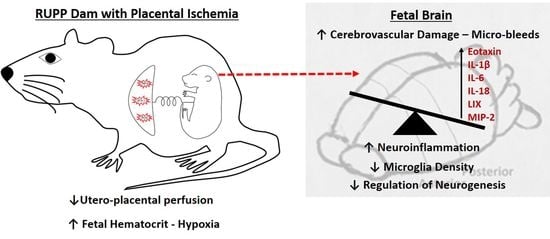
Perinatal Micro-Bleeds and Neuroinflammation in E19 Rat Fetuses Exposed to Utero-Placental Ischemia
Abstract
:1. Introduction
2. Results
2.1. General Characteristics and Pregnancy Outcomes:
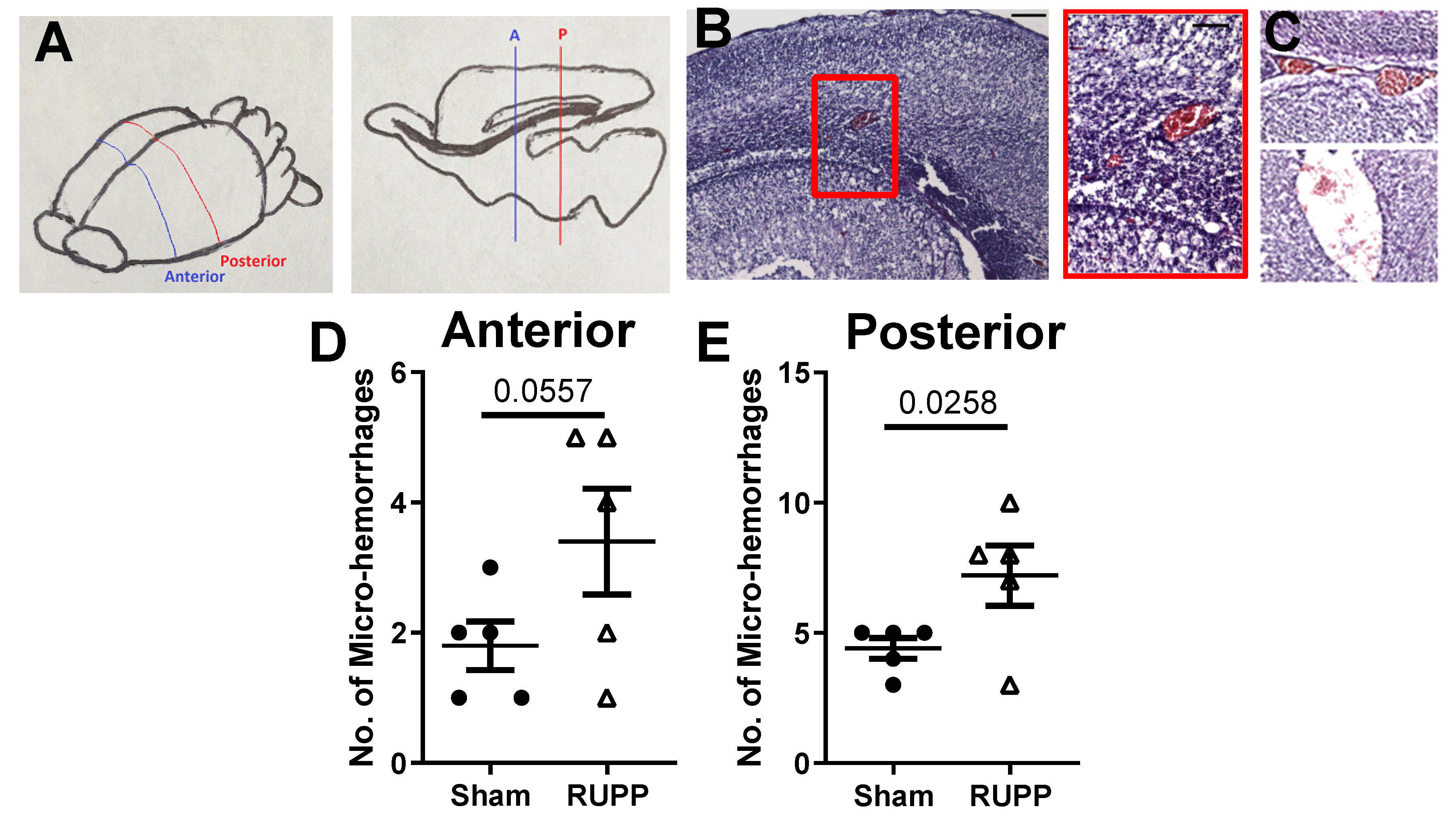
2.2. Micro-Hemorrhage in Fetal Brains
2.3. Inflammatory Profile in Fetal Brains
2.4. Microglia Changes in Fetal Brains
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Placental Ischemia Induction
4.3. Carotid Surgery and Blood Pressure Measurement
4.4. Harvest and Collection of Tissues
4.5. Micro-Bleed Detection
4.6. Fetal Brain Multiplex Array
4.7. Analysis of Microglia
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PE | Preeclampsia |
| RUPP | Reduced Uterine Perfusion Pressure |
| SVZ | Sub-ventricular zone |
| GD | Gestational day |
| BBB | Blood-brain barrier |
References
- Roberts, J.M.; August, P.A.; Bakris, G.; Barton, J.R.; Bernstein, I.M.; Druzin, M.; Gaiser, R.R.; Granger, J.P.; Jeyabalan, A.; Johnson, D.D.; et al. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obs. Gynecol 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Many, A.; Fattal, A.; Leitner, Y.; Kupferminc, M.J.; Harel, S.; Jaffa, A. Neurodevelopmental and cognitive assessment of children born growth restricted to mothers with and without preeclampsia. Hypertens Pregnancy 2003, 22, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Rätsep, M.T.; Hickman, A.F.; Maser, B.; Pudwell, J.; Smith, G.N.; Brien, D.; Stroman, P.W.; Adams, M.A.; Reynolds, J.N.; Croy, B.A.; et al. Impact of preeclampsia on cognitive function in the offspring. Behav. Brain Res. 2016, 302, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Moreno, E.; Fischi-Gomez, E.; Batalle, D.; Borradori-Tolsa, C.; Eixarch, E.; Thiran, J.P.; Gratacós, E.; Hüppi, P.S. Structural Brain Network Reorganization and Social Cognition Related to Adverse Perinatal Condition from Infancy to Early Adolescence. Front. Neurosci. 2016, 10, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Chen, P.; Bo, T.; Luo, K. Cognitive and Behavioral Outcomes of Intrauterine Growth Restriction School-Age Children. Pediatrics 2016, 137. [Google Scholar] [CrossRef] [PubMed]
- Tuovinen, S.; Aalto-Viljakainen, T.; Eriksson, J.G.; Kajantie, E.; Lahti, J.; Pesonen, A.K.; Heinonen, K.; Lahti, M.; Osmond, C.; Barker, D.J.; et al. Maternal hypertensive disorders during pregnancy: Adaptive functioning and psychiatric and psychological problems of the older offspring. BJOG 2014, 121, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Raikkonen, K.; Kajantie, E.; Pesonen, A.K.; Heinonen, K.; Alastalo, H.; Leskinen, J.T.; Nyman, K.; Henriksson, M.; Lahti, J.; Lahti, M.; et al. Early life origins cognitive decline: Findings in elderly men in the Helsinki Birth Cohort Study. PLoS ONE 2013, 8, e54707. [Google Scholar] [CrossRef] [PubMed]
- Kaukola, T.; Räsänen, J.; Herva, R.; Patel, D.D.; Hallman, M. Suboptimal neurodevelopment in very preterm infants is related to fetal cardiovascular compromise in placental insufficiency. Am. J. Obs. Gynecol. 2005, 193, 414–420. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Chaemsaithong, P.; Yeo, L.; Romero, R. Pre-eclampsia part 1: Current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014, 10, 466–480. [Google Scholar] [CrossRef]
- Lo, J.O.; Roberts, V.H.J.; Schabel, M.C.; Wang, X.; Morgan, T.K.; Liu, Z.; Studholme, C.; Kroenke, C.D.; Frias, A.E. Novel Detection of Placental Insufficiency by Magnetic Resonance Imaging in the Nonhuman Primate. Reprod. Sci. 2018, 25, 64–73. [Google Scholar] [CrossRef]
- Maher, G.M.; O'Keeffe, G.W.; Kearney, P.M.; Kenny, L.C.; Dinan, T.G.; Mattsson, M.; Khashan, A.S. Association of Hypertensive Disorders of Pregnancy With Risk of Neurodevelopmental Disorders in Offspring: A Systematic Review and Meta-analysis. Jama Psychiatry 2018, 75, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Dachew, B.A.; Mamun, A.; Maravilla, J.C.; Alati, R. Pre-eclampsia and the risk of autism-spectrum disorder in offspring: Meta-analysis. Br. J. Psychiatry 2018, 212, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.K.; Krakowiak, P.; Baker, A.; Hansen, R.L.; Ozonoff, S.; Hertz-Picciotto, I. Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. Jama Pediatr 2015, 169, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Curran, E.A.; O'Keeffe, G.W.; Looney, A.M.; Moloney, G.; Hegarty, S.V.; Murray, D.M.; Khashan, A.S.; Kenny, L.C. Exposure to Hypertensive Disorders of Pregnancy Increases the Risk of Autism Spectrum Disorder in Affected Offspring. Mol. Neurobiol 2018, 55, 5557–5564. [Google Scholar] [CrossRef] [PubMed]
- Nahum Sacks, K.; Friger, M.; Shoham-Vardi, I.; Sergienko, R.; Spiegel, E.; Landau, D.; Sheiner, E. Long-term neuropsychiatric morbidity in children exposed prenatally to preeclampsia. Early Hum. Dev. 2019, 130, 96–100. [Google Scholar] [CrossRef]
- Parks, W.T. Manifestations of Hypoxia in the Second and Third Trimester Placenta. Birth Defects Res. 2017, 109, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Harati-Sadegh, M.; Kohan, L.; Teimoori, B.; Mehrabani, M.; Salimi, S. The association of the placental Hypoxia-inducible factor1-alpha polymorphisms and HIF1-alpha mRNA expression with preeclampsia. Placenta 2018, 67, 31–37. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Weng, Z.; Zhang, S.; Ning, H.; Li, B. Expression and role of microRNA 18b and hypoxia inducible factor-1alpha in placental tissues of preeclampsia patients. Exp. Med. 2017, 14, 4554–4560. [Google Scholar] [CrossRef]
- Alexander, B.T.; Kassab, S.E.; Miller, M.T.; Abram, S.R.; Reckelhoff, J.F.; Bennett, W.A.; Granger, J.P. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 2001, 37, 1191–1195. [Google Scholar] [CrossRef]
- LaMarca, B.B.; Bennett, W.A.; Alexander, B.T.; Cockrell, K.; Granger, J.P. Hypertension produced by reductions in uterine perfusion in the pregnant rat: Role of tumor necrosis factor-alpha. Hypertension 2005, 46, 1022–1025. [Google Scholar] [CrossRef]
- Gadonski, G.; LaMarca, B.B.; Sullivan, E.; Bennett, W.; Chandler, D.; Granger, J.P. Hypertension produced by reductions in uterine perfusion in the pregnant rat: Role of interleukin 6. Hypertension 2006, 48, 711–716. [Google Scholar] [CrossRef]
- Gilbert, J.S.; Gilbert, S.A.; Arany, M.; Granger, J.P. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension 2009, 53, 399–403. [Google Scholar] [CrossRef]
- Gilbert, J.S.; Babcock, S.A.; Granger, J.P. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 2007, 50, 1142–1147. [Google Scholar] [CrossRef]
- Warrington, J.P.; Fan, F.; Murphy, S.R.; Roman, R.J.; Drummond, H.A.; Granger, J.P.; Ryan, M.J. Placental ischemia in pregnant rats impairs cerebral blood flow autoregulation and increases blood-brain barrier permeability. Physiol. Rep. 2014, 2. [Google Scholar] [CrossRef]
- Warrington, J.P.; Drummond, H.A.; Granger, J.P.; Ryan, M.J. Placental Ischemia-induced Increases in Brain Water Content and Cerebrovascular Permeability: Role of TNFα. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1425–R1431. [Google Scholar] [CrossRef]
- Granger, J.P.; LaMarca, B.B.; Cockrell, K.; Sedeek, M.; Balzi, C.; Chandler, D.; Bennett, W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol. Med. 2006, 122, 383–392. [Google Scholar]
- LaMarca, B.; Wallukat, G.; Llinas, M.; Herse, F.; Dechend, R.; Granger, J.P. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension 2008, 52, 1168–1172. [Google Scholar] [CrossRef]
- Chhor, V.; Moretti, R.; Le Charpentier, T.; Sigaut, S.; Lebon, S.; Schwendimann, L.; Oré, M.V.; Zuiani, C.; Milan, V.; Josserand, J.; et al. Role of microglia in a mouse model of paediatric traumatic brain injury. Brain Behav. Immun. 2017, 63, 197–209. [Google Scholar] [CrossRef]
- Ahn, S.J.; Anrather, J.; Nishimura, N.; Schaffer, C.B. Diverse Inflammatory Response After Cerebral Microbleeds Includes Coordinated Microglial Migration and Proliferation. Stroke 2018, 49, 1719–1726. [Google Scholar] [CrossRef]
- Menassa, D.A.; Gomez-Nicola, D. Microglial Dynamics During Human Brain Development. Front. Immunol. 2018, 9, 1014. [Google Scholar] [CrossRef] [Green Version]
- Dalmau, I.; Finsen, B.; Tønder, N.; Zimmer, J.; González, B.; Castellano, B. Development of microglia in the prenatal rat hippocampus. J. Comp. Neurol. 1997, 377, 70–84. [Google Scholar] [CrossRef]
- Luo, L.; Chen, D.; Qu, Y.; Wu, J.; Li, X.; Mu, D. Association between hypoxia and perinatal arterial ischemic stroke: A meta-analysis. PLoS ONE 2014, 9, e90106. [Google Scholar] [CrossRef]
- Lee, J.; Croen, L.A.; Backstrand, K.H.; Yoshida, C.K.; Henning, L.H.; Lindan, C.; Ferriero, D.M.; Fullerton, H.J.; Barkovich, A.J.; Wu, Y.W. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA 2005, 293, 723–729. [Google Scholar] [CrossRef]
- Wu, Y.W.; March, W.M.; Croen, L.A.; Grether, J.K.; Escobar, G.J.; Newman, T.B. Perinatal stroke in children with motor impairment: A population-based study. Pediatrics 2004, 114, 612–619. [Google Scholar] [CrossRef]
- Li, J.; Lamarca, B.; Reckelhoff, J.F. A Model of Pre-eclampsia in Rats: The Reduced Uterine Perfusion Pressure (RUPP) Model. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1–H8. [Google Scholar]
- Martinez-Ramirez, S.; Greenberg, S.M.; Viswanathan, A. Cerebral microbleeds: Overview and implications in cognitive impairment. Alzheimers Res. 2014, 6, 33. [Google Scholar] [CrossRef]
- Molvarec, A.; Szarka, A.; Walentin, S.; Beko, G.; Karádi, I.; Prohászka, Z.; Rigó, J. Serum leptin levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in normal pregnancy and preeclampsia. Reprod. Biol. Endocrinol. 2011, 9, 124. [Google Scholar] [CrossRef]
- Szarka, A.; Rigó, J.; Lázár, L.; Beko, G.; Molvarec, A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010, 11, 59. [Google Scholar] [CrossRef]
- Darmochwal-Kolarz, D.; Kludka-Sternik, M.; Tabarkiewicz, J.; Kolarz, B.; Rolinski, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J. Reprod. Immunol. 2012, 93, 75–81. [Google Scholar] [CrossRef]
- Toldi, G.; Rigó, J.; Stenczer, B.; Vásárhelyi, B.; Molvarec, A. Increased prevalence of IL-17-producing peripheral blood lymphocytes in pre-eclampsia. Am. J. Reprod. Immunol. 2011, 66, 223–229. [Google Scholar] [CrossRef]
- Martínez-García, E.A.; Chávez-Robles, B.; Sánchez-Hernández, P.E.; Núñez-Atahualpa, L.; Martín-Máquez, B.T.; Muñoz-Gómez, A.; González-López, L.; Gámez-Nava, J.I.; Salazar-Páramo, M.; Dávalos-Rodríguez, I.; et al. IL-17 increased in the third trimester in healthy women with term labor. Am. J. Reprod. Immunol. 2011, 65, 99–103. [Google Scholar] [CrossRef]
- Warrington, J.P. Placental ischemia increases seizure susceptibility and cerebrospinal fluid cytokines. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef]
- LaMarca, B.B.; Cockrell, K.; Sullivan, E.; Bennett, W.; Granger, J.P. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension 2005, 46, 82–86. [Google Scholar] [CrossRef]
- Amash, A.; Holcberg, G.; Sapir, O.; Huleihel, M. Placental secretion of interleukin-1 and interleukin-1 receptor antagonist in preeclampsia: Effect of magnesium sulfate. J. Interferon Cytokine Res. 2012, 32, 432–441. [Google Scholar] [CrossRef]
- Anthony, D.; Dempster, R.; Fearn, S.; Clements, J.; Wells, G.; Perry, V.H.; Walker, K. CXC chemokines generate age-related increases in neutrophil-mediated brain inflammation and blood-brain barrier breakdown. Curr. Biol. 1998, 8, 923–926. [Google Scholar] [CrossRef]
- Miwa, K.; Tanaka, M.; Okazaki, S.; Furukado, S.; Sakaguchi, M.; Kitagawa, K. Relations of blood inflammatory marker levels with cerebral microbleeds. Stroke 2011, 42, 3202–3206. [Google Scholar] [CrossRef]
- Gu, Y.; Gutierrez, J.; Meier, I.B.; Guzman, V.A.; Manly, J.J.; Schupf, N.; Brickman, A.M.; Mayeux, R. Circulating inflammatory biomarkers are related to cerebrovascular disease in older adults. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e521. [Google Scholar] [CrossRef]
- Theriault, B.C.; Woo, S.K.; Karimy, J.K.; Keledjian, K.; Stokum, J.A.; Sarkar, A.; Coksaygan, T.; Ivanova, S.; Gerzanich, V.; Simard, J.M. Cerebral microbleeds in a neonatal rat model. PLoS ONE 2017, 12, e0171163. [Google Scholar] [CrossRef]
- Zhang, L.W.; Warrington, J.P. Magnesium Sulfate Prevents Placental Ischemia-Induced Increases in Brain Water Content and Cerebrospinal Fluid Cytokines in Pregnant Rats. Front. Neurosci. 2016, 10, 561. [Google Scholar] [CrossRef]
- Parajuli, B.; Horiuchi, H.; Mizuno, T.; Takeuchi, H.; Suzumura, A. CCL11 enhances excitotoxic neuronal death by producing reactive oxygen species in microglia. Glia 2015, 63, 2274–2284. [Google Scholar] [CrossRef]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Shigemoto-Mogami, Y.; Hoshikawa, K.; Goldman, J.E.; Sekino, Y.; Sato, K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J. Neurosci. 2014, 34, 2231–2243. [Google Scholar] [CrossRef]
- Xiong, X.Y.; Liu, L.; Yang, Q.W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol. 2016, 142, 23–44. [Google Scholar] [CrossRef]
- Fernández-López, D.; Faustino, J.; Klibanov, A.L.; Derugin, N.; Blanchard, E.; Simon, F.; Leib, S.L.; Vexler, Z.S. Microglial Cells Prevent Hemorrhage in Neonatal Focal Arterial Stroke. J. Neurosci. 2016, 36, 2881–2893. [Google Scholar] [CrossRef] [Green Version]
- Thion, M.S.; Ginhoux, F.; Garel, S. Microglia and early brain development: An intimate journey. Science 2018, 362, 185–189. [Google Scholar] [CrossRef] [Green Version]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giambrone, A.B.; Logue, O.C.; Shao, Q.; Bidwell, G.L., III; Warrington, J.P. Perinatal Micro-Bleeds and Neuroinflammation in E19 Rat Fetuses Exposed to Utero-Placental Ischemia. Int. J. Mol. Sci. 2019, 20, 4051. https://doi.org/10.3390/ijms20164051
Giambrone AB, Logue OC, Shao Q, Bidwell GL III, Warrington JP. Perinatal Micro-Bleeds and Neuroinflammation in E19 Rat Fetuses Exposed to Utero-Placental Ischemia. International Journal of Molecular Sciences. 2019; 20(16):4051. https://doi.org/10.3390/ijms20164051
Chicago/Turabian StyleGiambrone, Ashtin B., Omar C. Logue, Qingmei Shao, Gene L. Bidwell, III, and Junie P. Warrington. 2019. "Perinatal Micro-Bleeds and Neuroinflammation in E19 Rat Fetuses Exposed to Utero-Placental Ischemia" International Journal of Molecular Sciences 20, no. 16: 4051. https://doi.org/10.3390/ijms20164051
APA StyleGiambrone, A. B., Logue, O. C., Shao, Q., Bidwell, G. L., III, & Warrington, J. P. (2019). Perinatal Micro-Bleeds and Neuroinflammation in E19 Rat Fetuses Exposed to Utero-Placental Ischemia. International Journal of Molecular Sciences, 20(16), 4051. https://doi.org/10.3390/ijms20164051