A Zinpyr-1-based Fluorimetric Microassay for Free Zinc in Human Serum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Excitation and Emission of Zinpyr-1
2.2. Optimization of Incubation Time for F
2.3. Optimization of Incubation Time, Chelator Type and Concentration for Fmin
2.4. Optimization of Incubation Time and Zinc Concentration for Fmax
2.5. Zinpyr-1 Concentration
2.6. Saturation of the Fluorescent Probe
2.7. Determination of Free Zinc in Human Serum Samples
3. Materials and Methods
3.1. Materials
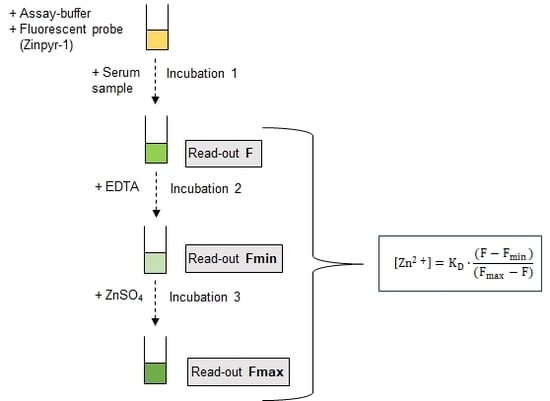
3.2. Assay Principle
3.3. Optimization of Assay Parameters
3.4. Application of the Final Assay Procedure
3.5. Determination of Zinc, Iron, Copper, Manganese and Selenium by Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| EDTA | Ethylenediaminetetraacetic acid |
| FA2 | fatty acid binding site 2 |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| HS | human AB serum |
| ICP-MS | inductively coupled plasma mass spectrometry |
| MBS | Multi-Metal Binding Site |
| NTS | N-terminal site |
| TPEN | N,N,N′,N′-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine |
References
- Prasad, A.S. Importance of Zinc in Human Nutrition. Am. J. Clin. Nutr. 1967, 20, 648–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, J.E. Zinc Proteins: Enzymes, storage proteins, transcription factors, and replication proteins. Annu. Rev. Biochem. 1992, 61, 897–946. [Google Scholar] [CrossRef] [PubMed]
- Maares, M.; Haase, H. Zinc and immunity: An essential interrelation. Arch. Biochem. Biophys. 2016, 611, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, J.L.; Bowen, C.N. Zinc deficiency and toxicity in pediatric practice. Curr. Opin. Pediatrics 2014, 26, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, M.J.; Zubillaga, M.; Lysionek, A.; Sarabia, M.I.; Caro, R.; De Paoli, T.; Hager, A.; Weill, R.; Boccio, J. Zinc as an essential micronutrient: A review. Nutr. Res. 2000, 20, 737–755. [Google Scholar] [CrossRef]
- Hess, S.Y.; Peerson, J.M.; King, J.C.; Brown, K.H. Use of serum zinc concentration as an indicator of population zinc status. Food Nutr. Bull. 2007, 28, S403–S429. [Google Scholar] [CrossRef] [PubMed]
- Chilvers, D.C.; Dawson, J.B.; Bahreyni-Toosi, M.H.; Hodgkinson, A. Identification and determination of copper and zinc protein complexes in blood plasma after chromatographic separation on DEAE-Sepharose CL-6B. Analyst 1984, 109, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Giroux, E.L.; Durieux, M.; Schechter, P.J. A study of zinc distribution in human serum. Bioinorg. Chem. 1976, 5, 211–218. [Google Scholar] [CrossRef]
- Foote, J.W.; Delves, H.T. Albumin bound and alpha 2-macroglobulin bound zinc concentrations in the sera of healthy adults. J. Clin. Pathol. 1984, 37, 1050–1054. [Google Scholar] [CrossRef]
- Prasad, A.S.; Oberleas, D. Binding of zinc to amino acids and serum proteins in vitro. J. Lab. Clin. Med. 1970, 76, 416–425. [Google Scholar]
- Giroux, E.L.; Henkin, R.I. Competition for zinc among serum albumin and amino acids. Biochim. et Biophys. Acta—Gen. Subj. 1972, 273, 64–72. [Google Scholar] [CrossRef]
- Zalewski, P.; Truong-Tran, A.; Lincoln, S.; Ward, D.; Shankar, A.; Coyle, P.; Jayaram, L.; Copley, A.; Grosser, D.; Murgia, C.; et al. Use of a zinc fluorophore to measure labile pools of zinc in body fluids and cell-conditioned media. Bio Tech. 2006, 40, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Bozym, R.A.; Chimienti, F.; Giblin, L.J.; Gross, G.W.; Korichneva, I.; Li, Y.; Libert, S.; Maret, W.; Parviz, M.; Frederickson, C.J.; et al. Free zinc ions outside a narrow concentration range are toxic to a variety of cells in vitro. Exp. Biol. Med. 2010, 235, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Driessen, C.; Hirv, K.; Kirchner, H.; Rink, L. Zinc regulates cytokine induction by superantigens and lipopolysaccharide. Immunology 1995, 84, 272–277. [Google Scholar] [PubMed]
- Haase, H.; Hebel, S.; Engelhardt, G.; Rink, L. The biochemical effects of extracellular Zn 2+ and other metal ions are severely affected by their speciation in cell culture media. Metallomics 2015, 7, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Bornhorst, J.; Kipp, A.P.; Haase, H.; Meyer, S.; Schwerdtle, T. The crux of inept biomarkers for risks and benefits of trace elements. Trends Anal. Chem. 2018, 104, 183–190. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Walkup, G.K.; Burdette, S.C.; Lippard, S.J.; Tsien, R.Y. A New cell-permeable fluorescent probe for Zn2+. J. Am. Chem. Soc. 2000, 122, 5644–5645. [Google Scholar] [CrossRef]
- Radford, R.J.; Lippard, S.J. Chelators for investigating zinc metalloneurochemistry. Curr. Opin. Chem. Biol. 2013, 17, 129–136. [Google Scholar] [CrossRef]
- Dineley, K.E.; Malaiyandi, L.M.; Reynolds, I.J. A reevaluation of neuronal zinc measurements: Artifacts associated with high intracellular dye concentration. Mol. Pharmacol. 2002, 62, 618. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J. Biol Inorg. Chem. 2006, 11, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Foote, J.W.; Delves, H.T. Determination of non-protein-bound zinc in human serum using ultrafiltration and atomic absorption spectrometry with electrothermal atomisation. Analyst 1988, 109, 709–711. [Google Scholar] [CrossRef]
- Bloxam, D.L.; Tan, J.C.Y.; Parkinson, C.E. Non-protein bound zinc concentration in human plasma and amniotic fluid measured by ultrafiltration. Clin. Chim. Acta 1984, 144, 81–93. [Google Scholar] [CrossRef]
- Magneson, G.R.; Puvathingal, J.M.; Ray, W.J. The concentrations of free Mg2+ and free Zn2+ in equine blood plasma. J. Biol. Chem. 1987, 262, 11140–11148. [Google Scholar] [PubMed]
- Zhang, P.; Allen, J.C. A Novel Dialysis Procedure Measuring Free Zn2+ in Bovine Milk and Plasma. J. Nutr. 1995, 125, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.; Mathew, J.; Kohler, J.E.; Blass, A.L.; Soybel, D.I. Redistribution of labile plasma zinc during mild surgical stress in the rat. Transl. Res. 2011, 157, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Hoeger, J.; Simon, T.-P.; Doemming, S.; Thiele, C.; Marx, G.; Schuerholz, T.; Haase, H. Alterations in zinc binding capacity, free zinc levels and total serum zinc in a porcine model of sepsis. Biometals 2015, 28, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Stewart, A.J.; Sleep, D.; Sadler, P.J.; Pinheiro, T.J.T.; Blindauer, C.A. A molecular mechanism for modulating plasma Zn speciation by fatty acids. J. Am. Chem. Soc. 2012, 134, 1454–1457. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef] [Green Version]
- Besecker, B.Y.; Exline, M.C.; Hollyfield, J.; Phillips, G.; DiSilvestro, R.A.; Wewers, M.D.; Knoell, D.L. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission123. Am. J. Clin. Nutr. 2011, 93, 1356–1364. [Google Scholar] [CrossRef]
- Wessels, I.; Cousins, R.J. Zinc dyshomeostasis during polymicrobial sepsis in mice involves zinc transporter Zip14 and can be overcome by zinc supplementation. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G768–778. [Google Scholar] [CrossRef]
- Lindeman, R.D.; Clark, M.L.; Colmore, J.P. Influence of Age and Sex on Plasma and Red-Cell Zinc Concentrations. J. Gerontol. 1971, 26, 358–363. [Google Scholar] [CrossRef]
- Hotz, C.; Peerson, J.M.; Brown, K.H. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: Reanalysis of the second National Health and Nutrition Examination Survey data (1976–1980). Am. J. Clin. Nutr. 2003, 78, 756–764. [Google Scholar] [CrossRef]
- Quinn, J.F.; Harris, C.; Kaye, J.A.; Lind, B.; Carter, R.; Anekonda, T.; Ralle, M. Gender effects on plasma and brain copper. Int. J. Alzheimer’s Dis. 2011, 2011, 150916. [Google Scholar] [CrossRef]
- Marques, A.G.; Sarni, R.O.S.; Lopes, L.A.; Lopes, E.; Amancio, O.M.S. Erythrocyte zinc and serum copper in male and female adolescents according to puberty stage at different growth phases. Nutrire 2016, 41, 9. [Google Scholar] [CrossRef]
- Hall, A.C.; Young, B.W.; Bremner, I. Intestinal metallothionein and the mutual antagonism between copper and zinc in the rat. J. Inorg. Biochem. 1979, 11, 57–66. [Google Scholar] [CrossRef]
- Bal, W.; Sokołowska, M.; Kurowska, E.; Faller, P. Binding of transition metal ions to albumin: Sites, affinities and rates. Biochim. et Biophys. Acta—Gen. Subj. 2013, 1830, 5444–5455. [Google Scholar] [CrossRef]
- Burdette, S.C.; Walkup, G.K.; Spingler, B.; Tsien, R.Y.; Lippard, S.J. Fluorescent sensors for Zn2+ based on a fluorescein platform: synthesis, properties and intracellular distribution. J. Am. Chem. Soc. 2001, 123, 7831–7841. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar]
- Meyer, S.; Markova, M.; Pohl, G.; Marschall, T.A.; Pivovarova, O.; Pfeiffer, A.F.H.; Schwerdtle, T. Development, validation and application of an ICP-MS/MS method to quantify minerals and (ultra-)trace elements in human serum. J. Trace Elem. Med. Biol. 2018, 49, 157–163. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alker, W.; Schwerdtle, T.; Schomburg, L.; Haase, H. A Zinpyr-1-based Fluorimetric Microassay for Free Zinc in Human Serum. Int. J. Mol. Sci. 2019, 20, 4006. https://doi.org/10.3390/ijms20164006
Alker W, Schwerdtle T, Schomburg L, Haase H. A Zinpyr-1-based Fluorimetric Microassay for Free Zinc in Human Serum. International Journal of Molecular Sciences. 2019; 20(16):4006. https://doi.org/10.3390/ijms20164006
Chicago/Turabian StyleAlker, Wiebke, Tanja Schwerdtle, Lutz Schomburg, and Hajo Haase. 2019. "A Zinpyr-1-based Fluorimetric Microassay for Free Zinc in Human Serum" International Journal of Molecular Sciences 20, no. 16: 4006. https://doi.org/10.3390/ijms20164006
APA StyleAlker, W., Schwerdtle, T., Schomburg, L., & Haase, H. (2019). A Zinpyr-1-based Fluorimetric Microassay for Free Zinc in Human Serum. International Journal of Molecular Sciences, 20(16), 4006. https://doi.org/10.3390/ijms20164006