Angiogenesis, Lymphangiogenesis, and the Immune Response in South African Preeclamptic Women Receiving HAART
Abstract
:1. Problem Identification
Maternal Mortality and Hypertension in South Africa
2. Human Immunodeficiency Virus Infection in South Africa
3. Angiogenesis
3.1. Soluble Fms-Like Tyrosine Kinase 1 (sFlt1), Placental Growth Factor (PlGF), and Soluble Endoglin (Eng)
3.2. Vascular Endothelial Growth Factor (VEGF)
3.3. Platelet Endothelial Cell Adhesion Molecule 1 (PECAM-1)
3.4. Angiopoietin (Ang)-2
3.5. sTie-2
3.6. Vascular Endothelial Growth Factor and HIV Tat protein
4. Lymphangiogenesis
4.1. Lymphatic System in the Placenta
4.2. Lymphangiogenesis and Preeclampsia
4.3. Lymphangiogenesis and HIV Infection
4.4. Lymphangiogenesis in the Duration of HAART and the Risk of Preeclampsia
5. Highly Active Anti-Retroviral Therapy
6. Immune Maladaptation
6.1. Natural Killer Cells in Normal versus Preeclamptic Pregnancies
6.2. Role of HAART on NK Cells and Risk of Preeclampsia Development
7. Cytokines in Normal Pregnancy, Preeclampsia, HIV Infection, and in the Duration of HAART
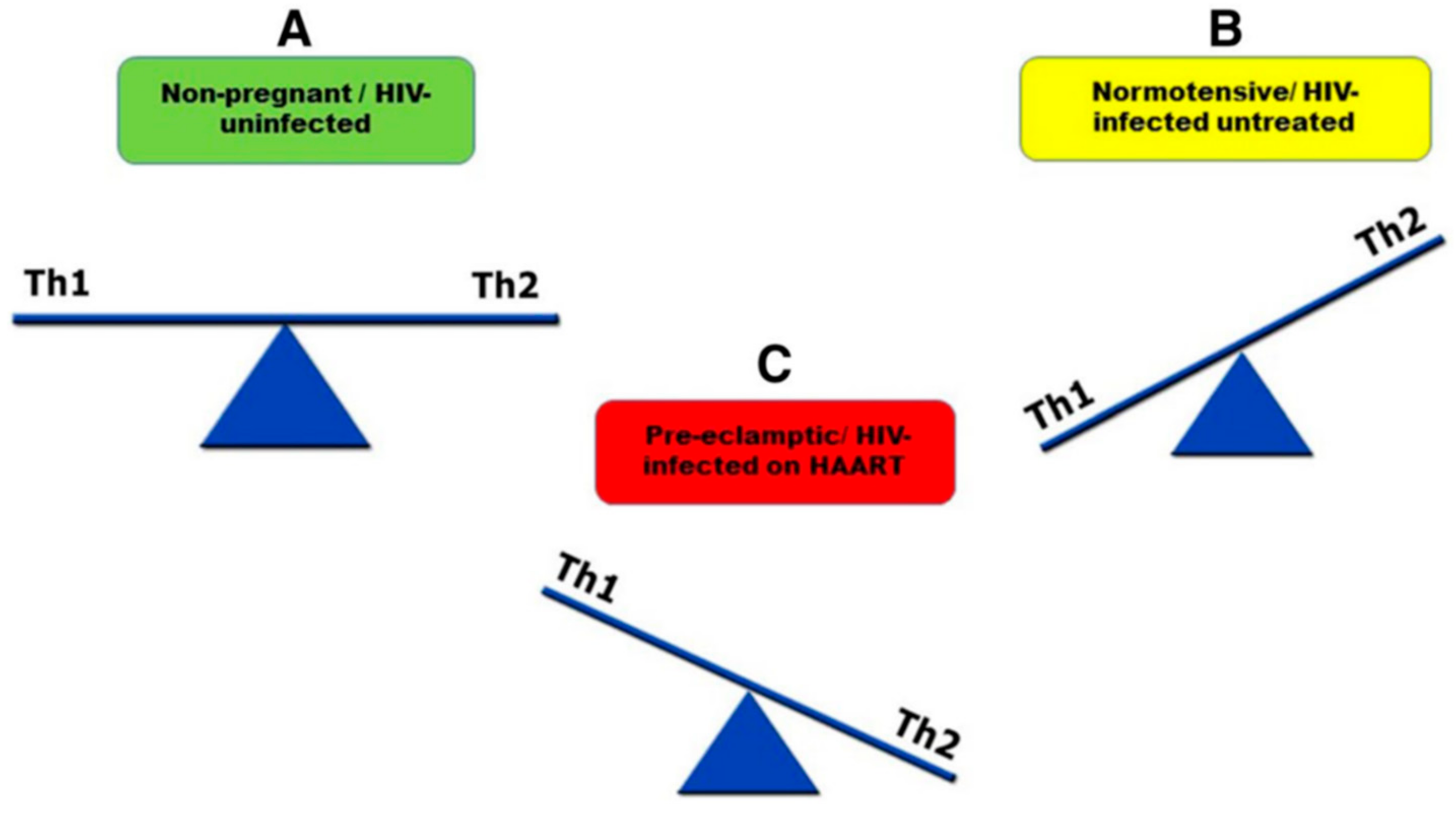
7.1. T Helper Cell 1 and T Helper Cell 2 (Th1 and Th2)
7.2. T Helper Cell 17 (Th17) and T Regulatory Cells (Treg)
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ang-1 | Angiopoietin-1 |
| Ang-2 | Angiopoietin-2 |
| Ang-3 | Angiopoietin-3 |
| Ang-4 | Angiopoietin-4 |
| AP-1 | Activator protein 1 |
| ARV | Anti-retroviral therapy |
| CD94 | Cluster of differentiation 94 |
| CTLA-4 | Cytotoxic T-lymphocyte antigen 4 |
| CXCR1 | Chemokine (C–X–C motif) receptor 1 |
| CXCR2 | Chemokine (C–X–C motif) receptor 2 |
| ENOS | Endothelial nitric oxide synthase |
| FGF-2 | Fibroblast growth factor |
| FIK-1 | Vascular endothelial growth factor receptor 2 (VEGFR22, kinase domain receptor) |
| FOXP3 | Forkhead box P3 |
| Gp120 | Glycoprotein 120 |
| HIF-1 | Hypoxic-inducible factor 1 |
| HAART | Highly active anti-retroviral therapy |
| HDP | Hypertensive disorders of pregnancy |
| HELLP | Hemolysis, elevated liver enzymes, and low platelets |
| HIV | Human immuno-deficiency virus |
| IL-17 | Interleukin-17 |
| KDR | Kinase insert domain receptor |
| KIR2DS | Killer-cell immunoglobulin-like receptor 2DS |
| KZN | KwaZulu-Natal |
| LAIR-1 | Leukocyte-associated immunoglobulin-like receptor 1 |
| LIR1 | Leukocyte immunoglobulin-like receptor 1 |
| MAPKs | Mitogen-activated protein kinases |
| MMP | Matrix metalloproteases |
| NF-κB | Nuclear factor kappa B |
| NKG2 | Natural killer cell G2 |
| NKG2C | Natural killer cell G2A |
| NKG2D | Natural killer cell G2D |
| NKp30 | Natural killer cell precursor 30 |
| NKp44 | Natural killer cell precursor 44 |
| NKp46 | Natural killer cell precursor 46 |
| PE | Preeclampsia |
| PECAM-1 | Platelet endothelial cell adhesion molecule 1 |
| PLGF | Placental growth factor |
| SEng | Soluble endoglin |
| SFlt1 | Soluble fms-like tyrosine kinase 1 |
| Slit2/Robo4 | Slit/Roundabout (Robo) |
| Sp-1 | Specificity protein 1 |
| Tat | Transactivating regulatory protein |
| TGF-ß | Transforming growth factor beta |
| TIE1 | Tyrosine protein kinase receptor 1 |
| TIE2 | Tyrosine protein kinase receptor 2 |
| Th1 | T helper cell type 1 |
| Th2 | T helper cell type 2 |
| Th17 | T helper type 17 |
| Treg | Regulatory T cells |
| UNAIDS | United Nations Program on HIV/AIDS |
| VE cadherin | Vascular endothelial cadherin |
| VEGF | Vascular endothelial growth factor |
| VEGFR-1 | Vascular endothelial growth factor receptor 1 |
| VEGFR-2 | Vascular endothelial growth factor receptor 2 |
| VEGFR-3 | Vascular endothelial growth factor receptor 3 |
| WHO | World Health Organization |
References
- World Health Organization. World Health Statistics 2016: Monitoring Health for the Sdgs Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Pretoria: National Department of Health. Saving Mothers 2014–2016: Seventh Triennial Report on Confidential Enquiries into Maternal Deaths in South Africa: Executive Summary; National Department of Health: Pretoria, South Africa, 2018.
- Moodley, J. Maternal deaths due to hypertensive disorders in pregnancy: Saving mothers report 2002–2004. Cardiovasc. J. Afr. 2007, 18, 358–361. [Google Scholar] [PubMed]
- Payne, B.; Hanson, C.; Sharma, S.; Magee, L.; von Dadelszen, P. Epidemiology of the hypertensive disorders of pregnancy. In The FIGO textbook of pregnancy hypertension; Magee, L.A., von Dadelszen, P., Stones, W., Mathai, M., Eds.; Global Library of Women’s Medicine: London, UK, 2016. [Google Scholar]
- Moodley, J.; Onyangunga, O.; Maharaj, N. Hypertensive disorders in primigravid black south african women: A one-year descriptive analysis. Hypertens. Pregnancy 2016, 35, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; van Look, P.F. Who analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- Osungbade, K.O.; Ige, O.K. Public health perspectives of preeclampsia in developing countries: Implication for health system strengthening. J. Pregnancy 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Lakew, Y.; Reda, A.A.; Tamene, H.; Benedict, S.; Deribe, K. Geographical variation and factors influencing modern contraceptive use among married women in ethiopia: Evidence from a national population based survey. Reprod. Health 2013, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. The Joint United Nations Programme on HIV/AIDS. Available online: https://www.unAIDS.org/en (accessed on 19 December 2018).
- UNAIDS. Global HIV & AIDS Statistics—2018 Fact Sheet. Available online: https://www.unAIDS.org/en (accessed on 16 April 2019).
- Human Sciences Research Council. South African National HIV Prevalence Incidence and Behaviour Survey. Human Sciences Research Council: Pretoria, South Africa, 2008. [Google Scholar]
- National Department of Health. National Antenatal Sentinel HIV & Syphilis Survey Report; National Department of Health: Pretoria, South Africa, 2017.
- Kalumba, V.M.; Moodley, J.; Naidoo, T.D. Is the prevalence of pre-eclampsia affected by HIV/AIDS? A retrospective case-control study. Cardiovasc. J. Afr. 2013, 24, 24–27. [Google Scholar] [CrossRef]
- Kubis, N.; Levy, B.I. Vasculogenesis and angiogenesis: Molecular and cellular controls. Part 1: Growth factors. Int. Neuroradiol. J. Perith. Neuroradiol. Surg. Proc. Relat. Neurosci. 2003, 9, 227–237. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Killilea, S.; Redmer, D. Angiogenesis in the female reproductive system. FASEB J. 1992, 6, 886–892. [Google Scholar] [CrossRef] [Green Version]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671. [Google Scholar] [CrossRef]
- Naicker, T.; Khedun, S.M.; Moodley, J.; Pijnenborg, R. Quantitative analysis of trophoblast invasion in preeclampsia. Acta Obstet. Gynecol. Scand. 2003, 82, 722–729. [Google Scholar] [CrossRef]
- Paydas, S.; Ergin, M.; Seydaoglu, G.; Erdogan, S.; Yavuz, S. Pronostic significance of angiogenic/lymphangiogenic, anti-apoptotic, inflammatory and viral factors in 88 cases with diffuse large b cell lymphoma and review of the literature. Leuk. Res. 2009, 33, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Ribaudo, H.J.; Souda, S.; Parekh, N.; Ogwu, A.; Lockman, S.; Powis, K.; Dryden-Peterson, S.; Creek, T.; Jimbo, W. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in botswana. J. Infect. Dis. 2012, 206, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Wimalasundera, R.; Larbalestier, N.; Smith, J.; De Ruiter, A.; Thom, S.M.; Hughes, A.; Poulter, N.; Regan, L.; Taylor, G. Pre-eclampsia, antiretroviral therapy, and immune reconstitution. Lancet 2002, 360, 1152–1154. [Google Scholar] [CrossRef]
- Powis, K.M.; McElrath, T.F.; Hughes, M.D.; Ogwu, A.; Souda, S.; Datwyler, S.A.; von Widenfelt, E.; Moyo, S.; Nádas, M.; Makhema, J. High viral load and elevated angiogenic markers associated with increased risk of preeclampsia among women initiating highly active antiretroviral therapy (haart) in pregnancy in the Mma Bana study, Botswana. J. Acquir. Immune Defic. Syndr. 2013, 62, 517. [Google Scholar] [CrossRef] [PubMed]
- Conroy, A.L.; McDonald, C.R.; Gamble, J.L.; Olwoch, P.; Natureeba, P.; Cohan, D.; Kamya, M.R.; Havlir, D.V.; Dorsey, G.; Kain, K.C. Altered angiogenesis as a common mechanism underlying preterm birth, small for gestational age, and stillbirth in women living with HIV. Am. J. Obstet. Gynecol. 2017, 217, 684.e1–684.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nnabuike Chibuoke Ngene, J.M.a.T.N. The performance of pre-delivery serum concentrations of angiogenic factors in predicting postpartum antihypertensive drug therapy following abdominal delivery in severe preeclampsia and normotensive pregnancy. PLoS ONE 2019, 14, e0215807. [Google Scholar] [CrossRef]
- Roberts, J.M.; Rajakumar, A. Preeclampsia and soluble fms-like tyrosine kinase 1. J. Clin. Endocrinol. Metab. 2009, 94, 2252–2254. [Google Scholar] [CrossRef]
- Masuda, Y.; Shimizu, A.; Mori, T.; Ishiwata, T.; Kitamura, H.; Ohashi, R.; Ishizaki, M.; Asano, G.; Sugisaki, Y.; Yamanaka, N. Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am. J. Pathol. 2001, 159, 599–608. [Google Scholar] [CrossRef]
- Govender, N.; Moodley, J.; Gathiram, P.; Naicker, T. Soluble fms-like tyrosine kinase-1 in HIV infected pre-eclamptic south african black women. Placenta 2014, 35, 618–624. [Google Scholar] [CrossRef]
- Govender, N.; Naicker, T.; Rajakumar, A.; Moodley, J. Soluble fms-like tyrosine kinase-1 and soluble endoglin in HIV-associated preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 100–105. [Google Scholar] [CrossRef]
- Perucci, L.O.; Gomes, K.B.; Freitas, L.G.; Godoi, L.C.; Alpoim, P.N.; Pinheiro, M.B.; Miranda, A.S.; Teixeira, A.L.; Dusse, L.M.; Sousa, L.P. Soluble endoglin, transforming growth factor-beta 1 and soluble tumor necrosis factor alpha receptors in different clinical manifestations of preeclampsia. PLoS ONE 2014, 9, e97632. [Google Scholar] [CrossRef]
- Govender, N.; Moodley, J.; Naicker, T. The use of soluble fms-like tyrosine kinase 1/placental growth factor ratio in the clinical management of pre-eclampsia. Afr. J. Reprod. Health 2018, 22, 135–143. [Google Scholar] [PubMed]
- Bates, D.O. An unexpected tail of vegf and plgf in pre-eclampsia. Biochem. Soc. Trans. 2011, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Helmo, F.R.; Lopes, A.M.M.; Carneiro, A.; Campos, C.G.; Silva, P.B.; dos Reis Monteiro, M.L.G.; Rocha, L.P.; dos Reis, M.A.; Etchebehere, R.M.; Machado, J.R.; et al. Angiogenic and antiangiogenic factors in preeclampsia. Pathol. Res. Pract. 2018, 214, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Cerdeira, A.S.; Agrawal, S.; Staff, A.C.; Redman, C.W.; Vatish, M. Angiogenic factors: Potential to change clinical practice in pre-eclampsia? BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Ngene, N.C.; Moodley, J. Role of angiogenic factors in the pathogenesis and management of pre-eclampsia. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2018, 141, 5–13. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 2013, 153, 13–19. [Google Scholar] [CrossRef]
- Coon, B.G.; Baeyens, N.; Han, J.; Budatha, M.; Ross, T.D.; Fang, J.S.; Yun, S.; Thomas, J.-L.; Schwartz, M.A. Intramembrane binding of ve-cadherin to vegfr2 and vegfr3 assembles the endothelial mechanosensory complex. J. Cell Biol. 2015, 208, 975–986. [Google Scholar] [CrossRef]
- Sahin, S.; Ozakpinar, O.B.; Eroglu, M.; Tetik, S. Platelets in preeclampsia: Function and role in the inflammation. Clin. Exp. Health Sci. 2014, 4, 111. [Google Scholar] [CrossRef]
- Thakoordeen, S.; Moodley, J.; Naicker, T. Serum levels of platelet endothelial cell adhesion molecule-1 (pecam-1) and soluble vascular endothelial growth factor receptor (svegfr)-1 and-2 in HIV associated preeclampsia. Hypertens. Pregnancy 2017, 36, 168–174. [Google Scholar] [CrossRef]
- Findley, C.M.; Cudmore, M.J.; Ahmed, A.; Kontos, C.D. Vegf induces tie2 shedding via a phosphoinositide 3-kinase/akt–dependent pathway to modulate tie2 signaling. Arterioscler. Thrombo. Vasc. Biol. 2007, 27, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Mbhele, N.; Moodley, J.; Naicker, T. Role of angiopoietin-2, endoglin, and placental growth factor in HIV-associated preeclampsia. Hypertens. Pregnancy 2017, 36, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Fagiani, E.; Christofori, G. Angiopoietins in angiogenesis. Cancer Lett. 2013, 328, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Findley, C.M.; Mitchell, R.G.; Duscha, B.D.; Annex, B.H.; Kontos, C.D. Plasma levels of soluble tie2 and vascular endothelial growth factor distinguish critical limb ischemia from intermittent claudication in patients with peripheral arterial disease. J. Am. Coll. Cardiol. 2008, 52, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Mazibuko, M.; Moodley, J.; Naicker, T. Dysregulation of circulating stie2 and sher2 in HIV-infected women with preeclampsia. Hypertens. Pregnancy 2019, 38, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.K.; Hung, M.C. Proteolytic cleavage, trafficking, and functions of nuclear receptor tyrosine kinases. FEBS J. 2015, 282, 3693–3721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, W.; Herbein, G. T-cell signaling in HIV-1 infection. Open Virol. J. 2013, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Romani, B.; Engelbrecht, S.; Glashoff, R.H. Functions of tat: The versatile protein of human immunodeficiency virus type 1. J. Gen. Virol. 2010, 91, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xue, M.; Qin, D.; Zhu, X.; Wang, C.; Zhu, J.; Hao, T.; Cheng, L.; Chen, X.; Bai, Z. HIV-1 tat promotes kaposi’s sarcoma-associated herpesvirus (kshv) vil-6-induced angiogenesis and tumorigenesis by regulating pi3k/pten/akt/gsk-3β signaling pathway. PLoS ONE 2013, 8, e53145. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Soldi, R.; Giunciuclio, D.; Giraudo, E.; Benelli, R.; Primo, L.; Noonan, D.; Salio, M.; Camussi, G.; Rock, W.; et al. The angiogenesis induced by HIV–1 tat protein is mediated by the flk–1/kdr receptor on vascular endothelial cells. Nat Med. 1996, 2, 1371. [Google Scholar] [CrossRef] [PubMed]
- Alghisi, G.C.; Rüegg, C. Vascular integrins in tumor angiogenesis: Mediators and therapeutic targets. Endothel. Cell Res. 2006, 13, 113–135. [Google Scholar] [CrossRef] [PubMed]
- Crublet, E.; Andrieu, J.P.; Vives, R.R.; Lortat-Jacob, H. The HIV-1 envelope glycoprotein gp120 features four heparan sulfate binding domains, including the co-receptor binding site. J. Biol. Chem. 2008, 283, 15193–15200. [Google Scholar] [CrossRef] [PubMed]
- Barillari, G.; Sgadari, C.; Palladino, C.; Gendelman, R.; Caputo, A.; Morris, C.B.; Nair, B.C.; Markham, P.; Nel, A.; Stürzl, M. Inflammatory cytokines synergize with the HIV-1 tat protein to promote angiogenesis and kaposi’s sarcoma via induction of basic fibroblast growth factor and the αvβ3 integrin. J. Immunol. 1999, 163, 1929–1935. [Google Scholar] [PubMed]
- Li, C.J.; Ueda, Y.; Shi, B.; Borodyansky, L.; Huang, L.; Li, Y.-Z.; Pardee, A.B. Tat protein induces self-perpetuating permissivity for productive HIV-1 infection. Proc. Natl. Acad. Sci. USA 1997, 94, 8116–8120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detry, B.; Bruyère, F.; Erpicum, C.; Paupert, J.; Lamaye, F.; Maillard, C.; Lenoir, B.; Foidart, J.-M.; Thiry, M.; Noël, A. Digging deeper into lymphatic vessel formation in vitro and in vivo. BMC Cell Biol. 2011, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Aspelund, A.; Alitalo, K. Lymphangiogenic factors, mechanisms, and applications. J. Clin. Investig. 2014, 124, 878–887. [Google Scholar] [CrossRef]
- Norrmén, C.; Tammela, T.; Petrova, T.V.; Alitalo, K. Biological basis of therapeutic lymphangiogenesis. Circulation 2011, 123, 1335–1351. [Google Scholar] [CrossRef]
- Lohela, M.; Bry, M.; Tammela, T.; Alitalo, K. Vegfs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 2009, 21, 154–165. [Google Scholar] [CrossRef]
- Kim, H.; Kataru, R.P.; Koh, G.Y. Inflammation-associated lymphangiogenesis: A double-edged sword? J. Clin. Investig. 2014, 124, 936–942. [Google Scholar] [CrossRef]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in development and human disease. Nature 2005, 438, 946. [Google Scholar] [CrossRef]
- Zampell, J.C.; Yan, A.; Avraham, T.; Daluvoy, S.; Weitman, E.S.; Mehrara, B.J. Hif-1α coordinates lymphangiogenesis during wound healing and in response to inflammation. FASEB J. 2012, 26, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.N.; Kumar, V.; Abbas, A.K.; Fausto, N. Robbins and cotran pathologic basis of disease. Saun 2010, 2011, 260–262. [Google Scholar]
- Jiang, W.G.; Davies, G.; Martin, T.A.; Parr, C.; Watkins, G.; Mansel, R.E.; Mason, M.D. The potential lymphangiogenic effects of hepatocyte growth factor/scatter factor in vitro and in vivo. Int. J. Mol. Med. 2005, 16, 723–728. [Google Scholar] [PubMed]
- Lohela, M.; Saaristo, A.; Veikkola, T.; Alitalo, K. Lymphangiogenic growth factors, receptors and therapies. Thromb. Haemost. 2003, 90, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, K.; Hirakawa, S.; Ma, B.; Drinnenberg, I.; Detmar, M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005, 24, 2885–2895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, R.; Björndahl, M.A.; Gallego, M.I.; Chen, S.; Religa, P.; Hansen, A.J.; Cao, Y. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood 2006, 107, 3531–3536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naghshvar, F.; Torabizadeh, Z.; Moslemi Zadeh, N.; Mirbaha, H.; Gheshlaghi, P. Investigating the relationship between serum level of s-met (soluble hepatic growth factor receptor) and preeclampsia in the first and second trimesters of pregnancy. ISRN Obstet. Gynecol. 2013, 2013, 925062. [Google Scholar] [CrossRef]
- Gu, B.; Alexander, J.S.; Gu, Y.; Zhang, Y.; Lewis, D.F.; Wang, Y. Expression of lymphatic vascular endothelial hyaluronan receptor-1 (lyve-1) in the human placenta. Lymphat. Res. Biol. 2006, 4, 11–17. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Gu, Y.; Zhao, S.; Groome, L.J.; Alexander, J.S. D2-40/podoplanin expression in the human placenta. Placenta 2011, 32, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, Y.; Zhang, J.; Rao, M.; Liang, H.; Liu, G. The defect of both angiogenesis and lymphangiogenesis is involved in preeclampsia. Placenta 2015, 36, 279–286. [Google Scholar] [CrossRef]
- Cele, S.; Odun-Ayo, F.; Onyangunga, O.; Moodley, J.; Naicker, T. Analysis of hepatocyte growth factor immunostaining in the placenta of HIV-infected normotensive versus preeclamptic pregnant women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 227, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Platonova, N.; Miquel, G.; Regenfuss, B.; Taouji, S.; Cursiefen, C.; Chevet, E.; Bikfalvi, A. Evidence for the interaction of fibroblast growth factor-2 with the lymphatic endothelial cell marker lyve-1. Blood 2013, 121, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.; Russell, D. Blood and lymphatic vasculature in the ovary: Development, function and disease. Hum. Reprod. Update 2013, 20, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Jerman, L.F.; Hey-Cunningham, A.J. The role of the lymphatic system in endometriosis: A comprehensive review of the literature. Biol. Reprod. 2015, 92, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Red-Horse, K. Lymphatic vessel dynamics in the uterine wall. Placenta 2008, 29, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Ji, H.; Feng, N.; Zhang, Y.; Yang, X.; Andersson, P.; Sun, Y.; Tritsaris, K.; Hansen, A.J.; Dissing, S. Collaborative interplay between fgf-2 and vegf-c promotes lymphangiogenesis and metastasis. Proc. Natl. Acad. Sci. USA 2012, 109, 15894–15899. [Google Scholar] [CrossRef] [PubMed]
- Shange, G.P.; Moodley, J.; Naicker, T. Effect of vascular endothelial growth factors a, c, and d in HIV-associated pre-eclampsia. Hypertens. Pregnancy 2017, 36, 196–203. [Google Scholar] [CrossRef]
- Volchek, M.; Girling, J.E.; Lash, G.E.; Cann, L.; Kumar, B.; Robson, S.C.; Bulmer, J.N.; Rogers, P.A. Lymphatics in the human endometrium disappear during decidualization. Hum. Reprod. 2010, 25, 2455–2464. [Google Scholar] [CrossRef] [Green Version]
- Lely, A.T.; Salahuddin, S.; Holwerda, K.M.; Karumanchi, S.A.; Rana, S. Circulating lymphangiogenic factors in preeclampsia. Hypertens. Pregnancy 2013, 32, 42–49. [Google Scholar] [CrossRef]
- Onyangunga, O.A.; Moodley, J.; Merhar, V.; Ofusori, D.A.; Naicker, T. Lymphatic vascular endothelial hyaluronan receptor-1 immunoexpression in placenta of HIV infected pre-eclamptic women. J. Reprod. Immunol. 2016, 117, 81–88. [Google Scholar] [CrossRef]
- Spradley, F.T.; Palei, A.C.; Anderson, C.D.; Granger, J.P. Melanocortin-4 receptor deficiency attenuates placental ischemia-induced hypertension in pregnant rats. Hypertension 2019, 73, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Morfoisse, F.; Renaud, E.; Hantelys, F.; Prats, A.-C.; Garmy-Susini, B. Role of hypoxia and vascular endothelial growth factors in lymphangiogenesis. Mol. Cell. Oncol. 2014, 1, e29907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tal, R. The role of hypoxia and hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biol. Reprod. 2012, 87, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, J.; Kuzontkoski, P.M.; Zhu, W.; Li, D.Y.; Groopman, J.E. Slit2/robo4 signaling modulates HIV-1 gp120-induced lymphatic hyperpermeability. PLoS Path. 2012, 8, e1002461. [Google Scholar] [CrossRef]
- Caccuri, F.; Rueckert, C.; Giagulli, C.; Schulze, K.; Basta, D.; Zicari, S.; Marsico, S.; Cervi, E.; Fiorentini, S.; Slevin, M. HIV-1 matrix protein p17 promotes lymphangiogenesis and activates the endothelin-1/endothelin b receptor axis. Arterioscl. Thromb. Vasc. Biol. 2014, 34, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Basta, D.; Latinovic, O.; Lafferty, M.K.; Sun, L.; Bryant, J.; Lu, W.; Caccuri, F.; Caruso, A.; Gallo, R.; Garzino-Demo, A. Angiogenic, lymphangiogenic and adipogenic effects of HIV-1 matrix protein p17. Path. Dis. 2015, 73, ftv062. [Google Scholar] [CrossRef] [Green Version]
- Park, K.W.; Morrison, C.M.; Sorensen, L.K.; Jones, C.A.; Rao, Y.; Chien, C.-B.; Wu, J.Y.; Urness, L.D.; Li, D.Y. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev. Biol. 2003, 261, 251–267. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhang, X.; Kuzontkoski, P.M.; Jiang, S.; Zhu, W.; Li, D.Y.; Groopman, J.E. Slit2n and robo4 regulate lymphangiogenesis through the vegf-c/vegfr-3 pathway. Cell Commun. Sign. 2014, 12, 25. [Google Scholar] [CrossRef]
- Khaliq, O.P.; Murugesan, S.; Moodley, J.; Mackraj, I. Differential expression of mirnas are associated with the insulin signaling pathway in preeclampsia and gestational hypertension. Clin. Exp. Hypertens. 2018, 40, 744–751. [Google Scholar] [CrossRef]
- Marincowitz, C. The Effects of HIV-1-Proteins and Antiretroviral Therapy on Aortic Endothelial Cells (Aecs)—A Mechanistic In Vitro Approach. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2019. [Google Scholar]
- Song, L.; Ding, S.; Ge, Z.; Zhu, X.; Qiu, C.; Wang, Y.; Lai, E.; Yang, W.; Sun, Y.; Chow, S.A. Nucleoside/nucleotide reverse transcriptase inhibitors attenuate angiogenesis and lymphangiogenesis by impairing receptor tyrosine kinases signalling in endothelial cells. Br. J. Pharm. 2018, 175, 1241–1259. [Google Scholar] [CrossRef]
- Sansone, M.; Sarno, L.; Saccone, G.; Berghella, V.; Maruotti, G.M.; Migliucci, A.; Capone, A.; Martinelli, P. Risk of preeclampsia in human immunodeficiency virus–infected pregnant women. Obstet. Gynecol. 2016, 127, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Krischer, J.; Rutschmann, O.; Hirschel, B.; Vollenweider-Roten, S.; Saurat, J.-H.; Pechère, M. Regression of kaposi’s sarcoma during therapy with HIV-1 protease inhibitors: A prospective pilot study. J. Am. Acad. Dermatol. 1998, 38, 594–598. [Google Scholar] [CrossRef]
- Sgadari, C.; Barillari, G.; Toschi, E.; Carlei, D.; Bacigalupo, I.; Baccarini, S.; Palladino, C.; Leone, P.; Bugarini, R.; Malavasi, L. HIV protease inhibitors are potent anti-angiogenic molecules and promote regression of kaposi sarcoma. Nat. Med. 2002, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Filardi, P.P.; Paolillo, S.; Marciano, C.; Iorio, A.; Losco, T.; Marsico, F.; Scala, O.; Ruggiero, D.; Ferraro, S.; Chiariello, M. Cardiovascular effects of antiretroviral drugs: Clinical review. Cardiovasc. Haematol. Disord. Drug Targets. 2008, 8, 238–244. [Google Scholar] [CrossRef]
- Fiala, M.; Murphy, T.; MacDougall, J.; Yang, W.; Luque, A.; Iruela-Arispe, L.; Cashman, J.; Buga, G.; Byrns, R.E.; Barbaro, G. Haart drugs induce mitochondrial damage and intercellular gaps and gp 120 causes apoptosis. Cardiovasc. Toxicol. 2004, 4, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.-s.; Lu, X.-h.; Conklin, B.S.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. HIV protease inhibitor ritonavir induces cytotoxicity of human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1560–1566. [Google Scholar] [CrossRef]
- Chai, H.; Yang, H.; Yan, S.; Li, M.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Effects of 5 HIV protease inhibitors on vasomotor function and superoxide anion production in porcine coronary arteries. J. Acquir. Immune Defic. Syndr. 2005, 40, 12–19. [Google Scholar] [CrossRef]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative stress in preeclampsia and placental diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef]
- Torriani, F.J.; Komarow, L.; Parker, R.A.; Cotter, B.R.; Currier, J.S.; Dubé, M.P.; Fichtenbaum, C.J.; Gerschenson, M.; Mitchell, C.K.; Murphy, R.L. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The actg (AIDS clinical trials group) study 5152’s. J. Am. Coll. Cardiol. 2008, 52, 569–576. [Google Scholar] [CrossRef]
- Savvidou, M.; Samuel, M.; Akolekar, R.; Poulton, M.; Nicolaides, K. First trimester maternal uterine artery doppler examination in HIV—positive women. HIV Med. 2011, 12, 632–636. [Google Scholar] [CrossRef]
- Sebitloane, H.M.; Moodley, J.; Sartorius, B. Associations between HIV, highly active anti-retroviral therapy, and hypertensive disorders of pregnancy among maternal deaths in south africa 2011–2013. Int. J. Gynecol. Obstet. 2017, 136, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Dosiou, C.; Giudice, L.C. Natural killer cells in pregnancy and recurrent pregnancy loss: Endocrine and immunologic perspectives. Endocr. Rev. 2005, 26, 44–62. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Allan, D.S.; Bowen, M.; Powis, S.J.; Joseph, S.; Verma, S.; Hiby, S.E.; McMichael, A.J.; Loke, Y.W.; Braud, V.M. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual nk cells. Eur. J. Immunol. 2000, 30, 1623–1631. [Google Scholar] [CrossRef]
- Wu, J.; Lanier, L.L. Natural killer cells and cancer. Adv. Cancer Res. 2003, 90, 127–156. [Google Scholar] [PubMed]
- Cerwenka, A.; Lanier, L.L. Natural killer cells, viruses and cancer. Nat. Rev. Immunol. 2001, 1, 41. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Viswanathan, C. Natural killer cells: In health and disease. Hematol. Oncol. Stem Cell Ther. 2015, 8, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Wallace, A.E.; Fraser, R.; Cartwright, J.E. Extravillous trophoblast and decidual natural killer cells: A remodelling partnership. Hum. Reprod. Update 2012, 18, 458–471. [Google Scholar] [CrossRef]
- Smith, C.; Jalbert, E.; de Almeida, V.; Canniff, J.; Lenz, L.L.; Mussi-Pinhata, M.M.; Cohen, R.A.; Yu, Q.; Amaral, F.R.; Pinto, J.; et al. Altered natural killer cell function in HIV-exposed uninfected infants. Front. Immun. 2017, 8, 470. [Google Scholar] [CrossRef]
- Mela, C.M.; Goodier, M.R. The contribution of cytomegalovirus to changes in nk cell receptor expression in HIV-1–infected individuals. J. Infect. Dis. 2007, 195, 158–159. [Google Scholar] [CrossRef]
- Alter, G.; Altfeld, M. Nk cells in HIV-1 infection: Evidence for their role in the control of HIV-1 infection. J. Int. Med. 2009, 265, 29–42. [Google Scholar] [CrossRef]
- Sharma, S. Natural killer cells and regulatory t cells in early pregnancy loss. Int. J. Dev. Biol. 2014, 58, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.-W.; Alfirevic, Z.; Quenby, S. Natural killer cells and pregnancy outcomes in women with recurrent miscarriage and infertility: A systematic review. Hum. Reprod. 2011, 26, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, V.; Dolati, S.; Hosseini, A.; Gharibi, T.; Danaii, S.; Yousefi, M. Natural killer t cells in preeclampsia: An updated review. Biomed. Pharmacother. 2017, 95, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Kottilil, S. Natural killer cells in HIV-1 infection: Role of nk cell-mediated non-cytolytic mechanisms in pathogenesis of HIV-1 infection. Indian J. Exp. Biol. 2003, 41, 1219–1225. [Google Scholar] [PubMed]
- Valentin, A.; Rosati, M.; Patenaude, D.J.; Hatzakis, A.; Kostrikis, L.G.; Lazanas, M.; Wyvill, K.M.; Yarchoan, R.; Pavlakis, G.N. Persistent HIV-1 infection of natural killer cells in patients receiving highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2002, 99, 7015–7020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaëlsson, J.; Long, B.R.; Loo, C.P.; Lanier, L.L.; Spotts, G.; Hecht, F.M.; Nixon, D.F. Immune reconstitution of cd56dim nk cells in individuals with primary HIV-1 infection treated with interleukin-2. J. Infect. Dis. 2008, 197, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ballan, W.M.; Vu, B.-A.N.; Long, B.R.; Loo, C.P.; Michaëlsson, J.; Barbour, J.D.; Lanier, L.L.; Wiznia, A.A.; Abadi, J.; Fennelly, G.J.; et al. Natural killer cells in perinatally HIV-1-infected children exhibit less degranulation compared to HIV-1-exposed uninfected children and their expression of KIR2DL3, NKG2C, and NKP46 correlates with disease severity. J. Immun. 2007, 179, 3362–3370. [Google Scholar] [CrossRef]
- Frias, M.; Rivero-Juarez, A.; Gordon, A.; Camacho, A.; Cantisan, S.; Cuenca-Lopez, F.; Torre-Cisneros, J.; Peña, J.; Rivero, A. Persistence of pathological distribution of nk cells in HIV-infected patients with prolonged use of haart and a sustained immune response. PLoS ONE 2015, 10, e0121019. [Google Scholar] [CrossRef]
- Bachmayer, N.; Sohlberg, E.; Sundström, Y.; Hamad, R.R.; Berg, L.; Bremme, K.; Sverremark-Ekström, E. Women with pre-eclampsia have an altered NKG2A and NKG2C receptor expression on peripheral blood natural killer cells. Am. J. Reprod. Immunol. 2009, 62, 147–157. [Google Scholar] [CrossRef]
- Laresgoiti-Servitje, E.; Gómez-López, N.; Olson, D.M. An immunological insight into the origins of pre-eclampsia. Hum. Reprod. Update 2010, 16, 510–524. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Wang, H.; Wang, Z.; Huang, H.; Dong, M. Elevated serum levels of interleukin-15 and interleukin-16 in preeclampsia. J. Reprod. Immunol. 2007, 73, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Fiore, S.; Newell, M.-L.; Trabattoni, D.; Thorne, C.; Gray, L.; Savasi, V.; Tibaldi, C.; Ferrazzi, E.; Clerici, M. Antiretroviral therapy-associated modulation of th1 and th2 immune responses in HIV-infected pregnant women. J. Reprod. Immunol. 2006, 70, 143–150. [Google Scholar] [CrossRef]
- Phoswa, W.N.; Naicker, T.; Ramsuran, V.; Moodley, J. Pre-eclampsia: the role of highly active antiretroviral therapy and immune markers. Inflamm. Res. 2019, 68, 47–57. [Google Scholar] [CrossRef]
- Maharaj, N.R.; Phulukdaree, A.; Nagiah, S.; Ramkaran, P.; Tiloke, C.; Chuturgoon, A.A. Pro-inflammatory cytokine levels in HIV infected and uninfected pregnant women with and without preeclampsia. PLoS ONE 2017, 12, e0170063. [Google Scholar] [CrossRef] [PubMed]
- Machado, E.S.; Krauss, M.R.; Megazzini, K.; Coutinho, C.M.; Kreitchmann, R.; Melo, V.H.; Pilotto, J.H.; Ceriotto, M.; Hofer, C.B.; Siberry, G.K. Hypertension, preeclampsia and eclampsia among HIV-infected pregnant women from latin america and caribbean countries. J. Infect. 2014, 68, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Nakashima, A.; Shima, T.; Ito, M. Th1/th2/th17 and regulatory t-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 2010, 63, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Darmochwal-Kolarz, D.; Kludka-Sternik, M.; Tabarkiewicz, J.; Kolarz, B.; Rolinski, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. The predominance of th17 lymphocytes and decreased number and function of treg cells in preeclampsia. J. Reprod. Immunol. 2012, 93, 75–81. [Google Scholar] [CrossRef]
- Al-Nafea, H.M.; Hamdy, N.M.; Aref, N.M. Evaluation of interleukin 17 level as a prognostic marker in active antiviral treated human immunodeficiency virus in saudi patients. Am. J. Biochem. 2017, 7, 13–22. [Google Scholar]
- Campillo-Gimenez, L.; Cumont, M.-C.; Fay, M.; Kared, H.; Monceaux, V.; Diop, O.; Müller-Trutwin, M.; Hurtrel, B.; Lévy, Y.; Zaunders, J. AIDS progression is associated with the emergence of il-17–producing cells early after simian immunodeficiency virus infection. J. Immunol. 2010, 184, 984–992. [Google Scholar] [CrossRef]
- Terness, P.; Kallikourdis, M.; Betz, A.G.; Rabinovich, G.A.; Saito, S.; Clark, D.A. Tolerance signaling molecules and pregnancy: Ido, galectins, and the renaissance of regulatory t cells. Am. J. Reprod. Immunol. 2007, 58, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Shima, T.; Nakashima, A.; Shiozaki, A.; Ito, M.; Sasaki, Y. What is the role of regulatory t cells in the success of implantation and early pregnancy? J. Assist. Reprod. Genet. 2007, 24, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Shiozaki, A.; Sasaki, Y.; Nakashima, A.; Shima, T.; Ito, M. Seminars in immunopathology. In Regulatory t Cells and Regulatory Natural Killer (nk) Cells Play Important Roles in Feto-Maternal Tolerance; Arck, P.C., Elkon, K.B., Hasler, P., Miyazaki, T., Eds.; Springer: Berlin, Germany, 2007; pp. 115–122. [Google Scholar]
- Sasaki, Y.; Darmochwal-Kolarz, D.; Suzuki, D.; Sakai, M.; Ito, M.; Shima, T.; Shiozaki, A.; Rolinski, J.; Saito, S. Proportion of peripheral blood and decidual cd4+ cd25bright regulatory t cells in pre-eclampsia. Clin. Exp. Immunol. 2007, 149, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Boasso, A.; Nilsson, J.; Zhang, R.; Shire, N.J.; Lindback, S.; Shearer, G.M.; Chougnet, C.A. Cutting edge: The prevalence of regulatory t cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J. Immunol. 2005, 174, 3143–3147. [Google Scholar] [CrossRef] [PubMed]
- Suchard, M.S.; Mayne, E.; Green, V.A.; Shalekoff, S.; Donninger, S.L.; Stevens, W.S.; Gray, C.M.; Tiemessen, C.T. Foxp3 expression is upregulated in cd4+ t cells in progressive HIV-1 infection and is a marker of disease severity. PLoS ONE 2010, 5, e11762. [Google Scholar] [CrossRef] [PubMed]
- Eggena, M.P.; Barugahare, B.; Jones, N.; Okello, M.; Mutalya, S.; Kityo, C.; Mugyenyi, P.; Cao, H. Depletion of regulatory t cells in HIV infection is associated with immune activation. J. Immunol. 2005, 174, 4407–4414. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Balado, M.M.; Martínez-Bonet, M.; Rosado, I.; Ruiz-Mateos, E.; Méndez-Lagares, G.; Rodríguez-Méndez, M.M.; Vidal, F.; Muñoz-Fernández, M.A.; Pacheco, Y.M.; Leal, M. Maraviroc reduces the regulatory t-cell frequency in antiretroviral-naive HIV-infected subjects. J. Infect. Dis. 2014, 210, 890–898. [Google Scholar] [CrossRef]
- Montes, M.; Sanchez, C.; Lewis, D.E.; Graviss, E.A.; Seas, C.; Gotuzzo, E.; White, A.C., Jr. Normalization of foxp3+ regulatory t cells in response to effective antiretroviral therapy. J. Inf. Dis. 2010, 203, 496–499. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naicker, T.; Phoswa, W.N.; Onyangunga, O.A.; Gathiram, P.; Moodley, J. Angiogenesis, Lymphangiogenesis, and the Immune Response in South African Preeclamptic Women Receiving HAART. Int. J. Mol. Sci. 2019, 20, 3728. https://doi.org/10.3390/ijms20153728
Naicker T, Phoswa WN, Onyangunga OA, Gathiram P, Moodley J. Angiogenesis, Lymphangiogenesis, and the Immune Response in South African Preeclamptic Women Receiving HAART. International Journal of Molecular Sciences. 2019; 20(15):3728. https://doi.org/10.3390/ijms20153728
Chicago/Turabian StyleNaicker, Thajasvarie, Wendy N. Phoswa, Onankoy A. Onyangunga, Premjith Gathiram, and Jagidesa Moodley. 2019. "Angiogenesis, Lymphangiogenesis, and the Immune Response in South African Preeclamptic Women Receiving HAART" International Journal of Molecular Sciences 20, no. 15: 3728. https://doi.org/10.3390/ijms20153728
APA StyleNaicker, T., Phoswa, W. N., Onyangunga, O. A., Gathiram, P., & Moodley, J. (2019). Angiogenesis, Lymphangiogenesis, and the Immune Response in South African Preeclamptic Women Receiving HAART. International Journal of Molecular Sciences, 20(15), 3728. https://doi.org/10.3390/ijms20153728




