Ionizing Radiation Increases the Activity of Exosomal Secretory Pathway in MCF-7 Human Breast Cancer Cells: A Possible Way to Communicate Resistance against Radiotherapy
Abstract
1. Introduction
2. Results
2.1. Ionizing Radiation Reduces the Viability of MCF-7 Cells
2.2. Ionizing Radiation Increases the Apoptosis Rate of MCF-7 Cells
2.3. Ionizing-Irradiated Cells Exhibit Increased Production of Reactive Oxygen Species
2.4. Ionizing Radiation Enhances the Expression of Genes Involved in Exosome Biogenesis/Secretion
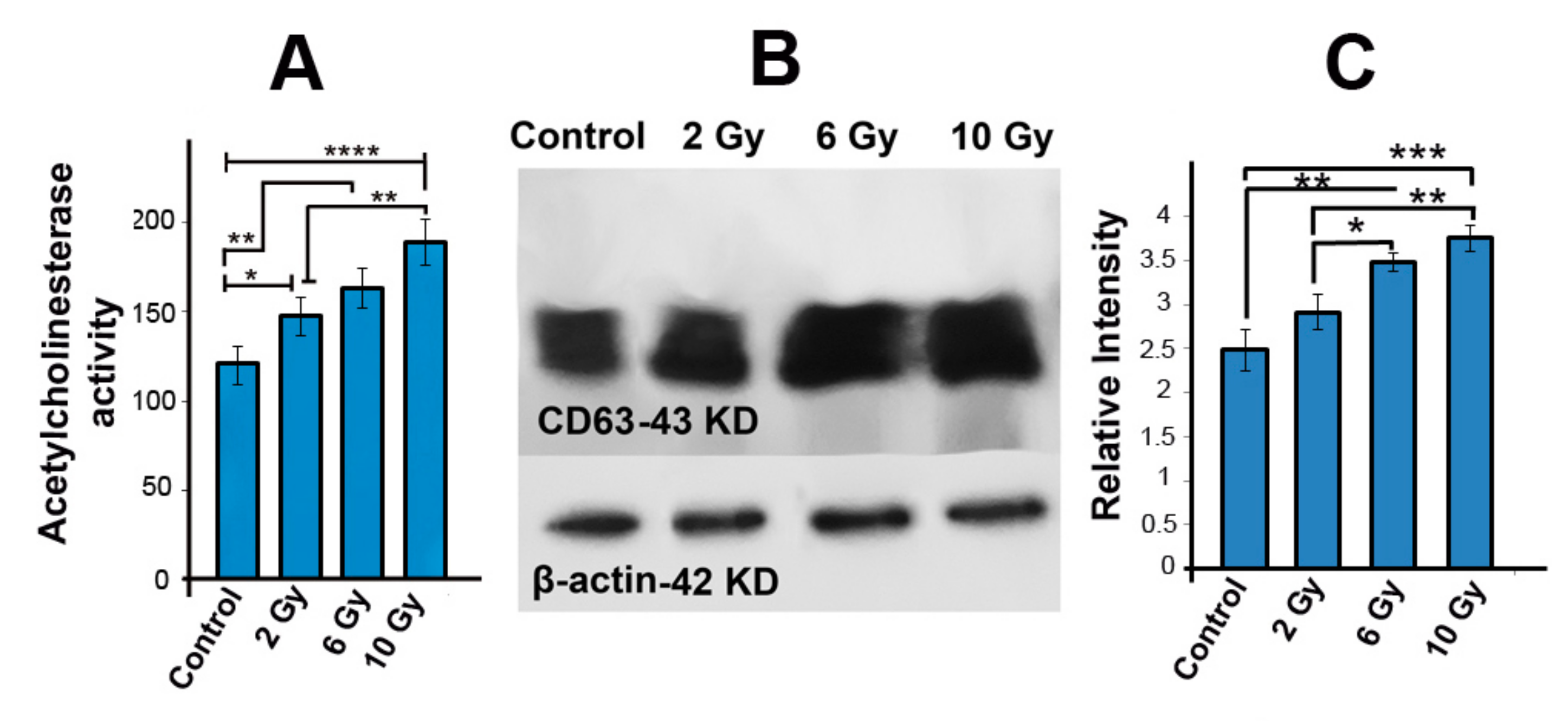
2.5. Acetyl Cholinesterase Activity Was Increased in Ionizing-Radiated MCF-7 Cells
2.6. The Protein Level of CD63 Was Increased in Irradiated MCF-7 Cells
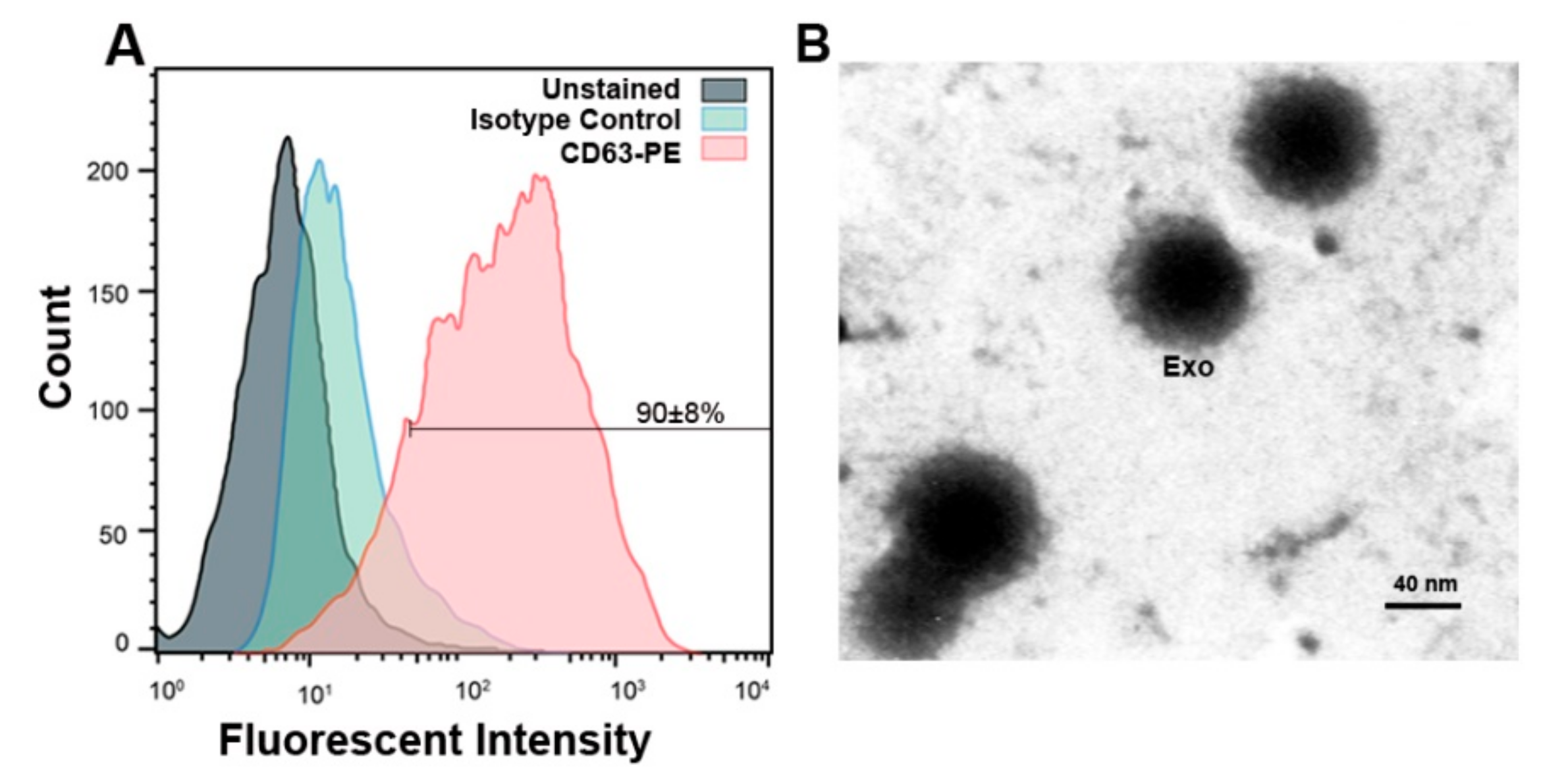
2.7. Confirmation of MCF-7 Derived Exosomes by Flow Cytometry and TEM
2.8. IR Alteres the Exosomes Size and Zeta-Potential
3. Discussion
4. Materials and Methods
4.1. Ethical Issue
4.2. Cell Culture
4.3. Irradiation Procedure
4.4. Cell Survival Assay
4.5. Apoptosis Test
4.6. Determination of ROS Production
4.7. Quantitative Real-Time PCR Analysis of Exosome Biogenesis Related Genes
4.8. Western Blotting Analysis
4.9. Acetylcholinesterase Assay
4.10. Exosome Purification
4.11. Confirmation of Exosomes
4.12. Evaluation the Size and Zeta Potential of Exosomes
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ESCRT | Endosomal Sorting Complex required for Transport |
| DLS | Dynamic Light scattering |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | Dimethyl Sulfoxide |
| DSBs | Double-Strand Breaks |
| PVDF | Polyvinylidene Difluoride |
| IR | Ionizing Radiation |
| Q-PCR | Quantitative real-time PCR |
| ROS | Reactive Oxygen Species |
| SSBs | Single-Strand Breaks |
| TEM | Transmission Electron Microscopy |
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in globocan 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Khalkhali, H.R.; Lotfnezhad Afshar, H.; Esnaashari, O.; Jabbari, N. Applying data mining techniques to extract hidden patterns about breast cancer survival in an iranian cohort study. J. Res. Health Sci. 2016, 16, 31–35. [Google Scholar] [PubMed]
- Esmaeili Govarchin Ghaleh, H.; Zarei, L.; Mansori Motlagh, B.; Jabbari, N. Using cuo nanoparticles and hyperthermia in radiotherapy of mcf-7 cell line: Synergistic effect in cancer therapy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, N.; Zarei, L.; Esmaeili Govarchin Galeh, H.; Mansori Motlagh, B. Assessment of synergistic effect of combining hyperthermia with irradiation and calcium carbonate nanoparticles on proliferation of human breast adenocarcinoma cell line (mcf-7 cells). Artif. Cells Nanomed. Biotechnol. 2018, 46, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.; Yarnold, J. Radiobiology of breast cancer. Clin. Oncol. 2006, 18, 166–178. [Google Scholar] [CrossRef]
- Nawaz, M.; Camussi, G.; Valadi, H.; Nazarenko, I.; Ekström, K.; Wang, X.; Principe, S.; Shah, N.; Ashraf, N.M.; Fatima, F. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat. Rev. Urol. 2014, 11, 688. [Google Scholar] [CrossRef]
- Nawaz, M.; Fatima, F.; Vallabhaneni, K.C.; Penfornis, P.; Valadi, H.; Ekström, K.; Kholia, S.; Whitt, J.D.; Fernandes, J.D.; Pochampally, R. Extracellular vesicles: Evolving factors in stem cell biology. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef]
- Van Niel, G.; D‘Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K. Minimal information for studies of extracellular vesicles 2018 (misev2018): A position statement of the international society for extracellular vesicles and update of the misev2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Bobrie, A.; Colombo, M.; Raposo, G.; Théry, C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Savina, A.; Fader, C.M.; Damiani, M.T.; Colombo, M.I. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 2005, 6, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P. Rab27a and rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Alenquer, M.; Amorim, M. Exosome biogenesis, regulation, and function in viral infection. Viruses 2015, 7, 5066–5083. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Fatima, F.; Nazarenko, I.; Ekström, K.; Murtaza, I.; Anees, M.; Sultan, A.; Neder, L.; Camussi, G.; Valadi, H. Extracellular vesicles in ovarian cancer: Applications to tumor biology, immunotherapy and biomarker discovery. Expert Rev. Proteom. 2016, 13, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Fatima, F.; Nawaz, M. Vesiculated long non-coding rnas: Offshore packages deciphering trans-regulation between cells, cancer progression and resistance to therapies. Non-Coding RNA 2017, 3, 10. [Google Scholar] [CrossRef]
- Wang, J.-S.; Wang, H.-J.; Qian, H.-L. Biological effects of radiation on cancer cells. Mil. Med. Res. 2018, 5, 20. [Google Scholar] [CrossRef]
- Neville, B.W.; Day, T.A. Oral cancer and precancerous lesions. CA: A Cancer J. Clin. 2002, 52, 195–215. [Google Scholar] [CrossRef]
- Al-Mayah, A.; Bright, S.; Chapman, K.; Irons, S.; Luo, P.; Carter, D.; Goodwin, E.; Kadhim, M. The non-targeted effects of radiation are perpetuated by exosomes. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2015, 772, 38–45. [Google Scholar] [CrossRef]
- Diamond, J.M.; Vanpouille-Box, C.; Spada, S.; Rudqvist, N.-P.; Chapman, J.R.; Ueberheide, B.M.; Pilones, K.A.; Sarfraz, Y.; Formenti, S.C.; Demaria, S. Exosomes shuttle trex1-sensitive ifn-stimulatory dsdna from irradiated cancer cells to dcs. Cancer Immunol. Res. 2018, 6, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Arscott, W.T.; Tandle, A.T.; Zhao, S.; Shabason, J.E.; Gordon, I.K.; Schlaff, C.D.; Zhang, G.; Tofilon, P.J.; Camphausen, K.A. Ionizing radiation and glioblastoma exosomes: Implications in tumor biology and cell migration. Transl. Oncol. 2013, 6, 638-IN636. [Google Scholar] [CrossRef] [PubMed]
- Mutschelknaus, L.; Peters, C.; Winkler, K.; Yentrapalli, R.; Heider, T.; Atkinson, M.J.; Moertl, S. Exosomes derived from squamous head and neck cancer promote cell survival after ionizing radiation. PLoS ONE 2016, 11, e0152213. [Google Scholar] [CrossRef] [PubMed]
- Ghisolfi, L.; Keates, A.C.; Hu, X.; Lee, D.-k.; Li, C.J. Ionizing radiation induces stemness in cancer cells. PLoS ONE 2012, 7, e43628. [Google Scholar] [CrossRef] [PubMed]
- Pickhard, A.C.; Margraf, J.; Knopf, A.; Stark, T.; Piontek, G.; Beck, C.; Boulesteix, A.-L.; Scherer, E.Q.; Pigorsch, S.; Schlegel, J. Inhibition of radiation induced migration of human head and neck squamous cell carcinoma cells by blocking of egf receptor pathways. BMC Cancer 2011, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-C.; Liu, J.-Y.; Li, J.; Zhang, J.; Xu, Y.-Q.; Zhang, H.-W.; Qiu, L.-B.; Ding, G.-R.; Su, X.-M.; Guo, G.-Z. Ionizing radiation promotes migration and invasion of cancer cells through transforming growth factor-beta–mediated epithelial–mesenchymal transition. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1530–1537. [Google Scholar] [CrossRef]
- Phillips, T.M.; McBride, W.H.; Pajonk, F. The response of cd24−/low/cd44+ breast cancer–initiating cells to radiation. J. Natl. Cancer Inst. 2006, 98, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeong, E.K.; Ju, M.K.; Jeon, H.M.; Kim, M.Y.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol. Cancer 2017, 16, 10. [Google Scholar] [CrossRef]
- Kumar Jella, K.; Rani, S.; O‘driscoll, L.; McClean, B.; Byrne, H.; Lyng, F. Exosomes are involved in mediating radiation induced bystander signaling in human keratinocyte cells. Radiat. Res. 2014, 181, 138–145. [Google Scholar] [CrossRef]
- De Jong, O.G.; Verhaar, M.C.; Chen, Y.; Vader, P.; Gremmels, H.; Posthuma, G.; Schiffelers, R.M.; Gucek, M.; van Balkom, B.W. Cellular stress conditions are reflected in the protein and rna content of endothelial cell-derived exosomes. J. Extracell. Vesicles 2012, 1, 18396. [Google Scholar] [CrossRef]
- Chin, C.; Bae, J.H.; Kim, M.J.; Hwang, J.Y.; Kim, S.J.; Yoon, M.S.; Lee, M.K.; Kim, D.W.; Chung, B.S.; Kang, C.D. Radiosensitization by targeting radioresistance-related genes with protein kinase a inhibitor in radioresistant cancer cells. Exp. Mol. Med. 2005, 37, 608. [Google Scholar] [CrossRef] [PubMed]
- Meir, O.; Dvash, E.; Werman, A.; Rubinstein, M. C/ebp-β regulates endoplasmic reticulum stress–triggered cell death in mouse and human models. PLoS ONE 2010, 5, e9516. [Google Scholar] [CrossRef]
- Gudkov, A.V.; Komarova, E.A. The role of p53 in determining sensitivity to radiotherapy. Nat. Rev. Cancer 2003, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- Sheard, M.A. Ionizing radiation as a response-enhancing agent for cd95-mediated apoptosis. Int. J. Cancer 2001, 96, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Yamamori, T.; Yasui, H.; Yamazumi, M.; Wada, Y.; Nakamura, Y.; Nakamura, H.; Inanami, O. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radic. Biol. Med. 2012, 53, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Wojakowska, A.; Marczak, L.; Muer, A.; Tinhofer-Keilholz, I.; Lysek-Gladysinska, M.; Widlak, P.; Pietrowska, M. Ionizing radiation affects protein composition of exosomes secreted in vitro from head and neck squamous cell carcinoma. Acta Biochim. Pol. 2015, 62. [Google Scholar] [CrossRef]
- Jelonek, K.; Widlak, P.; Pietrowska, M. The influence of ionizing radiation on exosome composition, secretion and intercellular communication. Protein Pept. Lett. 2016, 23, 656–663. [Google Scholar] [CrossRef]
- Lespagnol, A.; Duflaut, D.; Beekman, C.; Blanc, L.; Fiucci, G.; Marine, J.-C.; Vidal, M.; Amson, R.; Telerman, A. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in tsap6/steap3-null mice. Cell Death Differ. 2008, 15, 1723. [Google Scholar] [CrossRef]
- Blanc, L.; Vidal, M. New insights into the function of rab gtpases in the context of exosomal secretion. Small GTPases 2018, 9, 95–106. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Paine, M.S.; Brooks, A.M.; McCubrey, J.A.; Renegar, R.H.; Wang, R.; Terrian, D.M. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008, 68, 7864–7871. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.-T.; Teng, K.; Wu, C.; Adam, M.; Johnstone, R.M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985, 101, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Okada, R.; Nagao, K.; Kawamata, Y.; Hanyu, A.; Yoshimoto, S.; Takasugi, M.; Watanabe, S.; Kanemaki, M.T.; Obuse, C. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 2017, 8, 15287. [Google Scholar] [CrossRef] [PubMed]
- Jella, K.; Nasti, T.; Li, Z.; Lawson, D.; Ahmed, R.; Dynan, W.; Khan, M. Post-irradiated tumor-derived exosomes lead to melanoma tumor growth delay, potentially mediated by death associated molecular pattern (damps) proteins. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, S155. [Google Scholar] [CrossRef]
- Mutschelknaus, L.; Azimzadeh, O.; Heider, T.; Winkler, K.; Vetter, M.; Kell, R.; Tapio, S.; Merl-Pham, J.; Huber, S.M.; Edalat, L. Radiation alters the cargo of exosomes released from squamous head and neck cancer cells to promote migration of recipient cells. Sci. Rep. 2017, 7, 12423. [Google Scholar] [CrossRef] [PubMed]
- Bewicke-Copley, F.; Mulcahy, L.A.; Jacobs, L.A.; Samuel, P.; Akbar, N.; Pink, R.C.; Carter, D.R.F. Extracellular vesicles released following heat stress induce bystander effect in unstressed populations. J. Extracell. Vesicles 2017, 6, 1340746. [Google Scholar] [CrossRef]
- Samuel, P.; Mulcahy, L.A.; Furlong, F.; McCarthy, H.O.; Brooks, S.A.; Fabbri, M.; Pink, R.C.; Carter, D.R.F. Cisplatin induces the release of extracellular vesicles from ovarian cancer cells that can induce invasiveness and drug resistance in bystander cells. Philos. Trans. R. Soc. B: Biol. Sci. 2017, 373, 20170065. [Google Scholar] [CrossRef]
- Rezaie, J.; Nejati, V.; Khaksar, M.; Oryan, A.; Aghamohamadzadeh, N.; Shariatzadeh, M.A.; Rahbarghazi, R.; Mehranjani, M.S. Diabetic sera disrupted the normal exosome signaling pathway in human mesenchymal stem cells in vitro. Cell Tissue Res. 2018, 374, 555–565. [Google Scholar] [CrossRef]
- Bagheri, H.S.; Mousavi, M.; Rezabakhsh, A.; Rezaie, J.; Rasta, S.H.; Nourazarian, A.; Avci, Ç.B.; Tajalli, H.; Talebi, M.; Oryan, A. Low-level laser irradiation at a high power intensity increased human endothelial cell exosome secretion via wnt signaling. Lasers Med Sci. 2018, 33, 1131–1145. [Google Scholar] [CrossRef]
- Edgar, J.R.; Eden, E.R.; Futter, C.E. Hrs-and cd63-dependent competing mechanisms make different sized endosomal intraluminal vesicles. Traffic 2014, 15, 197–211. [Google Scholar] [CrossRef]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.M.; Sadik, M.; Alaarg, A.; Smith, C.E.; Lehtiö, J.; Andaloussi, S.E. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.S.; Catita, J.; Rosa, I.M.; e Silva, O.A.d.C.; Henriques, A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE 2018, 13, e0198820. [Google Scholar] [CrossRef] [PubMed]
- Al Musawi, M.S.; Jaafar, M.; Al-Gailani, B.; Ahmed, N.M.; Suhaimi, F.M. Laser-induced changes of in vitro erythrocyte sedimentation rate. Lasers Med Sci. 2017, 32, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Beit-Yannai, E.; Tabak, S.; Stamer, W.D. Physical exosome: Exosome interactions. J. Cell. Mol. Med. 2018, 22, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Ichiki, T. Evaluation of zeta-potential of individual exosomes secreted from biological cells using a microcapillary electrophoresis chip. In Encyclopedia of Biocolloid and Biointerface Science 2V Set; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 469–473. [Google Scholar]
- Van der Pol, E.; Coumans, F.; Varga, Z.; Krumrey, M.; Nieuwland, R. Innovation in detection of microparticles and exosomes. J. Thromb. Haemost. 2013, 11, 36–45. [Google Scholar] [CrossRef]




| Genes | Sequence (5′→3′) | Tm |
|---|---|---|
| Rab11 | F: CCTCAGCCTCTACGAAGCAAA R:CCGGAAGTTGATCTCCTCCTG | 59 |
| Rab27a | F: AGAGGAGGAAGCCATAGCAC R: CATGACCATTTGATCGCACCAC | 59 |
| Rab27b | F: GGAACTGGCTGACAAATATGG R: CAGTATCAGGGATTTGTGTCTT | 59 |
| TSAP6 | F: CCTCTACAGCTTCTGCTTGCC R: TAGATCTCCATCCGCCAGACC | 63 |
| CD63 | F: TCCTGAGTCAGACCATAATCC R: GATGGCAAACGTGATCATAAG | 63 |
| Alix | F: CTGGAAGGATGCTTTCGATAAAGG R: AGGCTGCACAATTGAACAACAC | 63 |
| GAPDH | F: CAAGTTCAACGGCACAGTCAAG R: ATACTCAGCACCAGCATCACC | 60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabbari, N.; Nawaz, M.; Rezaie, J. Ionizing Radiation Increases the Activity of Exosomal Secretory Pathway in MCF-7 Human Breast Cancer Cells: A Possible Way to Communicate Resistance against Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3649. https://doi.org/10.3390/ijms20153649
Jabbari N, Nawaz M, Rezaie J. Ionizing Radiation Increases the Activity of Exosomal Secretory Pathway in MCF-7 Human Breast Cancer Cells: A Possible Way to Communicate Resistance against Radiotherapy. International Journal of Molecular Sciences. 2019; 20(15):3649. https://doi.org/10.3390/ijms20153649
Chicago/Turabian StyleJabbari, Nasrollah, Muhammad Nawaz, and Jafar Rezaie. 2019. "Ionizing Radiation Increases the Activity of Exosomal Secretory Pathway in MCF-7 Human Breast Cancer Cells: A Possible Way to Communicate Resistance against Radiotherapy" International Journal of Molecular Sciences 20, no. 15: 3649. https://doi.org/10.3390/ijms20153649
APA StyleJabbari, N., Nawaz, M., & Rezaie, J. (2019). Ionizing Radiation Increases the Activity of Exosomal Secretory Pathway in MCF-7 Human Breast Cancer Cells: A Possible Way to Communicate Resistance against Radiotherapy. International Journal of Molecular Sciences, 20(15), 3649. https://doi.org/10.3390/ijms20153649





