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singh, K.; Almasan, A. Histone H2AX phosphorylation: a marker for DNA damage. Methods Mol. Biol. 2012, 920, 613–626. [Google Scholar]
- Tanaka, T.; Halicka, D.; Traganos, F.; Darzynkiewicz, Z. Cytometric analysis of DNA damage: phosphorylation of histone H2AX as a marker of DNA double-strand breaks (DSBs). Methods Mol. Biol. 2009, 523, 161–168. [Google Scholar]
- Weidele, K.; Kunzmann, A.; Schmitz, M.; Beneke, S.; Burkle, A. Ex vivo supplementation with nicotinic acid enhances cellular poly(ADP-ribosyl)ation and improves cell viability in human peripheral blood mononuclear cells. Biochem. Pharmacol. 2010, 80, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Albouaini, K.; Egred, M.; Alahmar, A.; Wright, D.J. Cardiopulmonary exercise testing and its application. Heart 2007, 93, 1285–1292. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Villanueva, M.; Kramer, A.; Hammes, T.; Venegas-Carro, M.; Thumm, P.; Bürkle, A.; Gruber, M. Influence of Acute Exercise on DNA Repair and PARP Activity before and after Irradiation in Lymphocytes from Trained and Untrained Individuals. Int. J. Mol. Sci. 2019, 20, 2999. https://doi.org/10.3390/ijms20122999
Moreno-Villanueva M, Kramer A, Hammes T, Venegas-Carro M, Thumm P, Bürkle A, Gruber M. Influence of Acute Exercise on DNA Repair and PARP Activity before and after Irradiation in Lymphocytes from Trained and Untrained Individuals. International Journal of Molecular Sciences. 2019; 20(12):2999. https://doi.org/10.3390/ijms20122999
Chicago/Turabian StyleMoreno-Villanueva, Maria, Andreas Kramer, Tabea Hammes, Maria Venegas-Carro, Patrick Thumm, Alexander Bürkle, and Markus Gruber. 2019. "Influence of Acute Exercise on DNA Repair and PARP Activity before and after Irradiation in Lymphocytes from Trained and Untrained Individuals" International Journal of Molecular Sciences 20, no. 12: 2999. https://doi.org/10.3390/ijms20122999
APA StyleMoreno-Villanueva, M., Kramer, A., Hammes, T., Venegas-Carro, M., Thumm, P., Bürkle, A., & Gruber, M. (2019). Influence of Acute Exercise on DNA Repair and PARP Activity before and after Irradiation in Lymphocytes from Trained and Untrained Individuals. International Journal of Molecular Sciences, 20(12), 2999. https://doi.org/10.3390/ijms20122999






