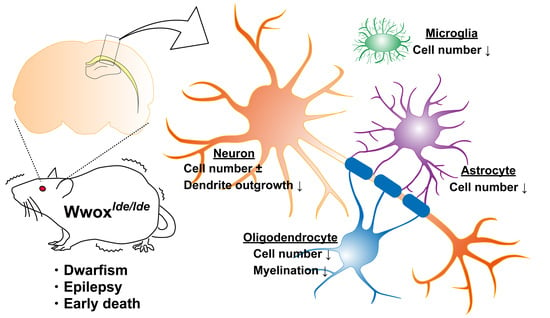
Loss of Wwox Causes Defective Development of Cerebral Cortex with Hypomyelination in a Rat Model of Lethal Dwarfism with Epilepsy
Abstract
1. Introduction
2. Results
2.1. Expression and Localization of Wwox Protein
2.2. Loss of Wwox Protein Affects Postnatal Development of the Cerebral Cortex
2.3. Cellular Localization of Wwox Protein in Cerebral Cortex and CC
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Reagents and Antibodies
4.3. Immunohistochemistry
4.4. Western Blot Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, N.-S.; Hsu, L.-J.; Lin, Y.-S.; Lai, F.-J.; Sheu, H.-M. WW domain-containing oxidoreductase: A candidate tumor suppressor. Trends Mol. Med. 2007, 13, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Ludes-Meyers, J.; Popescu, N.; Aldaz, C.; Bednarek, A.; Bedford, M. WWOX, the common chromosomal fragile site, FRA16D, cancer gene. Cytogenet. Genome Res. 2003, 100, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, S.K.; Del Mare, S.; Hussain, S.; Salah, Z.; Hagan, J.; Rawahneh, M.; Pu, X.-A.; Russell, S.; Stein, J.L.; Stein, G.S.; et al. Conditional inactivation of the mouse Wwox tumor suppressor gene recapitulates the null phenotype. J. Cell. Physiol. 2013, 228, 1377–1382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdeen, S.K.; Salah, Z.; Khawaled, S.; Aqeilan, R.I. Characterization of WWOX inactivation in murine mammary gland development. J. Cell. Physiol. 2013, 228, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Trapasso, F.; Hussain, S.; Costinean, S.; Marshall, D.; Pekarsky, Y.; Hagan, J.P.; Zanesi, N.; Kaou, M.; Stein, G.S.; et al. Targeted deletion of Wwox reveals a tumor suppressor function. Proc. Natl. Acad. Sci. USA 2007, 104, 3949–3954. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Hagan, J.P.; de Bruin, A.; Rawahneh, M.; Salah, Z.; Gaudio, E.; Siddiqui, H.; Volinia, S.; Lian, J.B.; Stein, G.S.; et al. Targeted ablation of the WW domain-containing oxidoreductase tumor suppressor leads to impaired steroidogenesis. Endocrinology 2009, 150, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Kil, H.; Nunez, M.I.; Conti, C.J.; Bedford, M.T.; Aldaz, C.M.; Nunñez, M.I.; Ludes-Meyers, J.H.; Parker-Thornburg, J.; Ludes-Meyers, J.H.; Parker-Thornburg, J. Wwox Hypomorphic Mice Display a Higher Incidence of B-Cell Lymphomas and Develop Testicular Atrophy. Genes Chromosom. Cancer 2007, 46, 1129–1136. [Google Scholar]
- Ludes-Meyers, J.H.; Kil, H.; Parker-Thornburg, J.; Kusewitt, D.F.; Bedford, M.T.; Aldaz, C.M. Generation and Characterization of Mice Carrying a Conditional Allele of the Wwox Tumor Suppressor Gene. PLoS ONE 2009, 4, e7775. [Google Scholar] [CrossRef]
- Ferguson, B.W.; Gao, X.; Kil, H.; Lee, J.; Benavides, F.; Abba, M.C.; Aldaz, C.M. Conditional Wwox Deletion in Mouse Mammary Gland by Means of Two Cre Recombinase Approaches. PLoS ONE 2012, 7, e36618. [Google Scholar] [CrossRef]
- Nunez, M.I.; Ludes-Meyers, J.; Aldaz, C.M. WWOX protein expression in normal human tissues. J. Mol. Histol. 2006, 37, 115–125. [Google Scholar] [CrossRef]
- Saluda-Gorgul, A.; Seta, K.; Nowakowsk, M.; Bednarek, A.K. WWOX Oxidoreductase—Substrate and Enzymatic Characterization. Z. Naturforsch. C 2011, 66, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Takenaka, M.; Suzuki, K. Phenotypic characterization of spontaneously mutated rats showing lethal dwarfism and epilepsy. Comp. Med. 2007, 57, 360–369. [Google Scholar]
- Suzuki, H.; Katayama, K.; Takenaka, M.; Amakasu, K.; Saito, K.; Suzuki, K. A spontaneous mutation of the Wwox gene and audiogenic seizures in rats with lethal dwarfism and epilepsy. Genes Brain Behav. 2009, 8, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Yagi, M.; Amakasu, K.; Suzuki, K.; Suzuki, H. Retarded Differentiation of Leydig Cells and Increased Apoptosis of Germ Cells in the Initial Round of Spermatogenesis of Rats with Lethal Dwarf and Epilepsy (lde/lde) Phenotypes. J. Androl. 2008, 29, 669–678. [Google Scholar] [CrossRef]
- White, S.; Hewitt, J.; Turbitt, E.; van der Zwan, Y.; Hersmus, R.; Drop, S.; Koopman, P.; Harley, V.; Cools, M.; Looijenga, L.; et al. A multi-exon deletion within WWOX is associated with a 46, XY disorder of sex development. Eur. J. Hum. Genet. 2012, 20, 348–351. [Google Scholar] [CrossRef]
- Lo, J.-Y.; Chou, Y.-T.; Lai, F.-J.; Hsu, L.-J. Regulation of cell signaling and apoptosis by tumor suppressor WWOX. Exp. Biol. Med. 2015, 240, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Abu-Remaileh, M.; Dodson, E.J.; Schueler-Furman, O.; Aqeilan, R.I. Pleiotropic Functions of Tumor Suppressor WWOX in Normal and Cancer Cells. J. Biol. Chem. 2015, 290, 30728–30735. [Google Scholar] [CrossRef]
- Schrock, M.S.; Batar, B.; Lee, J.; Druck, T.; Ferguson, B.; Cho, J.H.; Akakpo, K.; Hagrass, H.; Heerema, N.A.; Xia, F.; et al. Wwox-Brca1 interaction: Role in DNA repair pathway choice. Oncogene 2017, 36, 2215–2227. [Google Scholar] [CrossRef]
- Aldaz, C.M.; Ferguson, B.W.; Abba, M.C. WWOX at the crossroads of cancer, metabolic syndrome related traits and CNS pathologies. Biochim. Biophys. Acta 2014, 1846, 188–200. [Google Scholar] [CrossRef]
- Chen, S.; Chuang, J.; Wang, J.; Tsai, M.; Li, H.; Chang, N.-S. Expression of WW domain-containing oxidoreductase WOX1 in the developing murine nervous system. Neuroscience 2004, 124, 831–839. [Google Scholar] [CrossRef]
- Wang, H.Y.; Juo, L.I.; Lin, Y.T.; Hsiao, M.; Lin, J.T.; Tsai, C.H.; Tzeng, Y.H.; Chuang, Y.C.; Chang, N.S.; Yang, C.N.; et al. WW domain-containing oxidoreductase promotes neuronal differentiation via negative regulation of glycogen synthase kinase 3β. Cell Death Differ. 2012, 19, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Sze, C.-I.; Su, M.; Pugazhenthi, S.; Jambal, P.; Heath, J.; Schultz, L.; Hsu, L.-J.; Chang, N.-S. Down-regulation of WW Domain-containing Oxidoreductase Induces Tau Phosphorylationin Vitro. J. Biol. Chem. 2004, 279, 30498–30506. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, G.; Thoenes, M.; Afifi, H.H.; Körber, F.; Swan, D.; Bolz, H.J. The supposed tumor suppressor gene WWOX is mutated in an early lethal microcephaly syndrome with epilepsy, growth retardation and retinal degeneration. Orphanet J. Rare Dis. 2014, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Ben-Salem, S.; Al-Shamsi, A.M.; John, A.; Ali, B.R.; Al-Gazali, L. A novel whole exon deletion in WWOX gene causes early epilepsy, intellectual disability and optic atrophy. J. Mol. Neurosci. 2015, 56, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Mallaret, M.; Synofzik, M.; Lee, J.; Sagum, C.A.; Mahajnah, M.; Sharkia, R.; Drouot, N.; Renaud, M.; Klein, F.A.; Anheim, M.; et al. The tumour suppressor gene WWOX is mutated in autosomal recessive cerebellar ataxia with epilepsy and mental retardation. Brain 2014, 137, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Tabarki, B.; AIHashem, A.; AIShahwan, S.; Aikuraya, F.S.; Gedela, S.; Zuccoli, G. Severe CNS involvement in WWOX mutations: Description of five new cases. Am. J. Med. Genet. A 2015, 167, 3209–3213. [Google Scholar] [CrossRef] [PubMed]
- Ehaideb, S.N.; Ali, M.J.A.-B.; Al-Obaid, J.J.; AlJassim, K.M.; Alfadhel, M. Novel homozygous mutation in the WWOX gene causes seizures and global developmental delay: Report and review. Transl. Neurosci. 2018, 9, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Piard, J.; Hawkes, L.; Milh, M.; Villard, L.; Borgatti, R.; Romaniello, R.; Fradin, M.; Capri, Y.; Héron, D.; Nougues, M.C.; et al. The phenotypic spectrum of WWOX-related disorders: 20 additional cases of WOREE syndrome and review of the literature. Genet. Med. 2019, 21, 1308–1318. [Google Scholar] [CrossRef]
- Johannsen, J.; Kortüm, F.; Rosenberger, G.; Bokelmann, K.; Schirmer, M.A.; Denecke, J.; Santer, R. A novel missense variant in the SDR domain of the WWOX gene leads to complete loss of WWOX protein with early-onset epileptic encephalopathy and severe developmental delay. Neurogenetics 2018, 19, 151–156. [Google Scholar] [CrossRef]
- Tarta-Arsene, O.; Barca, D.; Craiu, D.; Iliescu, C. Practical clues for diagnosing WWOX encephalopathy. Epileptic Disord. 2017, 19, 357–361. [Google Scholar]
- Guler, G.; Uner, A.; Guler, N.; Han, S.-Y.; Iliopoulos, D.; Hauck, W.W.; McCue, P.; Huebner, K. The fragile genes FHIT and WWOX are inactivated coordinately in invasive breast carcinoma. Cancer 2004, 100, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Mullen, R.J.; Buck, C.R.; Smith, A.M. NeuN, a neuronal specific nuclear protein in vertebrates. Development 1992, 116, 201–211. [Google Scholar] [PubMed]
- Riederer, B.; Matus, A. Differential expression of distinct microtubule-associated proteins during brain development. Proc. Natl. Acad. Sci. USA 1985, 82, 6006–6009. [Google Scholar] [CrossRef] [PubMed]
- Chamak, B.; Fellous, A.; Glowinski, J.; Prochiantz, A. MAP2 expression and neuritic outgrowth and branching are coregulated through region-specific neuro-astroglial interactions. J. Neurosci. 1987, 7, 3163–3170. [Google Scholar] [CrossRef] [PubMed]
- Hartman, B.K.; Agrawal, H.C.; Agrawal, D.; Kalmbach, S. Development and maturation of central nervous system myelin: Comparison of immunohistochemical localization of proteolipid protein and basic protein in myelin and oligodendrocytes. Proc. Natl. Acad. Sci. USA 1982, 79, 4217–4220. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Gravel, M.; Zhang, R.; Thibault, P.; Braun, P.E. Process outgrowth in oligodendrocytes is mediated by CNP, a novel microtubule assembly myelin protein. J. Cell Biol. 2005, 170, 661–673. [Google Scholar] [CrossRef]
- Bhat, R.V.; Axt, K.J.; Fosnaugh, J.S.; Smith, K.J.; A Johnson, K.; Hill, D.E.; Kinzler, K.W.; Baraban, J.M. Expression of the APC tumor suppressor protein in oligodendroglia. Glia 1996, 17, 169–174. [Google Scholar] [CrossRef]
- Lang, J.; Maeda, Y.; Bannerman, P.; Xu, J.; Horiuchi, M.; Pleasure, D.; Guo, F. Adenomatous Polyposis Coli Regulates Oligodendroglial Development. J. Neurosci. 2013, 33, 3113–3130. [Google Scholar] [CrossRef]
- Klaman, M.; Hjos, F. Distribution of glial fibrillary acidic protein (GFAP)-immunoreactive astrocytes in the rat brain I. Forebrain. Exp. Brain Res. 1989, 78, 147–163. [Google Scholar]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-specific localization of a novel calcium binding protein, Iba1. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Bercury, K.K.; Macklin, W.B. Dynamics and Mechanisms of CNS Myelination. Dev. Cell 2015, 32, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Zuchero, J.B.; Barres, B.A. Intrinsic and extrinsic control of oligodendrocyte development. Curr. Opin. Neurobiol. 2013, 23, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Zuchero, J.B.; Barres, B.A. Glia in mammalian development and disease. Development 2015, 142, 3805–3809. [Google Scholar] [CrossRef] [PubMed]
- Downes, N.; Mullins, P. The development of myelin in the brain of the Juvenile rat. Toxicol. Pathol. 2014, 42, 913–922. [Google Scholar] [CrossRef]
- Griffiths, I.R. Myelin mutants: Model systems for the study of normal and abnormal myelination. BioEssays 1996, 18, 789–797. [Google Scholar] [CrossRef]
- Silva, J.; Sharma, S.; Hughes, B.; Yu, Y.E.; Cowell, J.K. Homozygous inactivation of the Lgi1 gene results in hypomyelination in the peripheral and central nervous system. J. Neurosci. Res. 2010, 88, 3328–3336. [Google Scholar] [CrossRef]
- Staats, K.A.; Pombal, D.; Schonefeldt, S.; Van Helleputte, L.; Maurin, H.; Dresselaers, T.; Govaerts, K.; Himmelreich, U.; Van Leuven, F.; Bosch, L.V.D.; et al. Transcriptional upregulation of myelin components in spontaneous myelin basic protein-deficient mice. Brain Res. 2015, 1606, 125–132. [Google Scholar] [CrossRef]
- Tamijani, S.M.S.; Karimi, B.; Amini, E.; Golpich, M.; Dargahi, L.; Ali, R.A.; Ibrahim, N.M.; Mohamed, Z.; Ghasemi, R.; Ahmadiani, A. Thyroid hormones: Possible roles in epilepsy pathology. Seizure 2015, 31, 155–164. [Google Scholar] [CrossRef]
- Watanabe, A.; Hippo, Y.; Taniguchi, H.; Iwanari, H.; Yashiro, M.; Hirakawa, K.; Kodama, T.; Aburatani, H. An opposing view on WWOX protein function as a tumor suppressor. Cancer Res. 2003, 63, 8629–8633. [Google Scholar]
- Ludes-Meyers, J.H.; Kil, H.; Bednarek, A.K.; Drake, J.; Bedford, M.T.; Aldaz, C.M. WWOX binds the specific proline-rich ligand PPXY: Identification of candidate interacting proteins. Oncogene 2004, 23, 5049–5055. [Google Scholar] [CrossRef]
- Domingues, H.S.; Portugal, C.C.; Socodato, R.; Relvas, J.B. Oligodendrocyte, Astrocyte, and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front. Cell Dev. Biol. 2016, 4, 71. [Google Scholar] [PubMed]
- Hussain, T.; Kil, H.; Hattiangady, B.; Lee, J.; Kodali, M.; Shuai, B.; Attaluri, S.; Takata, Y.; Shen, J.; Abba, M.C.; et al. Wwox deletion leads to reduced GABA-ergic inhibitory interneuron numbers and activation of microglia and astrocytes in mouse hippocampus. Neurobiol. Dis. 2019, 121, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Elsaadany, L.; El-Said, M.; Ali, R.; Kamel, H.; Ben-Omran, T. W44X mutation in the WWOX gene causes intractable seizures and developmental delay: A case report. BMC Med. Genet. 2016, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Yasuda, H.; Tochigi, Y.; Suzuki, H. The microtubule-associated protein astrin transgenesis rescues spermatogenesis and renal function in hypogonadic (hgn/hgn) rats. Andrology 2013, 1, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Tochigi, Y.; Iwasaki, Y.; Sano, M.; Yasuda, H.; Katayama, K.; Suzuki, H. Critical roles of Astrin in the mitosis of immature rat Sertoli cells. Biochem. Biophys. Res. Commun. 2017, 486, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Tochigi, Y.; Katayama, K.; Suzuki, H. Progression of renal fibrosis in congenital CKD model rats with reduced number of nephrons. Exp. Toxicol. Pathol. 2017, 69, 245–258. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tochigi, Y.; Takamatsu, Y.; Nakane, J.; Nakai, R.; Katayama, K.; Suzuki, H. Loss of Wwox Causes Defective Development of Cerebral Cortex with Hypomyelination in a Rat Model of Lethal Dwarfism with Epilepsy. Int. J. Mol. Sci. 2019, 20, 3596. https://doi.org/10.3390/ijms20143596
Tochigi Y, Takamatsu Y, Nakane J, Nakai R, Katayama K, Suzuki H. Loss of Wwox Causes Defective Development of Cerebral Cortex with Hypomyelination in a Rat Model of Lethal Dwarfism with Epilepsy. International Journal of Molecular Sciences. 2019; 20(14):3596. https://doi.org/10.3390/ijms20143596
Chicago/Turabian StyleTochigi, Yuki, Yutaka Takamatsu, Jun Nakane, Rika Nakai, Kentaro Katayama, and Hiroetsu Suzuki. 2019. "Loss of Wwox Causes Defective Development of Cerebral Cortex with Hypomyelination in a Rat Model of Lethal Dwarfism with Epilepsy" International Journal of Molecular Sciences 20, no. 14: 3596. https://doi.org/10.3390/ijms20143596
APA StyleTochigi, Y., Takamatsu, Y., Nakane, J., Nakai, R., Katayama, K., & Suzuki, H. (2019). Loss of Wwox Causes Defective Development of Cerebral Cortex with Hypomyelination in a Rat Model of Lethal Dwarfism with Epilepsy. International Journal of Molecular Sciences, 20(14), 3596. https://doi.org/10.3390/ijms20143596