Cell Death in the Kidney
Abstract
:1. Introduction
2. Kidney Injury and Cell Death
2.1. Tubular Cell Injury
2.2. Glomerular Cell Injury
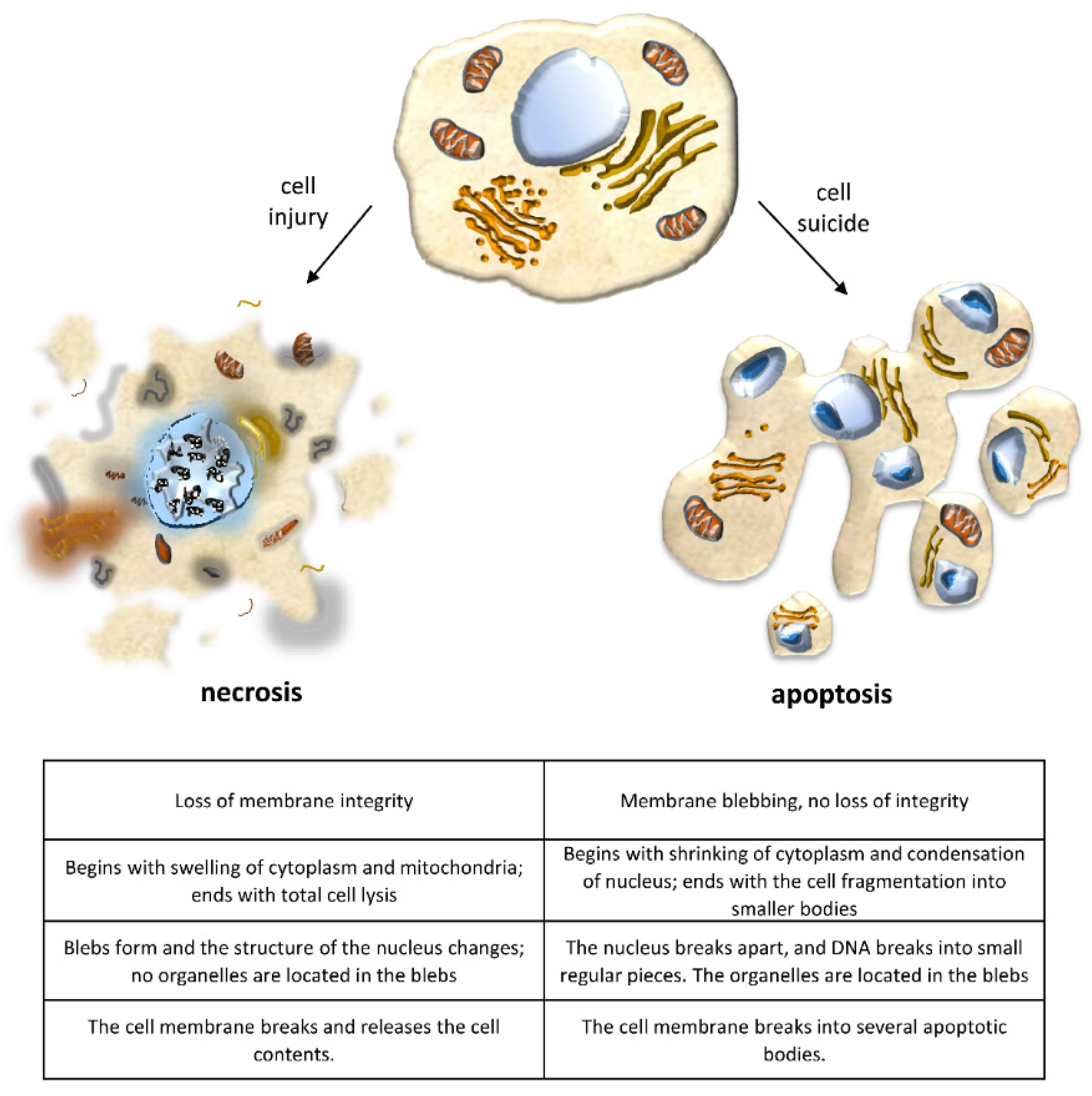
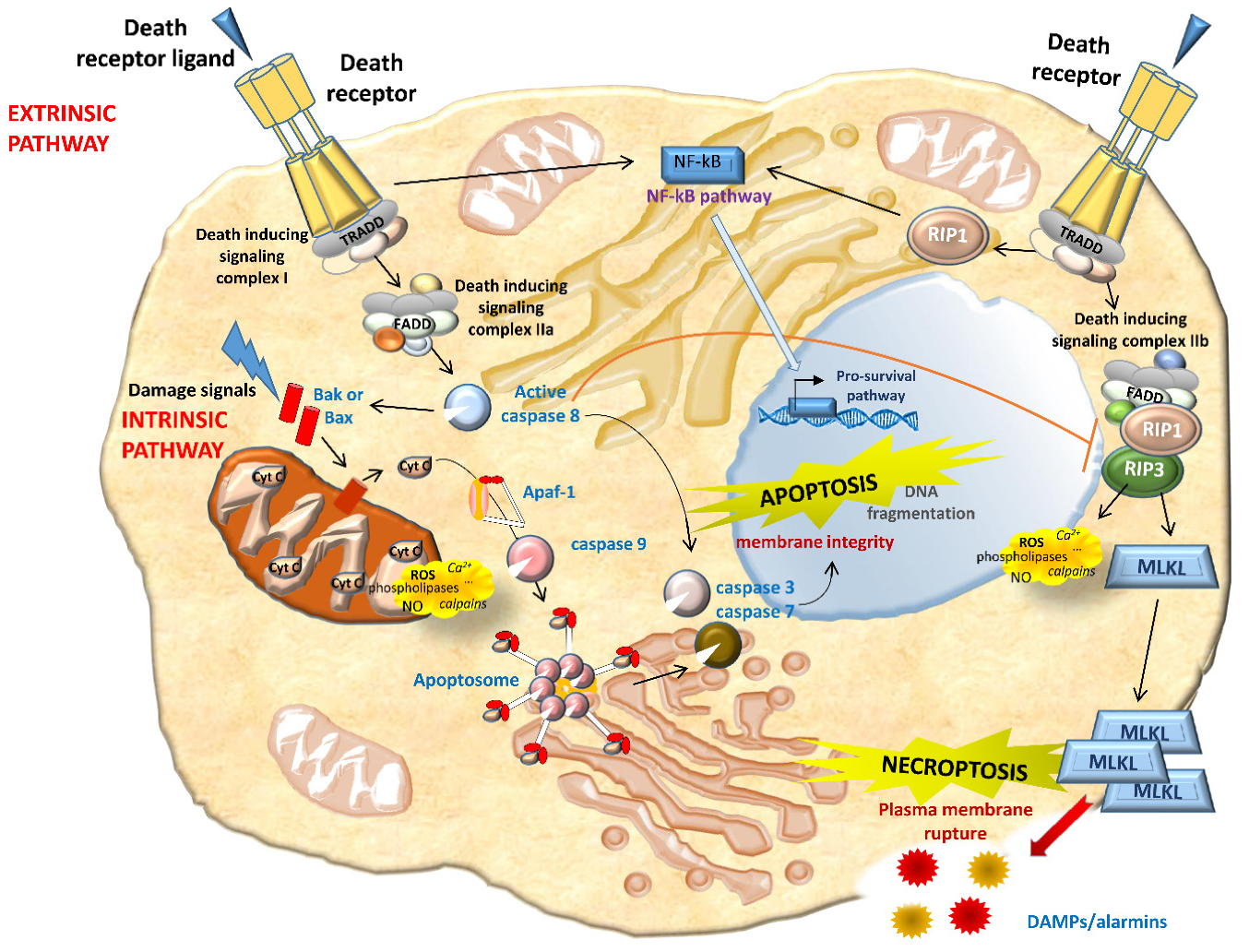
2.3. Necrosis/Regulated Necrosis and the Kidney
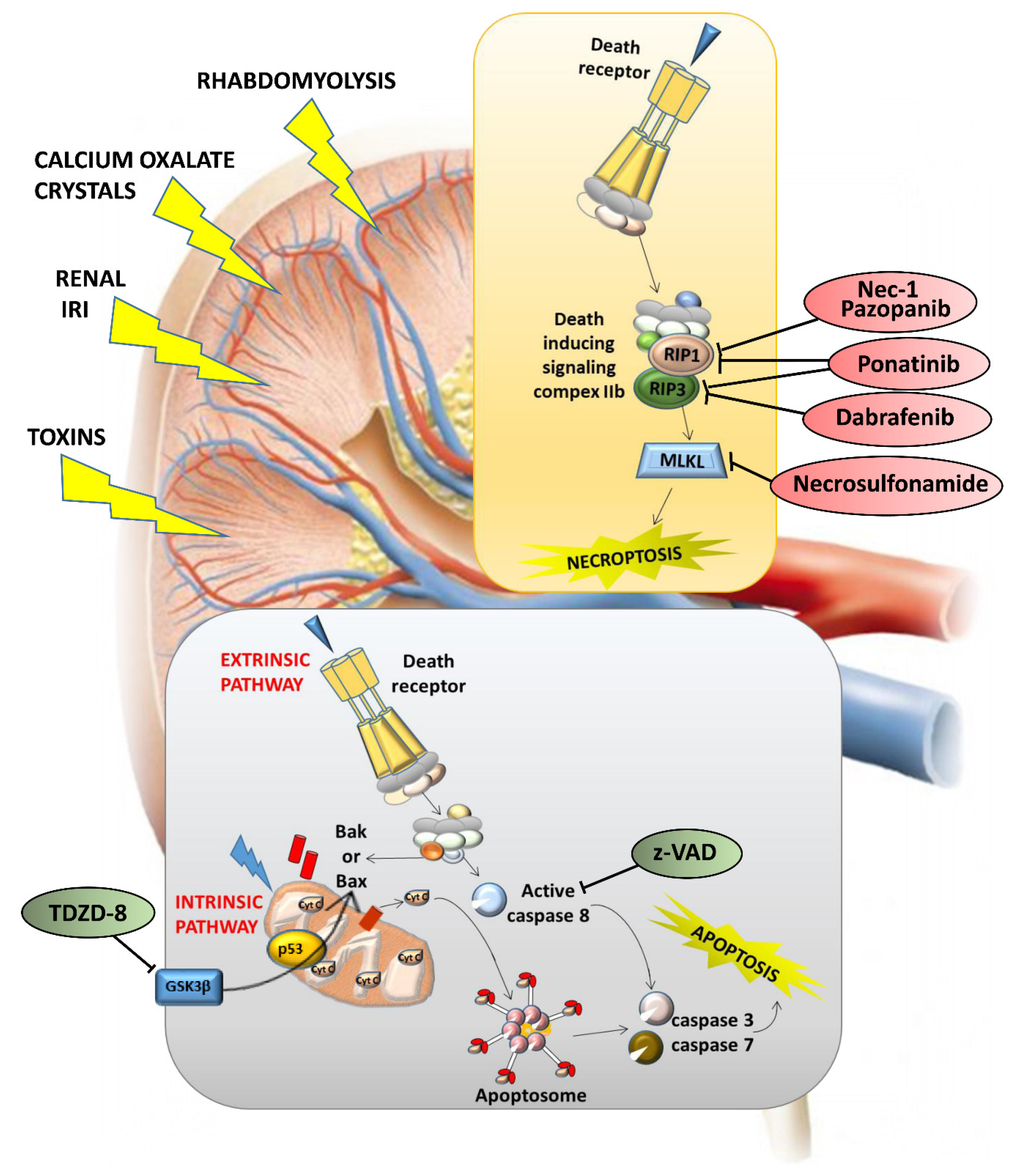
2.4. Cell Death and Crystal Nephropathies
3. Targeting Renal Cell Death
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Bessis, M. Studies on cell agony and death: An attempt at classification. In Ciba Foundation Symposium - Cellular Injury; de Reuck, A.V.S., Knight, J., Eds.; J&A Churchill: London, UK, 1964; ISBN 978-04-7072-277-0. [Google Scholar]
- Green, D.R. Means to an End: Apoptosis and Other Cell Death Mechanisms, 1st ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2010; ISBN 978-08-7969-888-1. [Google Scholar]
- Lynch, M.P.; Nawaz, S.; Gerschenson, L.E. Evidence for soluble factors regulating cell death and cell proliferation in primary cultures of rabbit endometrial cells grown on collagen. Proc. Natl. Acad. Sci. USA 1986, 83, 4784–4788. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, Y.; Steller, H. Programmed cell death in animal development and disease. Cell 2011, 147, 742–758. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Bravo-San Pedro, J.M.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Alnemri, E.S.; Altucci, L.; Andrews, D.; Annicchiarico-Petruzzelli, M.; et al. Essential versus accessory aspects of cell death: Recommendations of the NCCD 2015. Cell Death Differ. 2015, 22, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R. Cell Death: Apoptosis and Other Means to an End, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2018; ISBN 978-16-2182-214-1. [Google Scholar]
- Schwartz, L.M.; Smith, S.W.; Jones, M.E.; Osborne, B.A. Do all programmed cell deaths occur via apoptosis? Proc. Natl Acad. Sci. USA 1993, 90, 980–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, I.; Rayamajhi, M.; Miao, E.A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 2017, 17, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, T.; Linkermann, A.; Jouan-Lanhouet, S.; Walczak, H.; Vandenabeele, P. Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol 2014, 15, 135–147. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Krautwald, S.; Kroemer, G.; Linkermann, A. Molecular mechanisms of regulated necrosis. Semin Cell Dev. Biol. 2014, 35, 24–32. [Google Scholar] [CrossRef]
- Tait, S.W.; Oberst, A.; Quarato, G.; Milasta, S.; Haller, M.; Wang, R.; Karvela, M.; Ichim, G.; Yatim, N.; Albert, M.L.; et al. Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Reports 2013, 5, 878–885. [Google Scholar] [CrossRef]
- Vanden Berghe, T.; Hassannia, B.; Vandenabeele, P. An outline of necrosome triggers. Cell Mol. Life Sci. 2016, 73, 2137–2152. [Google Scholar] [CrossRef] [Green Version]
- Dillon, C.P.; Tummers, B.; Baran, K.; Green, D.R. Developmental checkpoints guarded by regulated necrosis. Cell Mol. Life Sci. 2016, 73, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Dondelinger, Y.; Darding, M.; Bertrand, M.J.M.; Walczak, H. Poly-ubiquitination in TNFR1-mediated necroptosis. Cell Mol. Life Sci. 2016, 73, 2165–2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, I.; Miao, E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015, 265, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.A.; Dawson, T.L.; Dawson, V.L. Mitochondrial and nuclear cross talk in cell death: Parthanatos. Ann. NY Acad. Sci. 2008, 1147, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.; Kraus, W. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nature Rev. Mol. Cell Biol. 2012, 13, 411–424. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Kroemer, G. Mitochondrial regulation of cell death: A phylogenetically conserved control. Microb. Cell 2016, 3, 101–108. [Google Scholar] [CrossRef]
- Cookson, B.T.; Brennan, M.A. Pro-inflammatory programmed cell death. Trends Microbiol 2001, 9, 113–114. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006, 8, 1812–1825. [Google Scholar] [CrossRef]
- Remijsen, Q.; Kuijpers, T.W.; Wirawan, E.; Lippens, S.; Vandenabeele, P.; Vanden Berghe, T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011, 18, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Maiuri, M.C.; Vitale, I.; Zischka, H.; Castedo, M.; Zitvogel, L.; Kroemer, G. Cell death modalities: Classification and pathophysiological implications. Cell Death Differ. 2007, 147, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Glucksmann, A. Cell deaths in normal verebrate ontogeny. Biol. Rev. 1951, 26, 59–86. [Google Scholar] [CrossRef] [PubMed]
- Havasi, A.; Borkan, S.C. Apoptosis and acute kidney injury. Kidney Int. 2011, 80, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Koseki, C.; Herzlinger, D.; al-Awqati, Q. Apoptosis in metanephric development. J. Cell Biol. 1992, 119, 1327–1333. [Google Scholar] [CrossRef]
- Meier, P.; Finch, A.; Evan, G. Apoptosis in development. Nature 2000, 407, 796–801. [Google Scholar] [CrossRef]
- Bard, J.B. Growth and death in the developing mammalian kidney: Signals, receptors and conversations. Bioessays 2002, 24, 72–82. [Google Scholar] [CrossRef]
- Bouchard, M. Transcriptional control of kidney development. Differentiation 2004, 72, 295–306. [Google Scholar] [CrossRef]
- Coles, H.S.R.; Burne, J.F.; Raff, M.C. Large scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development 1993, 118, 777–784. [Google Scholar]
- Lebrun, D.P.; Warncke, R.A.; Cleary, M.L. Expression of bcl-2 in fetal tissues suggests a role in morphogenesis. Am. J. Pathol. 1993, 142, 743–753. [Google Scholar]
- Lu, Q.L.; Poulsom, R.; Wong, L.; Hanby, A.M. Bcl-2 expression in adult and embryonic non hematopietic tissues. J. Pathol. 1993, 169, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Saifudeen, Z.; Dipp, S.; El-Dahr, S.S. A role for p53 in terminal epithelial cell differentiation. J. Clin. Invest. 2002, 109, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Ewings, K.E.; Wiggins, C.M.; Cook, S.J. Bim and the pro-survival Bcl-2 proteins: Opposites attract, ERK repels. Cell Cycle 2007, 6, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Saifudeen, Z.; Dipp, S.; Stefkova, J.; Yao, X.; Lookabaugh, S.; El-Dahr, S.S. p53 regulates metanephric development. J. Am. Soc. Nephrol. 2009, 20, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- El-Dahr, S.; Hilliard, S.; Aboudehen, K.; Saifudeen, Z. The MDM2-p53 pathway: Multiple roles in kidney evelopment. Pediatr. Nephrol. 2014, 29, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Ho, J. The regulation of apoptosis in kidney development: Implications for nephron number and pattern? Front. Pediatr. 2014, 2, 128. [Google Scholar] [CrossRef] [PubMed]
- Bussolati, B.; Bruno, S.; Grange, C.; Buttiglieri, S.; Deregibus, M.C.; Cantino, D.; Camussi, G. Isolation of renal progenitor cells from adult human kidney. Am. J. Pathol. 2005, 166, 545–555. [Google Scholar] [CrossRef]
- Kitamura, S.; Yamasaki, Y.; Kinomura, M.; Sugaya, T.; Sugiyama, H.; Maeshima, Y.; Makino, H. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J. 2005, 19, 1789–1797. [Google Scholar] [CrossRef]
- Dekel, B.; Zangi, L.; Shezen, E.; Reich-Zeliger, S.; Eventov-Friedman, S.; Katchman, H.; Jacob-Hirsch, J.; Amariglio, N.; Rechavi, G.; Margalit, R.; et al. Isolation and characterization of non tubular sca-1+lin_multipotent stem/progenitor cells from adult mouse kidney. J. Am. Soc. Nephrol. 2006, 17, 3300–3314. [Google Scholar] [CrossRef]
- Gupta, S.; Verfaillie, C.; Chmielewski, D.; Kren, S.; Eidman, K.; Connaire, J.; Heremans, Y.; Lund, T.; Blackstad, M.; Jiang, Y.; et al. Isolation and characterization of kidney-derived stem cells. J. Am. Soc. Nephrol. 2006, 17, 3028–3040. [Google Scholar] [CrossRef]
- Maeshima, A.; Sakurai, H.; Nigam, S.K. Adult kidney tubular cell population showing phenotypic plasticity, tubulogenic capacity, and integration capability into developing kidney. J. Am. Soc. Nephrol. 2006, 17, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.D.; Bonventre, J.V. Mesenchymal Stem Cells in Acute Kidney Injury. Annu Rev. Med. 2008, 59, 311–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, Y.H.; Pan, S.Y.; Yang, C.H.; Lin, S.L. Stem cells and kidney regeneration. J. Formos Med. Assoc. 2014, 113, 201–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, A.Q.; Bonventre, J.V. Regenerating the nephron with human pluripotent stem cells. Curr Opin Organ. Transplant. 2015, 20, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Thomasova, D.; Anders, H.J. Cell cycle control in the kidney. Nephrol Dial. Transplant. 2015, 30, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Havasi, A.; Dong, Z. Autophagy and Tubular Cell Death in the Kidney. Semin. Nephrol. 2016, 36, 174–188. [Google Scholar] [CrossRef] [Green Version]
- Gobe, G.C.; Axeisen, R.A. Genesís of renal tubular atrophy in experimental hydronephrosis in the rat. Lab. Invest. 1987, 56, 273–281. [Google Scholar]
- Gobe, G.C.; Axelson, R.A.; Searle, J.W. Cellular events in experimental unilateral ischemic renal atrophy and in regeneration after contralateral nephrectomy. Lab. Invest. 1990, 63, 770–779. [Google Scholar]
- Todd, D.; Yang, G.; Brown, R.W.; Cao, J.; D’Agati, V.; Thompson, T.S.; Truong, L.D. Apoptosis in renal cell carcinoma: Detection by in situ end-labeling of fragmented DNA and correlation with other prognostic factors. Hum. Pathol. 1996, 27, 1012–1017. [Google Scholar] [CrossRef]
- Tannapfel, A.; Hahn, H.A.; Katalinic, A.; Fietkau, R.J.; Kuhn, R.; Wittekind, C.W. Incidence of apoptosis, cell proliferation and p53 expression in renal cell carcinomas. Anticancer Res. 1997, 17, 1155–1162. [Google Scholar]
- Kennedy, W.A., II; Stenberg, A.; Lackgren, G.; Hensle, T.W.; Sawczuk, I.S. Renal tubular apoptosis after partial ureteral obstruction. J. Urol. 1994, 152, 658–664. [Google Scholar] [CrossRef]
- Truong, L.D.; Petrusevska, G.; Yang, G.; Gurpinar, T.; Shappell, S.; Lechago, J.; Rouse, D.; Suki, W.N. Cell apoptosis and proliferation in experimental chronic obstructive uropathy. Kidney Int. 1996, 50, 200–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, W.A., 2nd; Buttyan, R.; Garcia-Montes, E.; D’Agati, V.; Olsson, C.A.; Sawczuk, I.S. Epidermal growth factor suppresses renal tubular apoptosis following ureteral obstruction. Urology 1997, 49, 973–980. [Google Scholar] [CrossRef]
- Klahr, S.; Morrissey, J. Obstructive nephropathy and renal fibrosis. Am. J. Physiol. Renal Physiol. 2002, 283, F861–F875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.S.; Padanilam, B.J. Simultaneous deletion of Bax and Bak is required to prevent apoptosis and interstitial fibrosis in obstructive nephropathy. Am. J. Physiol Renal Physiol. 2015, 309, F540–F550. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.; Madsen, K.; Krag, S.; Frokiaer, J.; Jensen, B.L.; Norregaard, R. Disruption of cyclooxygenase type 2 exacerbates apoptosis and renal damage during obstructive nephropathy. Am. J. Physiol. Renal Physiol. 2015, 309, F1035–F1048. [Google Scholar] [CrossRef] [Green Version]
- Docherty, N.G.; O’Sullivan, O.E.; Healy, D.A.; Fitzpatrick, J.M.; Watson, R.W. Evidence that inhibition of tubular cell apoptosis protects against renal damage and development of fibrosis following ureteric obstruction. Am. J. Physiol. Renal Physiol. 2006 2006, 290, F4–F13. [Google Scholar] [CrossRef] [Green Version]
- Mei, W.; Peng, Z.; Lu, M.; Liu, C.; Deng, Z.; Xiao, Y.; Liu, J.; He, Y.; Yuan, Q.; Yuan, X.; et al. Peroxiredoxin 1 inhibits the oxidative stress induced apoptosis in renal tubulointerstitial fibrosis. Nephrology 2015, 20, 832–842. [Google Scholar] [CrossRef]
- Saikumar, P.; Venkatachalam, M.A. Role of apoptosis in hypoxic/ischemic damage in the kidney. Semin Nephrol. 2003, 23, 511–521. [Google Scholar] [CrossRef]
- Luo, S.; Rubinsztein, D.C. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: An effect rescued by Bcl-xL. Cell Death Differ. 2010, 17, 268–277. [Google Scholar] [CrossRef]
- Xu, Y.; Ruan, S.; Wu, X.; Chen, H.; Zheng, K.; Fu, B. Autophagy and apoptosis in tubular cells following unilateral ureteral obstruction are associated with mitochondrial oxidative stress. Int. J. Mol. Med. 2013, 31, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Schumer, M.; Colombel, M.C.; Sawczuk, I.S.; Gobé, G.; Connor, J.; O’Toole, K.M.; Olsson, C.A.; Wise, G.J.; Buttyan, R. Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am. J. Pathol. 1992, 140, 831–838. [Google Scholar] [PubMed]
- Price, P.M.; Hodeify, R. A possible mechanism of renal cell death after ischemia/reperfusion. Kidney Int. 2012, 81, 720–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linkermann, A.; Chen, G.; Dong, G.; Kunzendorf, U.; Krautwald, S.; Dong, Z. Regulated cell death in AKI. J. Am. Soc. Nephrol. 2014, 25, 2689–2701. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.; Clermont, G.; Kersten, A.; Venkataraman, R.; Angus, D.C.; De Bacquer, D.; Kellum, J.A. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care 2006, 10, R73:1-73-10. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Unruh, M.L.; Murugan, R. Acute kidney injury. BMJ Clin. Evid. 2011, 2011, 2001:1–2001:36. [Google Scholar] [CrossRef] [PubMed]
- Decuypere, J.P.; Ceulemans, L.J.; Agostinis, P.; Monbaliu, D.; Naesens, M.; Pirenne, J.; Jochmans, I. Autophagy and the Kidney: Implications for Ischemia-Reperfusion Injury and Therapy. Am. J. Kidney Dis. 2015, 66, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.; Burdick, J.F.; Keown, P.A.; Wallace, A.C.; Racussen, L.C.; Solez, K. Primary acute renal failure (“acute tubular necrosis”) in the transplanted kidney: Morphology and pathogenesis. Medicine (Baltimore) 1989, 68, 173–187. [Google Scholar] [CrossRef]
- Ito, H.; Kasagi, N.; Shomori, K.; Osaki, M.; Adachi, H. Apoptosis in the human allografted kidney. Analysis by terminal deoxynucleotidyl transferase-mediated DUTP-botin nick end labeling. Transplantation 1995, 60, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Akasaka, Y.; Kawamura, S. Fas antigen expression and its relationship with apoptosis in transplanted kidney. Pathol. Int. 1997, 47, 230–237. [Google Scholar] [CrossRef]
- Seron, D.; Moreso, F.; Bover, J.; Condom, E.; Gil-Vernet, S.; Canas, C.; Fulladosa, X.; Torras, J.; Carrera, M.; Grinyo, J.M.; et al. Early protocol renal allograft biopsies and graft outcome. Kidney Int. 1997, 51, 310–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castaneda, M.P.; Swiatecka-Urban, A.; Mitsnefes, M.M.; Feuerstein, D.; Kaskel, F.J.; Tellis, V.; Devarajan, P. Activation of mitochondrial apoptotic pathways in human renal allografts after ischemiareperfusion injury. Transplantation 2003, 76, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Santamaría, B.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. Mechanisms of Renal Apoptosis in Health and Disease. J. Am. Soc. Nephrol. 2008, 19, 1634–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallet, N.; Dieudé, M.; Cailhier, J.; Hébert, M. The Molecular Legacy of Apoptosis in Transplantation. Am. J. Transplant. 2012, 12, 1378–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonegio, R.; Lieberthal, W. Role of apoptosis in the pathogenesis of acute renal failure. Curr. Opin. Nephrol. Hypertens 2002, 11, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.C.; Wenham, P.W.; Rowe, P.A.; Burden, R.P.; Morgan, A.G.; Cotton, R.E.; Blamey, R.W. The late results of renal transplantation and the importance of chronic rejection as a cause of graft loss. Ann. R Coll Surg. Engl. 1989, 71, 44–47. [Google Scholar] [PubMed]
- Laine, J.; Etelamaki, P.; Holmberg, C.; Dunkel, L. Apoptotic cell death in human chronic renal allograft rejection. Transplantation 1997, 63, 101–105. [Google Scholar] [CrossRef]
- Thomas, G.L.; Yang, B.; Wagner, B.E.; Savill., J.; El Nahas, A.M. Cellular apoptosis and proliferation in experimental renal fibrosis. Nephrol Dial. Transplant. 1998, 13, 2216–2226. [Google Scholar] [CrossRef]
- Schelling, J.R.; Nkemere, N.; Kopp, J.B.; Cleveland, R.P. Fas-dependent fratricidal apoptosis is a mechanism of tubular epithelial cell deletion in chronic renal failure. Lab. Invest. 1998, 78, 813–824. [Google Scholar]
- Khan, S.; Cleveland, R.P.; Koch, C.J.; Schelling, J.R. Hypoxia induces renal tubular epithelial cell apoptosis in chronic renal disease. Lab. Invest. 1999, 79, 1089–1099. [Google Scholar]
- Choi, Y.J.; Baranowska-Daca, E.; Nguyen, V.; Kpji, T.; Ballantyne, C.M.; Sheikh-Hamand, D.; Suki, W.N.; Truong, L.D. Mechanism of chronic obstructive uropathy: Increased expression of apoptosis-promoting molecules. Kidney Int. 2000 58, 1481–1491. [CrossRef]
- Yang, B.; Johnson, T.S.; Thomas, G.L.; Watson, P.F.; Wagner, B.; Skill, N.J.; Haylor, J.L.; El Nahas, A.M. Expression of apoptosis related genes and proteins in experimental chronic renal scarring. J. Am. Soc. Nephrol. 2001, 12, 275–288. [Google Scholar] [PubMed]
- Zhu, Y.; Cui, H.; Xia, Y.; Gan, H. RIPK3-Mediated Necroptosis and Apoptosis Contributes toRenal Tubular Cell Progressive Loss and ChronicKidney Disease Progression in Rats. PLoS ONE 2016, 11, e0156729. [Google Scholar] [CrossRef]
- Barnes, D.J.; Pinto, J.R.; Davison, A.M.; Cameron, J.S.; Grunfeld, J.P.; Kerr, D.N.S.; Ritz, E.; Viberti, G.C. The patient with diabetes mellitus. In Oxford Textbook of Clinical Nephrology, 2nd ed.; Turner, N., Turner, N.N., Lameire, N., Goldsmith, D.J., Winearls, C.G., Himmelfarb, J., Remuzzi, G., Eds.; Oxford university Press: Oxford, UK, 1998; pp. 723–775. ISBN 978-01-9959-254-8. [Google Scholar]
- Bamri-Ezzine, S.; Ao, Z.J.; Londoño, I.; Gingras, D.; Bendayan, M. Apoptosis of Tubular Epithelial Cells in Glycogen Nephrosis During Diabetes. Lab. Invest. 2003, 83, 1069–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, S.L. Diabetes and renal tubular cell apoptosis. World J. Diabetes 2013, 4, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Nicotera, P.; . Zhivotovsky, B. Cell Death Mechanisms and Their Implications in Toxicology. Toxicological Sci. 2011, 119, 3–19. [Google Scholar] [CrossRef]
- Hamada, T.; Nakano, S.; Iwai, S.; Tanimoto, A.; Ariyoshi, K.; Koide, O. Pathological study on beagles after long-term oral administration of cadmium. Toxicol. Pathol. 1991, 19, 138–147. [Google Scholar] [CrossRef]
- Hamada, T.; Tanimoto, A.; Iwai, S.; Fujiwara, H.; Sasaguri, Y. Cytopathological changes induced by cadmium-exposure in canine proximal tubular cells: A cytochemical and ultrastructural study. Nephron 1994, 68, 104–111. [Google Scholar] [CrossRef]
- Duncan-Achanzar, K.B.; Jones, J.T.; Burke, M.F.; Carter, D.E.; Laird, H.E., II. Inorganic mercury chloride-induced apoptosis in the cultured porcine renal cell line LLC-PK1. J. Pharmacol. Exp. Ther. 1996, 277, 1726–1732. [Google Scholar]
- Nath, K.A.; Croatt, A.J.; Likely, S.; Behrens, T.W.; Warden, D. Renal oxidant injury and oxidant response induced by mercury. Kidney Int. 1996, 50, 1032–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabath, E.; Robles-Osorio, M.L. Renal health and the environment: Heavy metal nephrotoxicity. Nefrologia 2012, 32, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Dai, S.; Yin, Z.; Lu, H.; Jia, R.; Xu, J.; Song, X.; Li, L.; Shu, Y.; Zhao, X.; et al. Sub-chronic lead and cadmium co-induce apoptosis protein expression in liver and kidney of rats. Int J. Clin. Exp. Pathol. 2014, 7, 2905–2914. [Google Scholar] [PubMed]
- Eid, R.A. Apoptosis of Rat Renal Cells by Organophosphate Pesticide, Quinalphos: Ultrastructural Study. Saudi J. Kidney Dis. Transpl. 2017, 28, 725–736. [Google Scholar] [PubMed]
- Lieberthal, W.; Triaca, V.; Levine, J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: Apoptosis vs. necrosis. Am. J. Physiol. 1996, 270, F700–F708. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin Nephrotoxicity. Toxins (Basel) 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz, A.; Lorz, C.; Catalán, M.P.; Danoff, T.M.; Yamasaki, Y.; Egido, J.; Neilson, E.G. Expression of apoptosis regulatory proteins in tubular epithelium stressed in culture or following acute renal failure. Kidney Int. 2000, 57, 969–981. [Google Scholar] [CrossRef] [Green Version]
- Van de Water, B.; Kruidering, M.; Nagelkerke, J.F. F-actin disorganization in apoptotic cell death of cultured rat renal proximal tubular cells. Am. J. Physiol. 1996, 270, F593–F603. [Google Scholar] [CrossRef]
- Desouza, M.; Gunning, P.W.; Stehn, J.R. The actin cytoskeleton as a sensor and mediator of apoptosis. Bioarchitecture 2012, 2, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Nouwen, E.J.; Verstrepen, W.A.; Buyssens, N.; Zhu, M.Q.; De Broe, M.E. Hyperplasia, hypertrophy, and phenotypic alterations in the distal nephron after acute proximal tubular injury in the rat. Lab. Invest. 1994, 70, 479–493. [Google Scholar]
- Abuelezz, S.A.; Hendawy, N.; Abdel Gawad, S. Alleviation of renal mitochondrial dysfunction and apoptosis underlies the protective effect of sitagliptin in gentamicin-induced nephrotoxicity. J. Pharm. Pharmacol. 2016, 68, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Vervaet, B.A.; D’Haese, P.C.; Verhulst, A. Environmental toxin-induced acute kidney injury. Clin. Kidney J. 2017, 10, 747–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, D.J. Cell death in the diseased glomerulus. Histopathology 1998, 12, 679–683. [Google Scholar] [CrossRef]
- Savill, J.; Smith, J.; Sarraf, C.; Ren, Y.; Abbott, F.; Rees, A. Glomerular mesangial cells and inflammatory macrophages ingest neutrophils undergoing apoptosis. Kidney Int. 1992, 42, 924–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, A.J.; Mooney, A.; Hughes, J.; Lombardi, D.; Johnson, R.J.; Savill, J. Mesangial cell apoptosis: The major mechanism for resolution of glomerular hypercellularity in experimental mesangial proliferative nephritis. J. Clin. Invest. 1994, 94, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Kitamura, H.; Masuda, Y.; Ishizaki, M.; Sugisaki, Y.; Yamanaka, N. Apoptosis in the repair process of experimental proliferative glomerulonephritis. Kidney Int. 1995, 47, 114–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, D.S.; Lee, S.H.; Kwak, S.J.; Li, J.J.; Kim, D.H.; Nam, B.Y.; Kang, H.Y.; Chang, T.I.; Park, J.T.; Han, S.H.; et al. Apoptosis occurs differentially according to glomerular size in diabetic kidney disease. Nephrol Dial. Transplant. 2012, 27, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Savill, J. Apoptosis and the kidney. J. Am. Soc. Nephrol 1994, 5, 12–21. [Google Scholar]
- Takemura, T.; Murakami, K.; Miyazato, H.; Yagi, K.; Yoshioka, K. Expression of Fas antigen and Bcl-2 in human glomerulonephritis. Kidney Int 1995, 48, 1886–1892. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, H.; Kashihara, N.; Makino, H.; Yamasaki, Y.; Ota, A. Apoptosis in glomerular sclerosis. Kidney Int 1996, 49, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, A.; Kitamura, H.; Masuda, Y.; Ishizaki, M.; Sugisaki, Y.; Yamanaka, N. Glomerular capillary regeneration and endothelial cell apoptosis in both reversible and progressive models of glomerulonephritis. Contrib Nephrol 1996, 118, 29–40. [Google Scholar] [PubMed]
- Shimizu, A.; Masuda, Y.; Kitamura, H.; Ishizaki, M.; Sugisaki, Y.; Yamanaka, N. Apoptosis in progressive crescentic glomerulonephritis. Lab. Invest. 1996, 74, 941–951. [Google Scholar] [PubMed]
- Soto, H.; Mosquera, J.; Rodriguez, I.B.; Henriquez, L.A.; Roche, C.; Pinto, A. Apoptosis in proliferative glomerulonephritis: Decreased apoptosis expression in lupus nephritis. Nephrol Dial. Transplant. 1997, 12, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Savill, J. Regulation of glomerular cell number by apoptosis. Kidney Int 1999, 56, 1216–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, P.; Soares, M.F. Acute postinfectious glomerulonephritis: An immune response gone bad? Hum. Pathol 2003, 34, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Schmiedeke, T.M.; Stockl, F.W.; Weber, R.; Sugisaki, Y.; Batsford, S.R.; Vogt, A. Histones have high affinity for the glomerular basement membrane. Relevance for immune complex formation in lupus nephritis. J. Exp. Med. 1989, 169, 1879–1894. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Schmiedeke, T.; Stockl, F.; Sugisaki, Y.; Mertz, A.; Batsford, S. The role of cationic proteins in the pathogenesis of immune complex glomerulonephritis. Nephrol Dial. Transplant. 1990, 5, 6–9. [Google Scholar] [CrossRef]
- Schmiedeke, T.; Stoeckl, F.; Muller, S.; Sugisaki, Y.; Batsford, S.; Woitas, R.; Vogt, A. Glomerular immune deposits in murine lupus models may contain histones. Clin. Exp. Immunol 1992, 90, 453–458. [Google Scholar] [CrossRef]
- Stockl, F.; Muller, S.; Batsford, S.; Schmiedeke, T.; Waldherr, R.; Andrassy, K.; Sugisaki, Y.; Nakabayashi, K.; Nagasawa, T.; Rodriguez- Iturbe, B.; et al. A role for histones and ubiquitin in lupus nephritis? Clin. Nephrol 1994, 41, 10–17. [Google Scholar]
- Kuenkele, S.; Beyer, T.D.; Voll, R.E.; Kalden, J.R.; Herrmann, M. Impaired clearance of apoptotic cells in systemic lupus erythematosus: Challenge of T and B cell tolerance. Curr Rheumatol Rep. 2003, 5, 175–177. [Google Scholar] [CrossRef]
- Dieker, J.W.; van der Vlag, J.; Berden, J.H. Deranged removal of apoptotic cells: Its role in the genesis of lupus. Nephrol Dial. Transplant. 2004, 19, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Gaipl, U.S.; Voll, R.E.; Sheriff, A.; Franz, S.; Kalden, J.R.; Herrmann, M. Impaired clearance of dying cells in systemic lupus erythematosus. Autoimmun Rev. 2005, 4, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Kalaaji, M.; Mortensen, E.; Jorgensen, L.; Olsen, R.; Rekvig, O.P. Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells. Am. J. Pathol 2006, 168, 1779–1792. [Google Scholar] [CrossRef] [PubMed]
- Gaipl, U.S.; Sheriff, A.; Franz, S.; Munoz, L.E.; Voll, R.E.; Kalden, J.R.; Herrmann, M. Inefficient clearance of dying cells and autoreactivity. Curr Top. Microbiol Immunol 2006, 305, 161–176. [Google Scholar] [PubMed]
- Chen, R.; Kang, R.; Fan, X.G.; Tang, D. Release and activity of histone in diseases. Cell Death Dis 2014, 5, e1370:1-1370:9. [Google Scholar] [CrossRef] [PubMed]
- Holzman, B.L.; Wiggins, R.C. Glomerular crescent formation. Semin Nephrol 1991, 11, 346–353. [Google Scholar] [PubMed]
- Ophascharoensuk, V.; Pippin, J.W.; Gordon, K.L.; Shankland, S.J.; Couser, W.G.; Johnson, R.J. Role of intrinsic renal cells versus infiltrating cells in glomerular crescent formation. Kidney Int 1998, 54, 416–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, H.; Chen, S.; He, F.F.; Wang, Y.M.; Bondzie, P.; Zhang, C. New Insights into Glomerular Parietal Epithelial Cell Activation and Its Signaling Pathways in Glomerular Diseases. Biomed. Res. Int 2015, 2015, 318935:1–318935:8. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.H.; Mampaso, F.; Zamboni, L. Glomerular podocyte degeneration in human renal disease. Lab. Invest. 1977, 37, 30–42. [Google Scholar] [PubMed]
- Susztak, K.; Raff, A.C.; Schiffer, M.; Böttinger, E.P. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 2006, 55, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Tharaux, P.L.; Huber, T.B. How Many Ways Can a Podocyte Die? Semin Nephrol 2012, 32, 394–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burlaka, I.; Nilsson, L.M.; Scott, L.; Holtbäck, U.; Eklöf, A.C.; Fogo, A.B.; Brismar, H.; Aperia, A. Prevention of apoptosis averts glomerular tubular disconnection and podocyte loss in proteinuric. kidney disease. Kidney Int 2016, 90, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Bagchus, W.M.; Hoedemaeker, P.J.; Rozing, J.; Bakker, W.W. Glomerulonephritis induced by monoclonal anti-Thy 1.1 antibodies. A sequential histological and ultrastructural study in the rat. Lab. Invest. 1986, 55, 680–687. [Google Scholar] [PubMed]
- Wörnle, M.; Schmid, H.; Merkle, M.; Banas, B. Effects of chemokines on proliferation and apoptosis of human mesangial cells. BMC Nephrology 2004, 5, 8:1–8:14. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Singh, V.P. Changing picture of renal cortical necrosis in acute kidney injury in developing country. World J. Nephrol 2015, 4, 480–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brix, A.E. Renal papillary necrosis. Toxicol Pathol 2002, 30, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Rock, K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol 2008, 8, 279–289. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Vandenabeele, P.; Krysko, D.V. Necroptosis: The release of damage-associated molecular patterns and its physiological relevance. Immunity 2013, 38, 209–223. [Google Scholar] [CrossRef]
- Mulay, S.R.; Linkermann, A.; Anders, H.J. Necroinflammation in kidney disease. J. Am. Soc. Nephrol 2016, 27, 27–39. [Google Scholar] [CrossRef]
- Sarhan, M.; von Mässenhausen, A.; Hugo, C.; Oberbauer, R.; Linkermann, A. Immunological consequences of kidney cell death. Cell Death Dis 2018, 9, 114:1–114:15. [Google Scholar] [CrossRef]
- Linkermann, A.; Bräsen, J.H.; Himmerkus, N.; Liu, S.; Huber, T.B.; Kunzendorf, U.; Krautwald, S. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 2012, 81, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Linkermann, A.; Bräsen, J.H.; Darding, M.; Jin, M.K.; Sanz, A.B.; Heller, J.O.; De Zen, F.; Weinlich, R.; Ortiz, A.; Walczak, H.; et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2013, 110, 12024–12029. [Google Scholar] [CrossRef] [PubMed]
- Linkermann, A.; Skouta, R.; Himmerkus, N.; Mulay, S.R.; Dewitz, C.; De Zen, F.; Prokai, A.; Zuchtriegel, G.; Krombach, F.; Welz, P.S.; et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl. Acad. Sci. USA 2014, 111, 16836–16841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulay, S.R.; Desay, J.; Kumar, S.V.R.; Eberhard, J.N.; Thomasova, D.; Romoli, S.; Grigorescu, M.; Kulkarni, O.P.; Popper, B.; Vielhauer, V.; et al. Cytotoxicity of crystals involves RIPK3- MLKL-mediated necroptosis. Nat. Commun 2016, 7, 10274:1–10274:15. [Google Scholar] [CrossRef] [PubMed]
- Allam, R.; Kumar, S.V.; Darisipudi, M.N.; Anders, H.J. Extracellular histones in tissue injury and inflammation. J. Mol. Med. (Berl) 2014, 92, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.D.; Valerius, M.T.; Kobayashi, A.; Mugford, J.W.; Soeung, S.; Duffield, J.S.; McMahon, A.P.; Bonventre, J.V. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2008, 2, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Bonsib, S.M. Glomerular basement membrane necrosis and crescent organization. Kidney Int 1988, 33, 966–974. [Google Scholar] [CrossRef] [Green Version]
- Smeets, B.; Angelotti, M.L.; Rizzo, P.; Dijkman, H.; Lazzeri, E.; Mooren, F.; Ballerini, L.; Parente, E.; Sagrinati, C.; Mazzinghi, B.; et al. Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J. Am. Soc. Nephrol 2009, 20, 2593–2603. [Google Scholar] [CrossRef]
- Schreiber, A.; Xiao, H.; Jennette, J.C.; Schneider, W.; Luft, F.C.; Kettritz, R. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J. Am. Soc. Nephrol 2009, 20, 289–298. [Google Scholar] [CrossRef]
- Ryu, M.; Migliorini, A.; Miosge, N.; Gross, O.; Shankland, S.; Brinkkoetter, P.T.; Hagmann, H.; Romagnani, P.; Liapis, H.; Anders, H.J. Plasma leakage through glomerular basement membrane ruptures triggers the proliferation of parietal epithelial cells and crescent formation in non-inflammatory glomerular injury. J. Pathol 2012, 228, 482–494. [Google Scholar] [CrossRef]
- Schepers, M.S.; van Ballegooijen, E.S.; Bangma, C.H.; Verkoelen, C.F. Crystals cause acute necrotic cell death in renal proximal tubule cells, but not in collecting tubule cells. Kidney Int 2005, 68, 1543–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulay, S.R.; Evan, A.; Anders, H.J. Molecular mechanisms of crystal-related kidney inflammation and injury. Implications for cholesterol embolism, crystalline nephropathies and kidney stone disease. Nephrol Dial. Transplant. 2014, 29, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Ouyang, J.M. New view in cell death mode: Effect of crystal size in renal epithelial cells. Cell Death Dis 2015, 6, e2013:1-2013:3. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Ouyang, J.M.; Yu, K. Shape-dependent cellular toxicity on renal epithelial cells and stone risk of calcium oxalate dihydrate crystals. Sci Rep. 2017, 7, 7250:1–7250:13. [Google Scholar] [CrossRef] [PubMed]
- Honarpisheh, M.; Foresto-Neto, O.; Desai, J.; Steiger, S.; Gómez, L.A.; Popper, B.; Boor, P.; Anders, H.J.; Mulay, S.R. Phagocytosis of environmental or metabolic crystalline particles induces cytotoxicity by triggering necroptosis across a broad range of particle size and shape. Sci Rep. 2017, 7, 15523:1–15523:11. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R.; Anders, H.J. Crystal nephropathies: Mechanisms of crystal-induced kidney injury. Nat. Rev. Nephrol 2017, 13, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Gan, Q.Z.; Ouyang, J.M. Calcium oxalate toxicity in renal epithelial cells: The mediation of crystal size on cell death mode. Cell Death Discov 2015, 1, 15055:1–15055:8. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R.; Kulkarni, O.P.; Rupanagudi, K.V.; Migliorini, A.; Darisipudi, M.N.; Vilaysane, A.; Muruve, D.; Shi, Y.; Munro, F.; Liapis, H.; et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1beta secretion. J. Clin. Invest. 2013, 123, 236–246. [Google Scholar] [CrossRef]
- Kirsch, T. Determinants of pathological mineralization. Curr Opin Rheumatol 2006, 18, 174–180. [Google Scholar] [CrossRef]
- Schepens, D.; Verswijvel, G.; Kuypers, D.; Vanrenterghem, Y. Renal cortical nephrocalcinosis. Nephrol Dial. Transplant. 2000, 15, 1080–1082. [Google Scholar] [CrossRef] [Green Version]
- Priante, G.; Quaggio, F.; Gianesello, L.; Ceol, M.; Cristofaro, R.; Terrin, L.; Furlan, C.; Del Prete, D.; Anglani, F. Caspase-independent programmed cell death triggers Ca2PO4 deposition in an in vitro model of nephrocalcinosis. Biosci Rep. 2018, 38, BSR20171228:1-BSR20171228:19. [Google Scholar] [CrossRef] [PubMed]
- Priante, G.; Ceol, M.; Gianesello, L.; Furlan, C.; Del Prete, D.; Anglani, F. Human proximal tubular cells can form calcium phosphate deposits in osteogenic culture: Role of cell death and osteoblast-like transdifferentiation. Cell Death Discov 2019, 5, 57:1–57:14. [Google Scholar] [CrossRef]
- Proudfoot, D.; Skepper, J.N.; Hegyi, L.; Bennett, M.R.; Shanahan, C.M.; Weissberg, P.L. Apoptosis regulates human vascular calcification in vitro: Evidence for initiation of vascular calcification by apoptotic bodies. Circ Res 2000, 87, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Giachelli, C.M. Vascular calcification mechanisms. J. Am. Soc. Nephrol 2004, 15, 2959–2964. [Google Scholar] [CrossRef] [PubMed]
- Evrard, S.; Delanaye, P.; Kamel, S.; Cristol, J.P.; Cavalier, E. Vascular calcification: From pathophysiology to biomarkers. Clin. Chim Acta 2015, 438, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc Med. 2015, 25, 267–274. [Google Scholar] [CrossRef]
- Priante, G.; Mezzabotta, F.; Cristofaro, R.; Quaggio, F.; Ceol, M.; Gianesello, L.; Del Prete, D.; Anglani, F. Cell death in ectopic calcification of the kidney. Cell Death Dis 2019, 10, 466. [Google Scholar] [CrossRef]
- Unal-Cevik, I.; Kilinç, M.; Can, A.; Gürsoy-Ozdemir, Y.; Dalkara, T. Apoptotic and necrotic death mechanisms are concomitantly activated in the same cell after cerebral ischemia. Stroke 2004, 35, 2189–2194. [Google Scholar] [CrossRef]
- Zychlinsky, A.; Zheng, L.M.; Liu, C.; Young, J.D. Cytolytic lymphocytes induce both apoptosis and necrosis in target celis. J. Immunol 1991, 146, 393–400. [Google Scholar]
- Vanden Berghe, T.; Vanlangenakker, N.; Parthoens, E.; Deckers, W.; Devos, M.; Festjens, N.; Guerin, C.J.; Brunk, U.T.; Declercq, W.; Vandenabeele, P. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 2010, 17, 922–930. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Hu, L.; Yang, C. Necroptosis in acute kidney injury: A shedding light. Cell Death Dis 2016, 7, e2125:1-2125:9. [Google Scholar] [CrossRef] [PubMed]
- Homsi, E.; Janino, P.; de Faria, J.B. Role of caspases on cell death, inflammation, and cell cycle in glycerol-induced acute renal failure. Kidney Int 2006, 69, 1385–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servais, H.; Ortiz, A.; Devuyst, O.; Denamur, S.; Tulkens, P.M.; Mingeot-Leclercq, M.P. Renal cell apoptosis induced by nephrotoxic drugs: Cellular and molecular mechanisms and potential approaches to modulation. Apoptosis 2008, 13, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.J.; Cantley, L. GSK3beta plays dirty in acute kidney injury. J. Am. Soc. Nephrol 2010, 21, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Havasi, A.; Gall, J.; Bonegio, R.; Li, Z.; Mao, H.; Schwartz, J.H.; Borkan, S.C. GSK3beta promotes apoptosis after renal ischemic injury. J. Am. Soc. Nephrol 2010, 21, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Whelan, R.S.; Konstantinidis, K.; Wei, A.C.; Chen, Y.; Reyna, D.E.; Jha, S.; Yang, Y.; Calvert, J.W.; Lindsten, T.; Thompson, C.B.; et al. Bax regulates primary necrosis through mitochondrial dynamics. Proc. Natl. Acad. Sci. USA 2012, 109, 6566–6571. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Zhang, Y.; Han, J. RIP3 is an upregulator of aerobic metabolism and the enhanced respiration by necrosomal RIP3 feeds back on necrosome to promote necroptosis. Cell Death & Differentiation 2018, 25, 821–824. [Google Scholar]
- Teng, X.; Degterev, A.; Jagtap, P.; Xing, X.; Choi, S.; Denu, R.; Yuan, J.; Cuny, G.D. Structure-activity relationship study of novel necroptosis inhibitors. Bioorg Med. Chem Lett 2005, 15, 5039–5044. [Google Scholar] [CrossRef]
- Degterev, A.; Hitomi, J.; Germscheid, M.; Ch’en, I.L.; Korkina, O.; Teng, X.; Abbott, D.; Cuny, G.D.; Yuan, C.; Wagner, G.; et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem Biol 2008, 4, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef]
- Kaiser, W.J.; Sridharan, H.; Huang, C.; Mandal, P.; Upton, J.W.; Gough, P.J.; Sehon, C.A.; Marquis, R.W.; Bertin, J.; Mocarski, E.S. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol Chem 2013, 288, 31268–31279. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Bandyopadhyay, D.; Berger, S.B.; Campobasso, N.; Capriotti, C.A.; Cox, J.A.; Dare, L.; Finger, J.N.; Hoffman, S.J.; Kahler, K.M.; et al. Discovery of small molecule RIP1 kinase inhibitors for the treatment of pathologies associated with necroptosis. ACS Med. Chem Lett 2013, 4, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, J.M.; Tanzer, M.C.; Lucet, I.S.; Young, S.N.; Spall, S.K.; Sharma, P.; Pierotti, C.; Garnier, J.M.; Dobson, R.C.; Webb, A.I.; et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc. Natl. Acad. Sci. USA 2014, 111, 15072–15077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, D.; Marty-Roix, R.; Ganesan, S.; Proulx, M.K.; Vladimer, G.I.; Kaiser, W.J.; Mocarski, E.S.; Pouliot, K.; Chan, F.K.; Kelliher, M.A.; et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc. Natl. Acad. Sci. USA 2014, 111, 7391–7396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, P.; Berger, S.B.; Pillay, S.; Moriwaki, K.; Huang, C.; Guo, H.; Lich, J.D.; Finger, J.; Kasparcova, V.; Votta, B.; et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol. Cell 2014, 56, 481–495. [Google Scholar] [CrossRef]
- Li, J.X.; Feng, J.M.; Wang, Y.; Li, X.H.; Chen, X.X.; Su, Y.; Shen, Y.Y.; Chen, Y.; Xiong, B.; Yang, C.H.; et al. The B-Raf(V600E) inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury. Cell Death Dis 2014, 5, e1278:1-1278:11. [Google Scholar] [CrossRef]
- Fauster, A.; Rebsamen, M.; Huber, K.V.; Bigenzahn, J.W.; Stukalov, A.; Lardeau, C.H.; Scorzoni, S.; Bruckner, M.; Gridling, M.; Parapatics, K.; et al. A cellular screen identifies ponatinib and pazopanib as inhibitors of necroptosis. Cell Death Dis 2015, 6, e1767:1-1767:10. [Google Scholar] [CrossRef]
- Rodriguez, D.A.; Weinlich, R.; Brown, S.; Guy, C.; Fitzgerald, P.; Dillon, C.P.; Oberst, A.; Quarato, G.; Low, J.; Cripps, J.G.; et al. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ. 2016, 23, 76–88. [Google Scholar] [CrossRef]
- Oppenheim, J.J.; Yang, D. Alarmins: Chemotactic activators of immune responses. Curr Opin Immunol 2005, 17, 359–365. [Google Scholar] [CrossRef]
- Chan, J.K.; Roth, J.; Oppenheim, J.J.; Tracey, K.J.; Vogl, T.; Feldmann, M.; Horwood, N.; Nanchahal, J. Alarmins: Awaiting a clinical response. J. Clin. Invest. 2012, 122, 2711–2719. [Google Scholar] [CrossRef]
- Silke, J.; Rickard, J.A.; Gerlic, M. The diverse role of RIP kinases in necroptosis and inflammation. Nat. Immunol 2015, 16, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Linkermann, A.; Hackl, M.J.; Kunzendorf, U.; Walczak, H.; Krautwald, S.; Jevnikar, A.M. Necroptosis in immunity and ischemia-reperfusion injury. Am. J. Transplant. 2013, 13, 2797–2804. [Google Scholar] [CrossRef] [PubMed]
- Linkermann, A.; Green, D.R. Necroptosis. N Engl J. Med. 2014, 370, 455–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degterev, A.; Linkermann, A. Generation of small molecules to interfere with regulated necrosis. Cell Mol. Life Sci. 2016, 73, 2251–2267. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priante, G.; Gianesello, L.; Ceol, M.; Del Prete, D.; Anglani, F. Cell Death in the Kidney. Int. J. Mol. Sci. 2019, 20, 3598. https://doi.org/10.3390/ijms20143598
Priante G, Gianesello L, Ceol M, Del Prete D, Anglani F. Cell Death in the Kidney. International Journal of Molecular Sciences. 2019; 20(14):3598. https://doi.org/10.3390/ijms20143598
Chicago/Turabian StylePriante, Giovanna, Lisa Gianesello, Monica Ceol, Dorella Del Prete, and Franca Anglani. 2019. "Cell Death in the Kidney" International Journal of Molecular Sciences 20, no. 14: 3598. https://doi.org/10.3390/ijms20143598
APA StylePriante, G., Gianesello, L., Ceol, M., Del Prete, D., & Anglani, F. (2019). Cell Death in the Kidney. International Journal of Molecular Sciences, 20(14), 3598. https://doi.org/10.3390/ijms20143598





