Molecular Targets of Aspirin and Prevention of Preeclampsia and Their Potential Association with Circulating Extracellular Vesicles during Pregnancy
Abstract
:1. Introduction
2. Physiology of Pregnancy
3. Pathogenesis of Preeclampsia
4. Pharmacology of Aspirin and Basis for Its Use in PE
5. Low Dose Aspirin (LDA) and Pregnancy
6. Effects of LDA on Placental and Maternal Body System Function
7. Complications of LDA for Fetuses and Mothers
8. Predictive Biomarkers for Preeclampsia Cases Treated with Low Dose Aspirin
- (i)
- Maternal serum concentrations of placental growth factor (PlGF) level are generally low in preeclampsia.
- (ii)
- (iii)
- Normotension in the first trimester is associated with reduced risk of PE [169].
- (iv)
- In a randomized controlled clinical trial conducted by Asemi Z. et al., low dose aspirin (80 mg) was administered with calcium supplementation (500 mg) in pregnant women who were at risk for PE. The treatment was continued for nine consecutive weeks before measuring high sensitivity C-reactive protein (hs-CRP), total antioxidant capacity (TAC), total glutathione (GSH) in plasma and serum glucose and insulin level. The study showed a significant difference in serum hs-CRP level and increased levels of plasma TAC and total GSH in pregnant women at risk for preeclampsia as compared to those that took placebo (did not receive any treatment), but serum insulin levels were not affected at all [170].
9. Extracellular Vesicles (EVs)
10. Extracellular Vesicles/Exosomes in Normal and Preeclamptic Pregnancies
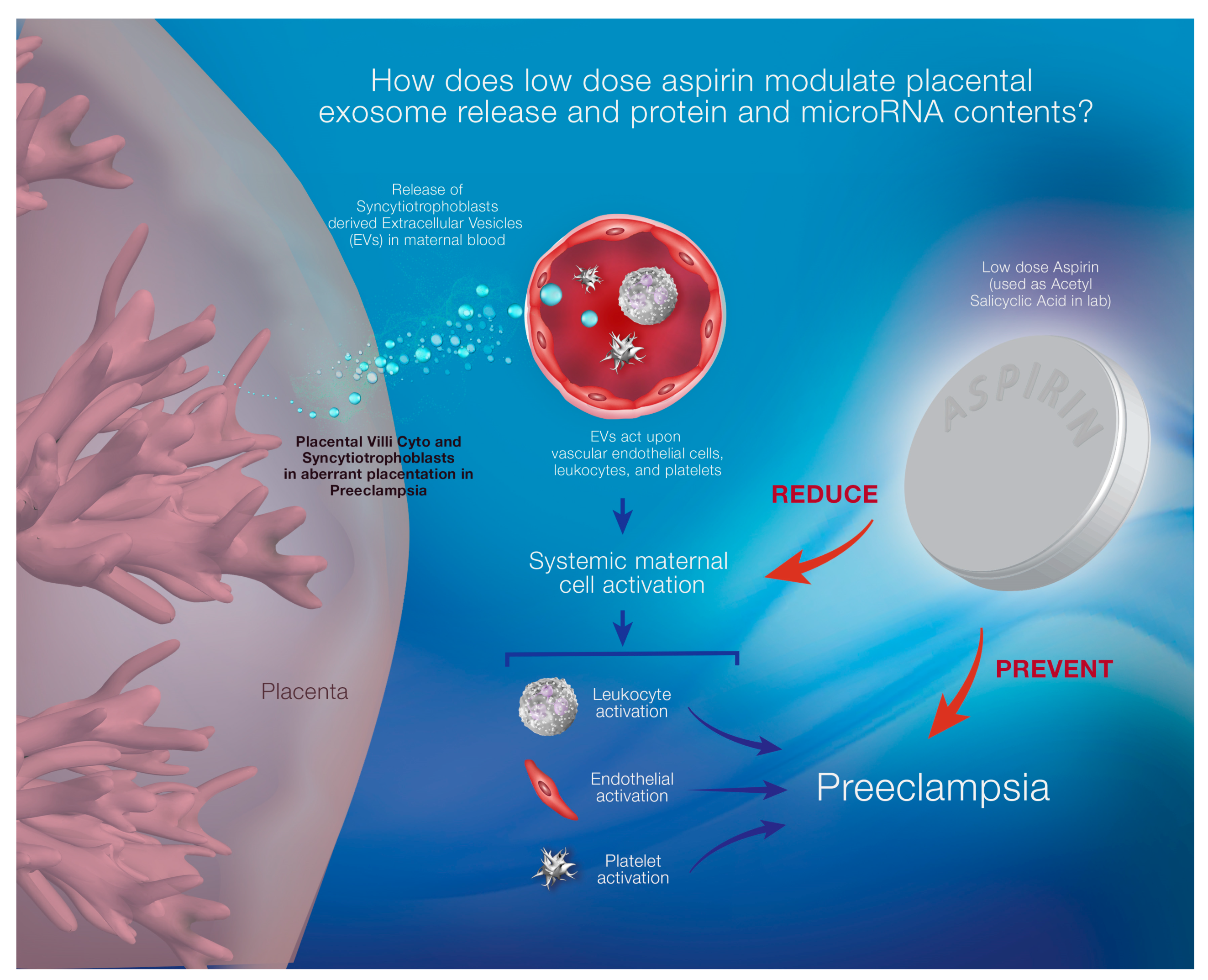
11. Effects of LDA on Exosomal Secretion
12. Summary
Funding
Conflicts of Interest
References
- Mendes, S. New Insights into the Process of Placentation and the Role of Oxidative Uterine Microenvironment. Oxid. Med. Cell. Longev. 2019, 2019, 9174521. [Google Scholar] [CrossRef]
- Solano, M.E. Decidual immune cells: Guardians of human pregnancies. Best Pr. Res. Clin. Obs. Gynaecol. 2019. [Google Scholar] [CrossRef]
- Burton, G.J. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, 12381. [Google Scholar] [CrossRef]
- Bose, P. Heparin and aspirin attenuate placental apoptosis in vitro: Implications for early pregnancy failure. Am. J. Obstet. Gynecol. 2005, 192, 23–30. [Google Scholar] [CrossRef]
- Panagodage, S. Low-Dose Acetylsalicylic Acid Treatment Modulates the Production of Cytokines and Improves Trophoblast Function in an in Vitro Model of Early-Onset Preeclampsia. Am. J. Pathol. 2016, 186, 3217–3224. [Google Scholar] [CrossRef] [Green Version]
- Villa, P.M.; Kajantie, E.; Laivuori, H. Acetylsalicylic acid and prevention of preeclampsia. Duodecim 2014, 130, 243–250. [Google Scholar]
- Oyola, S.; Kirley, K. Another good reason to recommend Low-Dose Aspirin. J. Fam. Pract. 2015, 64, 301–303. [Google Scholar]
- Yao, S.; Wu, H.; Yu, Y. Early intervention with aspirin for preventing preeclampsia in high-risk women: A meta-analysis. Nan Fang Yi Ke Da Xue Xue Bao 2015, 35, 868–873. [Google Scholar]
- Andreoli, L.; Bertsias, G.K.; Agmon-Levin, N.; Brown, S.; Cervera, R.; Costedoat-Chalumeau, N.; Doria, A.; Fischer-Betz, R.; Forger, F.; Moraes-Fontes, M.F.; et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann. Rheum. Dis. 2016, 76, 476–485. [Google Scholar] [CrossRef]
- Wang, L. Efficacy evaluation of low-dose aspirin in IVF/ICSI patients evidence from 13 RCTs: A systematic review and meta-analysis. Medicine (Baltimore) 2017, 96, e7720. [Google Scholar] [CrossRef]
- Bujold, E. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: A meta-analysis. Obstet. Gynecol. 2010, 116, 402–414. [Google Scholar] [CrossRef]
- Villa, P.M. Aspirin in the prevention of pre-eclampsia in high-risk women: A randomised placebo-controlled PREDO Trial and a meta-analysis of randomised trials. Bjog 2013, 120, 64–74. [Google Scholar] [CrossRef]
- Fantasia, H.C. Low-Dose Aspirin for the Prevention of Preeclampsia. Nurs. Womens Health 2018, 22, 87–92. [Google Scholar] [CrossRef]
- Roberge, S. Early administration of low-dose aspirin for the prevention of severe and mild preeclampsia: A systematic review and meta-analysis. Am. J. Perinatol. 2012, 29, 551–556. [Google Scholar] [CrossRef]
- Xu, T.T.; Zhou, F.; Deng, C.Y.; Huang, G.Q.; Li, J.K.; Wang, X.D. Low-Dose Aspirin for Preventing Preeclampsia and Its Complications: A Meta-Analysis. J. Clin. Hypertens (Greenwich) 2015, 17, 567–573. [Google Scholar] [CrossRef]
- Ahrens, K.A. Complications and Safety of Preconception Low-Dose Aspirin Among Women WITH Prior Pregnancy Losses. Obstet. Gynecol. 2016, 127, 689–698. [Google Scholar] [CrossRef]
- Cronqvist, T. Syncytiotrophoblast derived extracellular vesicles transfer functional placental miRNAs to primary human endothelial cells. Sci. Rep. 2017, 7, 4558. [Google Scholar] [CrossRef] [Green Version]
- Holcberg, G. Implantation, Physiology of Placentation. In Recurrent Pregnancy Loss; Springer International Publishing: Basel, Switzerland, 2016. [Google Scholar]
- Haller-Kikkatalo, K. Autoimmune activation toward embryo implantation is rare in immune-privileged human endometrium. Semin. Reprod. Med. 2014, 32, 376–384. [Google Scholar] [CrossRef]
- Moser, G.; Huppertz, B. Implantation and extravillous trophoblast invasion: From rare archival specimens to modern biobanking. Placenta 2017, 56, 19–26. [Google Scholar] [CrossRef]
- Sato, Y.; Fujiwara, H.; Konishi, I. Role of platelets in placentation. Med. Mol. Morphol. 2010, 43, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S. Predominant basal directional release of thromboxane, but not prostacyclin, by placental trophoblasts from normal and preeclamptic pregnancies. Placenta 2008, 29, 81–88. [Google Scholar] [CrossRef]
- Mierzynski, R. Anticoagulant therapy in pregnant patients with metabolic syndrome: A review. Curr. Pharm. Biotechnol. 2014, 15, 47–63. [Google Scholar] [CrossRef]
- Graves, J.C.; Miller, K.E.; Sellers, A.D. Maternal serum triple analyte screening in pregnancy. Am. Fam. Physician 2002, 65, 915–920. [Google Scholar]
- Maternal Serum Marker Screening. In Understanding Genetics; Genetic Alliance: Washington, DC, USA, 2010.
- Nwanodi, O.B. Preeclampsia-Eclampsia Adverse Outcomes Reduction: The Preeclampsia-Eclampsia Checklist. Healthcare 2016, 4, 26. [Google Scholar] [CrossRef]
- Helou, A. Management of pregnancies complicated by hypertensive disorders of pregnancy: Could we do better? Aust. N. Z. J. Obstet. Gynaecol. 2016, 57, 253–269. [Google Scholar] [CrossRef]
- Pai, C.H. Lack of Thromboxane Synthase Prevents Hypertension and Fetal Growth Restriction after High Salt Treatment during Pregnancy. PLoS ONE 2016, 11, e0151617. [Google Scholar] [CrossRef]
- Bakhti, A.; Vaiman, D. Prevention of gravidic endothelial hypertension by aspirin treatment administered from the 8th week of gestation. Hypertens Res. 2011, 34, 1116–1120. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, E. Clinical risk factors for pre-eclampsia determined in early pregnancy: Systematic review and meta-analysis of large cohort studies. BMJ 2016, 353, 1753. [Google Scholar] [CrossRef]
- Nicolaides, K.H. A model for a new pyramid of prenatal care based on the 11 to 13 weeks’ assessment. Prenat. Diagn. 2011, 31, 3–6. [Google Scholar] [CrossRef]
- McCoy, S.; Baldwin, K. Pharmacotherapeutic options for the treatment of preeclampsia. Am. J. Health Syst. Pharm. 2009, 66, 337–344. [Google Scholar] [CrossRef]
- Gilani, S.I. Preeclampsia and Extracellular Vesicles. Curr. Hypertens. Rep. 2016, 18, 1–11. [Google Scholar] [CrossRef]
- Burton, G.J. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef]
- Friedman, A.M.; Cleary, K.L. Prediction and prevention of ischemic placental disease. Semin. Perinatol. 2014, 38, 177–182. [Google Scholar] [CrossRef]
- Cuckle, H.; von Dadelszen, P.; Ghidini, A. Current controversies in prenatal diagnosis 4: Pregnancy complications due to placental vascular disease (pre-eclampsia, FGR): Are we ready for prevention? Prenat. Diagn. 2013, 33, 17–20. [Google Scholar] [CrossRef]
- Armant, D.R. Human trophoblast survival at low oxygen concentrations requires metalloproteinase-mediated shedding of heparin-binding EGF-like growth factor. Development 2006, 133, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Redman, C.W.; Sargent, I.L. Placental stress and pre-eclampsia: A revised view. Placenta 2009, 30, S38–S42. [Google Scholar] [CrossRef]
- Blumenstein, M. A proteomic approach identifies early pregnancy biomarkers for preeclampsia: Novel linkages between a predisposition to preeclampsia and cardiovascular disease. Proteomics 2009, 9, 2929–2945. [Google Scholar] [CrossRef]
- Auer, J. Serum profile in preeclampsia and intra-uterine growth restriction revealed by iTRAQ technology. J. Proteom. 2010, 73, 1004–1017. [Google Scholar] [CrossRef]
- Chelbi, S.T. Expressional and epigenetic alterations of placental serine protease inhibitors: SERPINA3 is a potential marker of preeclampsia. Hypertension 2007, 49, 76–83. [Google Scholar] [CrossRef]
- Larsen, T.G. Fetal human leukocyte antigen-C and maternal killer-cell immunoglobulin-like receptors in cases of severe preeclampsia. Placenta 2019, 75, 27–33. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L. Placental debris, oxidative stress and pre-eclampsia. Placenta 2000, 21, 597–602. [Google Scholar] [CrossRef]
- Furuya, M. Pathophysiology of placentation abnormalities in pregnancy-induced hypertension. Vasc. Health Risk Manag. 2008, 4, 1301–1313. [Google Scholar] [CrossRef]
- Granger, J.P. Pathophysiology of preeclampsia: Linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation 2002, 9, 147–160. [Google Scholar] [CrossRef]
- Lyall, F.; Myatt, L. The role of the placenta in pre-eclampsia--a workshop report. Placenta 2002, 23, 142–145. [Google Scholar] [CrossRef]
- Lockwood, C.J. Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. Am. J. Pathol. 2008, 172, 1571–1579. [Google Scholar] [CrossRef]
- Benyo, D.F. Expression of inflammatory cytokines in placentas from women with preeclampsia. J. Clin. Endocrinol. Metab. 2001, 86, 2505–2512. [Google Scholar]
- Raghupathy, R. Cytokines as key players in the pathophysiology of preeclampsia. Med. Princ. Pr. 2013, 22, 8–19. [Google Scholar] [CrossRef]
- Vaughan, J.E.; Walsh, S.W.; Ford, G.D. Thromboxane mediates neutrophil superoxide production in pregnancy. Am. J. Obstet. Gynecol. 2006, 195, 1415–1420. [Google Scholar] [CrossRef]
- Walsh, S.W. Eicosanoids in preeclampsia. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 223–232. [Google Scholar] [CrossRef]
- Walsh, S.W. Preeclampsia: An imbalance in placental prostacyclin and thromboxane Production. Am. J. Obstet. Gynecol. 1985, 152, 335–340. [Google Scholar] [CrossRef]
- Reslan, O.M.; Khalil, R.A. Molecular and vascular targets in the pathogenesis and management of the hypertension associated with preeclampsia. Cardiovasc. Hematol. Agents Med. Chem. 2010, 8, 204–226. [Google Scholar] [CrossRef]
- Yusuf, K. Thromboxane A2 Limits Differentiation and Enhances Apoptosis of Cultured Human Trophoblasts. Pediatr. Res. 2001, 50, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Ally, A.I.; Horrobin, D.F. Thromboxane A2 in blood vessel walls and its physiological significance: Relevance to thrombosis and hypertension. Prostaglandins Med. 1980, 4, 431–438. [Google Scholar] [CrossRef]
- Sellers, M.M.; Stallone, J.N. Sympathy for the devil: The role of thromboxane in the regulation of vascular tone and blood pressure. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1978–H1986. [Google Scholar] [CrossRef]
- Gilbert, J.S. Pathophysiology of hypertension during preeclampsia: Linking placental ischemia with endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H541–H550. [Google Scholar] [CrossRef]
- Downing, J.W. Hypothesis: Selective phosphodiesterase-5 inhibition improves outcome in preeclampsia. Med. Hypotheses 2004, 63, 1057–1064. [Google Scholar] [CrossRef]
- Euser, A.G. Low-dose aspirin for pre-eclampsia prevention in twins with elevated human chorionic gonadotropin. J. Perinatol. 2016, 36, 601–605. [Google Scholar] [CrossRef] [Green Version]
- Di Simone, N. Pathogenic role of anti-beta2-glycoprotein I antibodies on human placenta: Functional effects related to implantation and roles of heparin. Hum. Reprod. Update 2007, 13, 189–196. [Google Scholar] [CrossRef]
- Anderson, U.D. First trimester prediction of preeclampsia. Curr. Hypertens Rep. 2015, 17, 584. [Google Scholar] [CrossRef]
- Meiri, H. Prediction of preeclampsia by placental protein 13 and background risk factors and its prevention by aspirin. J. Perinat. Med. 2014, 42, 591–601. [Google Scholar] [CrossRef]
- Seravalli, V. Relationship between first-trimester serum placental protein-13 and maternal characteristics, placental Doppler studies and pregnancy outcome. J. Perinat. Med. 2016, 44, 543–549. [Google Scholar] [CrossRef]
- Rolnik, D.L. Maternal plasma cell-free DNA in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2015, 45, 106–111. [Google Scholar] [CrossRef]
- Schrör, K. Acetylsalicylic Acid. In Acetylsalicylic Acid; John Wiley and Sons: Weinheim, Germany, 2010. [Google Scholar]
- Cadavid, A.P. Aspirin: The Mechanism of Action Revisited in the Context of Pregnancy Complications. Front. Immunol. 2017, 8, 261. [Google Scholar] [Green Version]
- Lafont, O. From the willow to aspirin. Rev. Hist. Pharm. (Paris) 2007, 55, 209–216. [Google Scholar] [CrossRef]
- Botting, R.M. Vane’s discovery of the mechanism of action of aspirin changed our understanding of its clinical pharmacology. Pharm. Rep. 2010, 62, 518–525. [Google Scholar] [CrossRef]
- West, G.B. Aspirin and the prostaglandins. Chem. Drug 1972, 198, 196–197. [Google Scholar]
- Finkel, R.M.A.C.; Luigi, X. (Eds.) Antiinflammatory Drugs and Autacoids in Lippincott’s Illustrated Reviews: Pharmacology, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009; p. 499. [Google Scholar]
- Walsh, S.W. Low-dose aspirin: Treatment for the imbalance of increased thromboxane and decreased prostacyclin in preeclampsia. Am. J. Perinatol. 1989, 6, 124–132. [Google Scholar] [CrossRef]
- Perneby, C. Thromboxane Metab. Excretion Dur. Pregnancy--Influ. Preeclampsia Aspirin Treat. Thromb. Res. 2011, 127, 605–606. [Google Scholar] [CrossRef]
- Sibai, B.M. Low-dose aspirin in pregnancy. Obstet. Gynecol. 1989, 74, 551–557. [Google Scholar] [CrossRef]
- Li, C. Aspirin inhibits expression of sFLT1 from human cytotrophoblasts induced by hypoxia, via cyclo-oxygenase 1. Placenta 2015, 36, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Costa, F. 274: Low-dose aspirin improves trophoblastic function in early-onset pre-eclampsia. Am. J. Obstet. Gynecol. 2014, 210, S145. [Google Scholar] [CrossRef]
- Gil-Villa, A.M. Aspirin triggered-lipoxin A4 reduces the adhesion of human polymorphonuclear neutrophils to endothelial cells initiated by preeclamptic plasma. Prostaglandins Leukot. Essent. Fat. Acids 2012, 87, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Sentilhes, L.; Azria, E.; Schmitz, T. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N. Engl. J. Med. 2017, 377, 2399–2400. [Google Scholar]
- Navaratnam, K.; Alfirevic, A.; Alfirevic, Z. Low dose aspirin and pregnancy: How important is aspirin resistance? Bjog 2016, 123, 1481–1487. [Google Scholar] [CrossRef]
- Bujold, E. Low-dose aspirin reduces morbidity and mortality in pregnant women at high-risk for preeclampsia. Evid. Based Nurs. 2015, 18, 71. [Google Scholar] [CrossRef]
- Sibai, B.M. Therapy: Low-dose aspirin to reduce the risk of pre-eclampsia? Nat. Rev. Endocrinol. 2015, 11, 6–8. [Google Scholar] [CrossRef]
- Henderson, J.T.; Whitlock, E.P.; O’Connor, E. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia. Ann. Intern. Med. 2014, 160, 695–703. [Google Scholar] [CrossRef]
- Roberge, S.; Demers, S.; Bujold, E. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia. Ann. Intern. Med. 2014, 161, 613. [Google Scholar] [CrossRef]
- Bartsch, E. Risk threshold for starting low dose aspirin in pregnancy to prevent preeclampsia: An opportunity at a low cost. PLoS ONE 2015, 10, e0116296. [Google Scholar] [CrossRef]
- Toyoda, K. Antithrombotic therapy for pregnant women. Neurol. Med. Chir. (Tokyo) 2013, 53, 526–530. [Google Scholar] [CrossRef]
- Yurdakok, M. Fetal and neonatal effects of anticoagulants used in pregnancy: A review. Turk. J. Pediatr. 2012, 54, 207–215. [Google Scholar]
- Askie, L.M. Antiplatelet agents for prevention of pre-eclampsia: A meta-analysis of individual patient data. Lancet 2007, 369, 1791–1798. [Google Scholar] [CrossRef]
- Roberge, S. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: Systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 110–120. [Google Scholar] [CrossRef]
- Summaries for patients: Aspirin to prevent preeclampsia-related complications and death: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 161, I28. [CrossRef]
- Roberge, S.; Demers, S.; Bujold, E. Initiation of aspirin in early gestation for the prevention of pre-eclampsia. Bjog 2013, 120, 773–774. [Google Scholar] [CrossRef]
- Ortved, D. Cost-effectiveness of first-trimester screening with early preventative use of aspirin in women at high risk of early-onset pre-eclampsia. Ultrasound Obstet. Gynecol. 2019, 53, 239–244. [Google Scholar] [CrossRef]
- LeFevre, M.L. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 161, 819–826. [Google Scholar] [CrossRef]
- Bond, S. US Preventive Services Task Force guideline supports low-dose aspirin for prevention of preeclampsia. J. Midwifery Womens Health 2015, 60, 222–223. [Google Scholar] [CrossRef]
- Sidaway, P. Pre-eclampsia: Low-dose aspirin for pre-eclampsia. Nat. Rev. Nephrol. 2014, 10, 613. [Google Scholar]
- Voelker, R. USPSTF: Low-dose aspirin may help reduce risk of preeclampsia. JAMA 2014, 311, 2055. [Google Scholar] [CrossRef]
- Ayala, D.E.; Ucieda, R.; Hermida, R.C. Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiol. Int. 2013, 30, 260–279. [Google Scholar] [CrossRef]
- Mutlu, I. Effects of anticoagulant therapy on pregnancy outcomes in patients with thrombophilia and previous poor obstetric history. Blood Coagul. Fibrinolysis 2015, 26, 267–273. [Google Scholar] [CrossRef]
- Dodd, J.M. Antithrombotic therapy for improving maternal or infant health outcomes in women considered at risk of placental dysfunction. Cochrane Database Syst. Rev. 2013, 24, Cd006780. [Google Scholar] [CrossRef]
- Chauleur, C. News on antithrombotic therapy and pregnancy. Therapie 2011, 66, 437–443. [Google Scholar] [CrossRef]
- De Vries, J.I. Low-molecular-weight heparin added to aspirin in the prevention of recurrent early-onset pre-eclampsia in women with inheritable thrombophilia: The FRUIT-RCT. J. Thromb. Haemost. 2012, 10, 64–72. [Google Scholar] [CrossRef]
- Gris, J.C. Addition of enoxaparin to aspirin for the secondary prevention of placental vascular complications in women with severe pre-eclampsia. The pilot randomised controlled NOH-PE trial. Thromb. Haemost. 2011, 106, 1053–1061. [Google Scholar]
- Rodger, M.A. Low-molecular-weight heparin and recurrent placenta-mediated pregnancy complications: A meta-analysis of individual patient data from randomised controlled trials. Lancet 2016, 388, 2629–2641. [Google Scholar] [CrossRef]
- Souza, E.V. Aspirin plus calcium supplementation to prevent superimposed preeclampsia: A randomized trial. Braz. J. Med. Biol. Res. 2014, 47, 419–425. [Google Scholar] [CrossRef]
- Browne, J.L. Prevention of Hypertensive Disorders of Pregnancy: A Novel Application of the Polypill Concept. Curr. Cardiol. Rep. 2016, 18, 1–11. [Google Scholar] [CrossRef]
- Grandone, E.; Villani, M.; Tiscia, G.L. Aspirin and heparin in pregnancy. Expert Opin. Pharm. 2015, 16, 1793–1803. [Google Scholar] [CrossRef]
- Ghesquiere, L. Can we prevent preeclampsia? Presse. Med. 2016, 45, 403–413. [Google Scholar]
- Kane, S.C.; Da Silva Costa, F.; Brennecke, S.P. New directions in the prediction of pre-eclampsia. Aust. N. Z. J. Obstet. Gynaecol. 2014, 54, 101–107. [Google Scholar] [CrossRef]
- Jiang, N. The effect of calcium channel blockers on prevention of preeclampsia in pregnant women with chronic hypertension. Clin. Exp. Obstet. Gynecol. 2015, 42, 79–81. [Google Scholar]
- Neykova, K. Antithrombotic Medication in Pregnant Women with Previous Intrauterine Growth Restriction. Akush Ginekol. (Sofiia) 2016, 55, 3–9. [Google Scholar]
- Maged, A.M. The role of prophylactic use of low dose aspirin and calheparin in patients with unexplained recurrent abortion. Gynecol. Endocrinol. 2016, 32, 970–972. [Google Scholar] [CrossRef]
- Gan, J.; He, H.; Qi, H. Preventing preeclampsia and its fetal complications with low-dose aspirin in East Asians and non-East Asians: A systematic review and meta-analysis. Hypertens Pregnancy 2016, 35, 426–435. [Google Scholar] [CrossRef]
- Tong, S.; Mol, B.W.; Walker, S.P. Preventing preeclampsia with aspirin: Does dose or timing matter? Am. J. Obstet. Gynecol. 2017, 216, 95–97. [Google Scholar] [CrossRef]
- Mone, F. Should we recommend universal aspirin for all pregnant women? Am. J. Obstet. Gynecol. 2017, 216, e1–e141. [Google Scholar] [CrossRef]
- Meher, S. Antiplatelet therapy before or after 16 weeks’ gestation for preventing preeclampsia: An individual participant data meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 121–128. [Google Scholar] [CrossRef]
- Roberge, S. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: A meta-analysis. Ultrasound Obstet. Gynecol. 2013, 41, 491–499. [Google Scholar] [CrossRef]
- O’Gorman, N. Study protocol for the randomised controlled trial: Combined multimarker screening and randomised patient treatment with ASpirin for evidence-based PREeclampsia prevention (ASPRE). BMJ Open 2016, 6, e011801. [Google Scholar] [CrossRef]
- Etwel, F.; Koren, G. When positive studies of novel therapies are subsequently nullified: Cumulative meta-analyses in preeclampsia. Clin. Investig. Med. 2015, 38, 274–283. [Google Scholar] [CrossRef]
- Truong, A. Subchorionic hematomas are increased in early pregnancy in women taking low-dose aspirin. Fertil. Steril. 2016, 105, 1241–1246. [Google Scholar] [CrossRef] [Green Version]
- Van Hoorn, M.E. Low-molecular-weight heparin and aspirin in the prevention of recurrent early-onset pre-eclampsia in women with antiphospholipid antibodies: The FRUIT-RCT. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 197, 168–173. [Google Scholar] [CrossRef]
- Hoffman, M.K. A description of the methods of the aspirin supplementation for pregnancy indicated risk reduction in nulliparas (ASPIRIN) study. BMC Pregnancy Childbirth 2017, 17, 135. [Google Scholar] [CrossRef]
- Mumford, S.L. Expanded findings from a randomized controlled trial of preconception low-dose aspirin and pregnancy loss. Hum. Reprod. 2016, 31, 657–665. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O’Gorman, N.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar] [CrossRef]
- Orendi, K. Effects of vitamins C and E, acetylsalicylic acid and heparin on fusion, beta-hCG and PP13 expression in BeWo cells. Placenta 2010, 31, 431–438. [Google Scholar] [CrossRef]
- Rigourd, V. Re-evaluation of the role of STOX1 transcription factor in placental development and preeclampsia. J. Reprod. Immunol. 2009, 82, 174–181. [Google Scholar] [CrossRef]
- Benigni, A. Effect of low-dose aspirin on fetal and maternal generation of thromboxane by platelets in women at risk for pregnancy-induced hypertension. N. Engl. J. Med. 1989, 321, 357–362. [Google Scholar] [CrossRef]
- Schiff, E. The use of aspirin to prevent pregnancy-induced hypertension and lower the ratio of thromboxane A2 to prostacyclin in relatively high risk pregnancies. N. Engl. J. Med. 1989, 321, 351–356. [Google Scholar] [CrossRef]
- Sibai, B.M. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N. Engl. J. Med. 1993, 329, 1213–1218. [Google Scholar] [CrossRef]
- Knight, M. Antiplatelet agents for preventing and treating pre-eclampsia. Cochrane Database Syst. Rev. 2000, Cd000492. [Google Scholar] [CrossRef]
- Woodworth, S.H. Eicosanoid biosynthetic enzymes in placental and decidual tissues from preeclamptic pregnancies: Increased expression of thromboxane-A2 synthase gene. J. Clin. Endocrinol. Metab. 1994, 78, 1225–1231. [Google Scholar]
- Katsi, V. Aspirin vs Heparin for the Prevention of Preeclampsia. Curr. Hypertens Rep. 2016, 18, 57. [Google Scholar] [CrossRef]
- Pijnenborg, R. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980, 1, 3–19. [Google Scholar] [CrossRef]
- Brosens, I. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef]
- Jamal, A.; Milani, F.; Al-Yasin, A. Evaluation of the effect of metformin and aspirin on utero placental circulation of pregnant women with PCOS. Iran. J. Reprod. Med. 2012, 10, 265–270. [Google Scholar]
- Turan, O. 284: Starting aspirin (ASA) in the first trimester (T1) promotes placental invasion in low-risk pregnancy. Am. J. Obstet. Gynecol. 2014, 210, S149–S150. [Google Scholar] [CrossRef]
- Haapsamo, M.; Martikainen, H.; Rasanen, J. Low-dose aspirin reduces uteroplacental vascular impedance in early and mid gestation in IVF and ICSI patients: A randomized, placebo-controlled double-blind study. Ultrasound Obstet. Gynecol. 2008, 32, 687–693. [Google Scholar] [CrossRef]
- Serhan, C.N.; Yacoubian, S.; Yang, R. Anti-inflammatory and proresolving lipid mediators. Annu. Rev. Pathol. 2008, 3, 279–312. [Google Scholar] [CrossRef]
- Nascimento-Silva, V. Aspirin-triggered lipoxin A4 blocks reactive oxygen species generation in endothelial cells: A novel antioxidative mechanism. Thromb. Haemost. 2007, 97, 88–98. [Google Scholar]
- Cezar-de-Mello, P.F. ATL-1, an analogue of aspirin-triggered lipoxin A4, is a potent inhibitor of several steps in angiogenesis induced by vascular endothelial growth factor. Br. J. Pharmcol. 2008, 153, 956–965. [Google Scholar] [CrossRef] [Green Version]
- Fiorucci, S. Evidence that 5-lipoxygenase and acetylated cyclooxygenase 2-derived eicosanoids regulate leukocyte-endothelial adherence in response to aspirin. Br. J. Pharmacol. 2003, 139, 1351–1359. [Google Scholar] [CrossRef] [Green Version]
- Morris, T. Effects of low-dose aspirin on acute inflammatory responses in humans. J. Immunol. 2009, 183, 2089–2096. [Google Scholar] [CrossRef]
- Morris, T. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 8842–8847. [Google Scholar] [CrossRef] [Green Version]
- Jozsef, L. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NF-kappa B and AP-1 activation, and IL-8 gene expression in human leukocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 13266–13271. [Google Scholar] [CrossRef]
- Ariel, A. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J. Immunol. 2003, 170, 6266–6272. [Google Scholar] [CrossRef]
- Levy, B.D. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef]
- Nascimento-Silva, V. Novel lipid mediator aspirin-triggered lipoxin A4 induces heme oxygenase-1 in endothelial cells. Am. J. Physiol. Cell Physiol. 2005, 289, C557–C563. [Google Scholar] [CrossRef]
- Kim, J. Aspirin prevents TNF-alpha-induced endothelial cell dysfunction by regulating the NF-kappaB-dependent miR-155/eNOS pathway: Role of a miR-155/eNOS axis in preeclampsia. Free Radic. Biol. Med. 2017, 104, 185–198. [Google Scholar] [CrossRef]
- Van Dijk, M. The STOX1 genotype associated with pre-eclampsia leads to a reduction of trophoblast invasion by alpha-T-catenin upregulation. Hum. Mol. Genet. 2010, 19, 2658–2667. [Google Scholar] [CrossRef]
- Van Dijk, M.; Drewlo, S.; Oudejans, C.B.M. Differential methylation of STOX1 in human placenta. Epigenetics 2010, 5, 736–742. [Google Scholar] [CrossRef]
- Fenstad, M.H. STOX2 but not STOX1 is differentially expressed in decidua from pre-eclamptic women: Data from the Second Nord-Trondelag Health Study. Mol. Hum. Reprod. 2010, 16, 960–968. [Google Scholar] [CrossRef]
- Van Dijk, M.; Oudejans, C.B. STOX1: Key player in trophoblast dysfunction underlying early onset preeclampsia with growth retardation. J. Pregnancy 2011, 2011, 521826. [Google Scholar] [CrossRef]
- Founds, S.A. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta 2009, 30, 15–24. [Google Scholar] [CrossRef]
- Erlandsson, L. Alpha-1 microglobulin as a potential therapeutic candidate for treatment of hypertension and oxidative stress in the STOX1 preeclampsia mouse model. Sci. Rep. 2019, 9, 8561. [Google Scholar] [CrossRef]
- Doridot, L. Preeclampsia-like symptoms induced in mice by fetoplacental expression of STOX1 are reversed by aspirin treatment. Hypertension 2013, 61, 662–668. [Google Scholar] [CrossRef]
- Schisterman, E.F. A randomised trial to evaluate the effects of low-dose aspirin in gestation and reproduction: Design and baseline characteristics. Paediatr Perinat Epidemiol. 2013, 27, 598–609. [Google Scholar] [CrossRef]
- Gizzo, S. Could empirical low-dose-aspirin administration during IVF cycle affect both the oocytes and embryos quality via COX 1-2 activity inhibition? J. Assist. Reprod. Genet. 2014, 31, 261–268. [Google Scholar] [CrossRef]
- Clark, D.A. Aspirin and heparin to improve live birth rate in IVF for unexplained implantation failure? Reprod. Biomed. Online 2013, 26, 538–541. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y. Effects of combining lowdose aspirin with a Chinese patent medicine on follicular blood flow and pregnancy outcome. Mol. Med. Rep. 2014, 10, 2372–2376. [Google Scholar] [CrossRef]
- Leeson, P. Updated review identifies no adverse impact on mother or offspring during the perinatal period of aspirin use for prevention of preeclampsia. Evid. Based Med. 2015, 20, 11. [Google Scholar] [CrossRef]
- Dasari, R. Effect of maternal low dose aspirin on neonatal platelet function. Indian Pediatr. 1998, 35, 507–511. [Google Scholar]
- Levy, G.; Garrettson, L.K. Kinetics of salicylate elimination by newborn infants of mothers who ingested aspirin before delivery. Pediatrics 1974, 53, 201–210. [Google Scholar]
- Zarek, S.M. Antimullerian hormone and pregnancy loss from the Effects of Aspirin in Gestation and Reproduction trial. Fertil. Steril. 2016, 105, 946–952. [Google Scholar] [CrossRef]
- Mazaud-Guittot, S. Paracetamol, aspirin, and indomethacin induce endocrine disturbances in the human fetal testis capable of interfering with testicular descent. J. Clin. Endocrinol. Metab. 2013, 98, E1757–E1767. [Google Scholar] [CrossRef]
- Chu, S. In Utero Exposure to Aspirin and Risk of Asthma in Childhood. Epidemiology 2016, 27, 726–731. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, D.M. Paracetamol (acetaminophen), aspirin (acetylsalicylic acid) and indomethacin are anti-androgenic in the rat foetal testis. Int. J. Androl. 2012, 35, 377–384. [Google Scholar] [CrossRef]
- Bloor, M.; Paech, M. Nonsteroidal anti-inflammatory drugs during pregnancy and the initiation of lactation. Anesth. Analg. 2013, 116, 1063–1075. [Google Scholar] [CrossRef]
- Jensen, M.S. Analgesics during pregnancy and cryptorchidism: Additional analyses. Epidemiology 2011, 22, 610–612. [Google Scholar] [CrossRef]
- De Berardis, G. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA 2012, 307, 2286–2294. [Google Scholar] [CrossRef]
- Khazardoost, S. Effect of aspirin in prevention of adverse pregnancy outcome in women with elevated alpha-fetoprotein. J. Matern. Fetal. Neonatal. Med. 2014, 27, 561–565. [Google Scholar] [CrossRef]
- Demers, S.; Roberge, S.; Bujold, E. Low-dose aspirin for the prevention of adverse pregnancy outcomes in women with elevated alpha-fetoprotein. J. Matern. Fetal. Neonatal. Med. 2015, 28, 726. [Google Scholar] [CrossRef]
- Block-Abraham, D.M. First-trimester risk factors for preeclampsia development in women initiating aspirin by 16 weeks of gestation. Obs. Gynecol. 2014, 123, 611–617. [Google Scholar] [CrossRef]
- Asemi, Z. A randomized controlled clinical trial investigating the effect of calcium supplement plus low-dose aspirin on hs-CRP, oxidative stress and insulin resistance in pregnant women at risk for pre-eclampsia. Pak. J. Biol. Sci. 2012, 15, 469–476. [Google Scholar]
- Durcin, M. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J. Extracell. Vesicles 2017, 6, 1305677. [Google Scholar] [CrossRef]
- Morel, O. Microparticles: A critical component in the nexus between inflammation, immunity, and thrombosis. Semin. Immunopathol. 2011, 33, 469–486. [Google Scholar] [CrossRef]
- Gho, Y.S.; Lee, C. Emergent properties of extracellular vesicles: A holistic approach to decode the complexity of intercellular communication networks. Mol. Biosyst. 2017, 13, 1291–1296. [Google Scholar] [CrossRef]
- Borges, F.T.; Reis, L.A.; Schor, N. Extracellular vesicles: Structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 2013, 46, 824–830. [Google Scholar] [CrossRef]
- Di Rocco, G.; Baldari, S.; Toietta, G. Towards Therapeutic Delivery of Extracellular Vesicles: Strategies for In Vivo Tracking and Biodistribution Analysis. Stem Cells Int. 2016, 2016, 5029619. [Google Scholar] [CrossRef]
- Thery, C. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Momen-Heravi, F. Current methods for the isolation of extracellular vesicles. Biol. Chem. 2013, 394, 1253–1262. [Google Scholar] [CrossRef]
- Huang-Doran, I.; Zhang, C.Y.; Vidal-Puig, A. Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol. Metab. 2017, 28, 3–18. [Google Scholar] [CrossRef]
- Mitchell, M.D. Placental exosomes in normal and complicated pregnancy. Am. J. Obstet. Gynecol. 2015, 213, S173–S181. [Google Scholar] [CrossRef]
- Adam, S. Review: Fetal-maternal communication via extracellular vesicles Implications for complications of pregnancies. Placenta 2017, 54, 83–88. [Google Scholar] [CrossRef]
- Salomon, C.; Rice, G.E. Role of Exosomes in Placental Homeostasis and Pregnancy Disorders. Prog. Mol. Biol. Transl. Sci. 2017, 145, 163–179. [Google Scholar]
- Nair, S.; Salomon, C. Extracellular vesicles and their immunomodulatory functions in pregnancy. Semin. Immunopathol. 2018, 40, 425–437. [Google Scholar] [CrossRef] [Green Version]
- Sarker, S. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J. Transl. Med. 2014, 12, 204. [Google Scholar] [CrossRef]
- Salomon, C.G.D.; Romero, K.S.; Longo, S.; Correa, P.; Illanes, S.E.; Rice, G.E. Placental exosomes as early biomarker of preeclampsia—Potential role of exosomal microRNAs across gestation. J. Clin. Endocrinol. Metab. 2017, 102, 3182–3194. [Google Scholar] [CrossRef]
- Salomon, C. The role of placental exosomes in gestational diabetes mellitus. In Gestational Diabetes-Causes, Diagnosis and Treatment; Sobrevia, L., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Radu, C.M. Origin and levels of circulating microparticles in normal pregnancy: A longitudinal observation in healthy women. Scand. J. Clin. Lab. Investig. 2015, 75, 487–495. [Google Scholar] [CrossRef]
- Biro, O. Circulating exosomal and Argonaute-bound microRNAs in preeclampsia. Gene 2019, 692, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Truong, G. Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells—Liquid biopsies for monitoring complications of pregnancy. PLoS ONE 2017, 12, e0174514. [Google Scholar] [CrossRef]
- Abou-Kheir, W. HTR-8/SVneo cell line contains a mixed population of cells. Placenta 2017, 50, 1–7. [Google Scholar] [CrossRef]
- Tannetta, D.S. Characterisation of syncytiotrophoblast vesicles in normal pregnancy and pre-eclampsia: Expression of Flt-1 and endoglin. PLoS ONE 2013, 8, e56754. [Google Scholar] [CrossRef]
- Germain, S.J. Systemic inflammatory priming in normal pregnancy and preeclampsia: The role of circulating syncytiotrophoblast microparticles. J. Immunol. 2007, 178, 5949–5956. [Google Scholar] [CrossRef]
- Redman, C.W. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta 2012, 33, S48–S54. [Google Scholar] [CrossRef]
- Tong, M.; Chamley, L.W. Placental Extracellular Vesicles and Feto-Maternal Communication. Cold Spring Harb. Perspect. Med. 2015, 5, 023028. [Google Scholar] [CrossRef]
- Chang, X. Exosomes From Women With Preeclampsia Induced Vascular Dysfunction by Delivering sFlt (Soluble Fms-Like Tyrosine Kinase)-1 and sEng (Soluble Endoglin) to Endothelial Cells. Hypertension 2018, 72, 1381–1390. [Google Scholar] [CrossRef]
- Sammar, M. Reduced placental protein 13 (PP13) in placental derived syncytiotrophoblast extracellular vesicles in preeclampsia A novel tool to study the impaired cargo transmission of the placenta to the maternal organs. Placenta 2018, 66, 17–25. [Google Scholar] [CrossRef]
- Tong, M. Antiphospholipid antibodies increase the levels of mitochondrial DNA in placental extracellular vesicles: Alarmin-g for preeclampsia. Sci. Rep. 2017, 7, 16556. [Google Scholar] [CrossRef]
- Aharon, A. The role of extracellular vesicles in placental vascular complications. Thromb. Res. 2015, 135, S23–S25. [Google Scholar] [CrossRef]
- Miranda, J. Placental exosomes profile in maternal and fetal circulation in intrauterine growth restriction—Liquid biopsies to monitoring fetal growth. Placenta 2018, 64, 34–43. [Google Scholar] [CrossRef]
- Campello, E. Circulating microparticles in umbilical cord blood in normal pregnancy and pregnancy with preeclampsia. Thromb. Res. 2015, 136, 427–431. [Google Scholar] [CrossRef]
- Jia, R. Comparative Proteomic Profile of the Human Umbilical Cord Blood Exosomes between Normal and Preeclampsia Pregnancies with High-Resolution Mass Spectrometry. Cell Physiol. Biochem. 2015, 36, 2299–2306. [Google Scholar] [CrossRef] [Green Version]
- Jadli, A. Combination of copeptin, placental growth factor and total annexin V microparticles for prediction of preeclampsia at 10-14 weeks of gestation. Placenta 2017, 58, 67–73. [Google Scholar] [CrossRef]
- Motta-Mejia, C. Placental Vesicles Carry Active Endothelial Nitric Oxide Synthase and Their Activity is Reduced in Preeclampsia. Hypertension 2017, 70, 372–381. [Google Scholar] [CrossRef]
- Gill, M. Placental Syncytiotrophoblast-Derived Extracellular Vesicles Carry Active NEP (Neprilysin) and Are Increased in Preeclampsia. Hypertension 2019, 73, 1112–1119. [Google Scholar] [CrossRef] [Green Version]
- Kohli, S. Maternal extracellular vesicles and platelets promote preeclampsia via inflammasome activation in trophoblasts. Blood 2016, 128, 2153–2164. [Google Scholar] [CrossRef]
- Motawi, T.M.K. Role of mesenchymal stem cells exosomes derived microRNAs; miR-136, miR-494 and miR-495 in pre-eclampsia diagnosis and evaluation. Arch. Biochem. Biophys. 2018, 659, 13–21. [Google Scholar] [CrossRef]
- Hu, C.C. Pre-eclampsia is associated with altered expression of the renal sodium transporters NKCC2, NCC and ENaC in urinary extracellular vesicles. PLoS ONE 2018, 13, e0204514. [Google Scholar] [CrossRef]
- Moro, L. Placental Microparticles and MicroRNAs in Pregnant Women with Plasmodium falciparum or HIV Infection. PLoS ONE 2016, 11, e0146361. [Google Scholar] [CrossRef]
- Xiong, Z.H. Protective effect of human umbilical cord mesenchymal stem cell exosomes on preserving the morphology and angiogenesis of placenta in rats with preeclampsia. Biomed. Pharmacother. 2018, 105, 1240–1247. [Google Scholar] [CrossRef]
- Zhao, M. Melatonin prevents preeclamptic sera and antiphospholipid antibodies inducing the production of reactive nitrogen species and extrusion of toxic trophoblastic debris from first trimester placentae. Placenta 2017, 58, 17–24. [Google Scholar] [CrossRef]
- Tong, M. Aggregated transthyretin is specifically packaged into placental nano-vesicles in preeclampsia. Sci. Rep. 2017, 7, 6694. [Google Scholar] [CrossRef]
- Gilani, S.I. Urinary Extracellular Vesicles of Podocyte Origin and Renal Injury in Preeclampsia. J. Am. Soc. Nephrol. 2017, 28, 3363–3372. [Google Scholar] [CrossRef]
- O’Brien, M.; Baczyk, D.; Kingdom, J.C. Endothelial Dysfunction in Severe Preeclampsia is Mediated by Soluble Factors, Rather than Extracellular Vesicles. Sci. Rep. 2017, 7, 5887. [Google Scholar] [CrossRef]
- Xu, J. Vitamin D Reduces Oxidative Stress-Induced Procaspase-3/ROCK1 Activation and MP Release by Placental Trophoblasts. J. Clin. Endocrinol. Metab. 2017, 102, 2100–2110. [Google Scholar] [CrossRef]
- Goetzl, E.J. Human plasma platelet-derived exosomes: Effects of aspirin. Faseb. J. 2016, 30, 2058–2063. [Google Scholar] [CrossRef]
- Connor, D.E. Effects of antiplatelet therapy on platelet extracellular vesicle release and procoagulant activity in health and in cardiovascular disease. Platelets 2016, 27, 805–811. [Google Scholar] [CrossRef]
- Chiva-Blanch, G. Microparticle Shedding by Erythrocytes, Monocytes and Vascular Smooth Muscular Cells Is Reduced by Aspirin in Diabetic Patients. Rev. Esp. Cardiol. 2016, 69, 672–680. [Google Scholar] [CrossRef]
- Ambrose, A.R. Comparison of the release of microRNAs and extracellular vesicles from platelets in response to different agonists. Platelets 2017, 29, 446–454. [Google Scholar] [CrossRef]
- Tannetta, D.S. Syncytiotrophoblast Extracellular Vesicles from Pre-Eclampsia Placentas Differentially Affect Platelet Function. PLoS ONE 2015, 10, e0142538. [Google Scholar] [CrossRef]
- Patil, R. Effect of anticoagulant therapy on cell-derived microparticles and pregnancy outcome in women with pregnancy loss. Br. J. Haematol. 2015, 171, 892–896. [Google Scholar] [CrossRef]
| Study Design | Mode of Treatment | Outcome of Study | Reference |
|---|---|---|---|
| Randomized controlled trial (RCT) | Low dose aspirin (LDA) and/or Low Molecular Weight Heparin (LMWH) | Improved pregnancy outcomes (Less PE and IUGR incidence) | [108] |
| Prospective case-control study | LDA and LMWH in first trimester | Reduced incidence of unexplained recurrent spontaneous abortion | [109] |
| Database searching for RCTs involving LDA and placebo in PE | LDA or Placebo | LDA reduces PE risk | [110] |
| Literature searching on LDA and PE | LDA at 100 mg/day <16 gestational weeks | Reduced PE incidence due to LDA prophylaxis | [111] |
| Systematic literature searches about aspirin and PE | LDA | LDA prophylaxis in at risk patients to develop PE have higher advantages compared to negligible disadvantages i.e., feto-maternal bleeding, aspirin resistance etc. | [112] |
| Systematic review and an individual participant data meta-analysis | Antiplatelet aspirin therapy in early pregnancy | 10–15% reduction in the risk of PE | [113] |
| A systematic review and meta-analysis of randomized controlled trials | 50–150 mg/day aspirin or no treatment at <16 or >16 gestational weeks | LDA at <16 weeks, there was a significant reduction and a dose-response effect for the prevention of preeclampsia | [87] |
| A systematic review and meta-analysis through electronic database searches (PubMed, Cochrane, Embase). | LDA or placebo at <16 or >16 gestational weeks | <16 weeks, significant reduction of PE. >16 weeks, negligible impact on PE and related disorders | [11] |
| Databases searching involving keywords ‘aspirin’ and ‘pregnancy’ | RCTs that evaluated the prophylactic use of LDA (50–150 mg/day) during pregnancy were included. | LDA initiated at ≤ 16 weeks of gestation is associated with a greater reduction of perinatal death and other adverse perinatal outcomes than when initiated at >16 weeks. | [114] |
| Meta-analysis of individual patient data recruited to 31 RCTs of PE primary prevention. | One or more antiplatelet agents (e.g., LDA or dipyridamole) versus a placebo or no antiplatelet agent. | Antiplatelet agents were associated with a significant 10% reduction in the relative risk of both PE (p = 0.004) and preterm birth before 34 weeks’ gestation (p = 0.011) compared to control cases. | [86] |
| Women at high risk for preterm PE were recruited to RCTs | 150 mg/day of aspirin was used to reduce the incidence of aspirin resistance and maximize the effect. | LDA reduced the incidence of preterm PE | [115] |
| A planned secondary analysis of the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial, a multicenter, block-randomized, double-blind, placebo-controlled trial investigating the effects of LDA on the incidence of live birth. | Daily LDA (81 mg, n = 615) or placebo (n = 613) and were followed for up to six menstrual cycles or through gestation if they became pregnant. | Preconception LDA appears to be well tolerated by women trying to conceive, women who become pregnant, and by their fetuses and neonates. | [16] |
| Chronological, cumulative meta-analyses of two recently published meta-analyses of RCTs examining the effects of antioxidant or LDA on the rates of PE. | Antioxidant or Low Dose Acetylsalicylic Acid (LDAA) therapy | Studies with smaller sample sizes are more likely to be biased against the null hypothesis. As such, cumulative meta-analysis is an effective tool in predicting potential bias against the null hypothesis and the need for additional studies. | [116] |
| Prospective cohort study involving 533 pregnant women in their first trimester | LDAA and LMWH | The use of ASA may be associated with an increased risk of developing a sub-chorionic hematoma (SCH) during the first trimester. | [117] |
| Multicentre RCTs involving 32 women with a previous delivery <34 weeks gestation with HD and/or SGA and aPLA were included before 12 weeks gestation. | The intervention was daily LMWH with aspirin or aspirin alone. | Combined LMWH and aspirin treatment started before 12 weeks gestation in a subsequent pregnancy did not show reduction of onset of recurrent HD either <34 weeks gestation or irrespective of gestational age, compared with aspirin alone. | [118] |
| Prospective randomized, placebo-controlled, double-blinded, multinational clinical trial | Daily administration of LDA (81 mg/day) initiated between 6 and 13 weeks of pregnancy and continued upto 36 weeks. | PTB, PE, SGA, perinatal mortality were reduced. | [119] |
| Prospective RCTs | Preconception LDA daily | It is not associated with reduction of pregnancy loss | [120] |
| Multicenter, double blind, placebo-controlled trial involving women at high risk for preterm PE | Some of them received 150 mg/day aspirin and some of them received placebo at 11–14 gestational weeks until 36 weeks of gestation | Primary outcome was delivery with PE before 37 weeks of gestation. Treatment with aspirin reduced the incidence of preterm preeclampsia. | [121] |
| EVs | Sample Type | Gestational Age | Isolation Method | Pregnancy Condition | Biological Process/Results | Reference |
|---|---|---|---|---|---|---|
| Maternal Blood Stream and other Body Fluids | ||||||
| Trophoblast derived exosomal micro RNA (has-miR-210) | Plasma and HTR-8 cell culture conditioned media | Third trimester | Membrane affinity spin column method | Normal and PE | This micro RNA is responsible for PE pathogenesis | [187] |
| Exosomes | Plasma | Third trimester (before cesarean section) | Commercial kit (ExoQuick) | Normal and PE | Vascular dysfunction | [194] |
| Exosomal micro RNAs | Placental mesenchymal stem cells culture conditioned media and peripheral blood | During cesarean section delivery | Ultracentrifugation followed by Real Time PCR | Normal Pregnancy (NP) and PE | High level of exosomal miRNA-136, 494, 495 in PE | [205] |
| Urinary Exosomal proteins | Urine | After 20 weeks | Centrifugation | Healthy non-pregnant, Normal pregnancy, PE | Phosphorylation of renal tubular sodium transporter proteins that enhance sodium reabsorption in PE compared to NP | [206] |
| Exosomes | Human umbilical cord mesenchymal stem cells (MSC) | After delivery | Flow cytometry based detection of MSC surface markers | PE | Effect on placental tissue morphology and angiogenesis in rat PE placenta | [207,208] |
| Placental syncytiotrophoblast derived extracellular vesicles (STBEVs) | Placental perfusate | Following cesarean section delivery | Centrifugation | Normal and PE pregnancy | Lower level of placental protein 13 was found in STBEVs of PE placenta | [195] |
| Placental extracellular vesicles | Cultured human placental villi explant and Maternal serum | First trimester placenta | Sequential centrifugation and ultracentrifugation | Normal pregnancy | Presence of antiphospholipid antibody increases the level of mitochondrial DNA in the placental EVs and increases the risk to develop PE | [196] |
| Microparticles | Maternal serum | 10–14 weeks | Centrifugation | Normal, PE, IUGR | Serum copeptin, annexin V were higher and placental growth factor was low in PE | [201] |
| Macovesicles/placental debris | Placental explant and maternal serum | First trimester (8–10 weeks) | Centrifugation | PE | Melatonin is secreted from placental explant that reduce PE sera induced production of endothelial cell activating placental EVs | [209] |
| Nanovesicles | Placenta | First trimester and term placenta | Differential centrifugation | PE | Transthyretin is increased in amount and incorporated in placental nanovesicles | [210] |
| EVs | Urine | Maternal urine | EVs were stained for annexin, nephrin and podocin proteins | PE and Normotensive pregnant women | Nephrin protein was packaged in increased amount in urinary EVs of PE women | [211] |
| Syncytiotrophoblast derived extracellular vesicles (STBEV) | Placental perfusion and maternal plasma | Gestational age matched | Differential centrifugation | Normal and PE | Less nitric oxide synthase in STBEVs of PE women | [202] |
| EVs | Placental explant | First and second trimester | Sequential centrifugation | Normal and PE | Endothelial dysfunction in severe early onset PE is via soluble angiogenic factors, not by EVs | [212] |
| Exosomes | Maternal plasma | First, second and third trimester | Differential centrifugation, ultracentrifugation followed by density gradient centrifugation | Normal and PE | The concentration of exosomes is higher and miRNA content is different in PE compared to normal pregnancy | [184] |
| Microparticles | Placental trophoblasts | At term (>37 weeks) | Two-step centrifugation | Uncomplicated and preeclamptic | Increase MP shedding from PE placenta; upregulation of caveolin-1 and downregulation of eNOS in these MPs which is modulated by vitamin-D | [213] |
| EVs | Endothelial cells and Platelets | Not mentioned | Differential centrifugation | Normal and PE | Inflammasome activation in placental trophoblasts results in PE development | [204] |
| Fetal Circulation | ||||||
| Exosomes | Umbilical cord blood | At delivery | Differential Centrifugation + Density gradient centrifugation | Normal | No difference in concentration of exosomes in term, small for gestational age, fetal growth restricted neonates | [198] |
| Microparticle (MPs) | Umbilical cord blood | At delivery | MPs were identified by size and annexin V fluorescein isothiocyanate (FITC) labelling | Normal and PE | MP levels is higher compared to maternal blood in PE | [199] |
| Exosomes | Umbilical cord blood | At delivery | Differential centrifugation + Filtration | Normal and PE | Altered protein expression profile that are involved with PE etiology | [200] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, S.; Kumar, S.; Hyett, J.; Salomon, C. Molecular Targets of Aspirin and Prevention of Preeclampsia and Their Potential Association with Circulating Extracellular Vesicles during Pregnancy. Int. J. Mol. Sci. 2019, 20, 4370. https://doi.org/10.3390/ijms20184370
Dutta S, Kumar S, Hyett J, Salomon C. Molecular Targets of Aspirin and Prevention of Preeclampsia and Their Potential Association with Circulating Extracellular Vesicles during Pregnancy. International Journal of Molecular Sciences. 2019; 20(18):4370. https://doi.org/10.3390/ijms20184370
Chicago/Turabian StyleDutta, Suchismita, Sathish Kumar, Jon Hyett, and Carlos Salomon. 2019. "Molecular Targets of Aspirin and Prevention of Preeclampsia and Their Potential Association with Circulating Extracellular Vesicles during Pregnancy" International Journal of Molecular Sciences 20, no. 18: 4370. https://doi.org/10.3390/ijms20184370
APA StyleDutta, S., Kumar, S., Hyett, J., & Salomon, C. (2019). Molecular Targets of Aspirin and Prevention of Preeclampsia and Their Potential Association with Circulating Extracellular Vesicles during Pregnancy. International Journal of Molecular Sciences, 20(18), 4370. https://doi.org/10.3390/ijms20184370






