VceC Mediated IRE1 Pathway and Inhibited CHOP-induced Apoptosis to Support Brucella Replication in Goat Trophoblast Cells
Abstract
:1. Introduction
2. Results
2.1. Compare to VceC Amino Acid Sequences Derived from Different Brucella Strains
2.2. Mutant Strains ΔVceC Intracellular Survival in GTCs
2.3. Effect of ΔVceC on GTC Apoptosis
2.4. Deletion of VceC Decreases Brucella-Mediated ER Stress
2.5. The Replication of ΔVceC Was More Sensitive after Changing ER Stress in GTCs
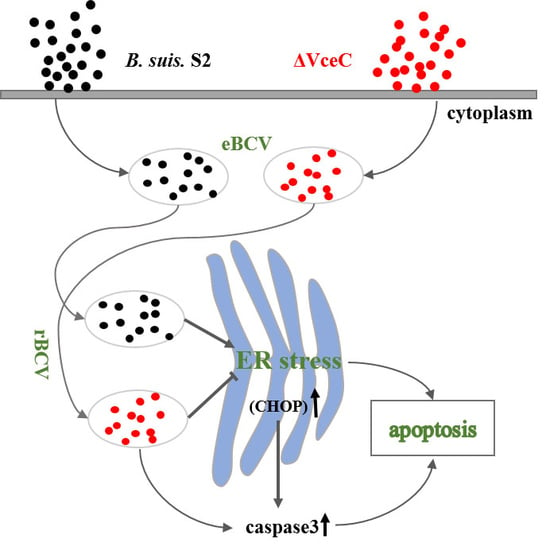
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Construction of the Mutant Strain ΔVceC
4.3. Cell Infection Assay
4.4. Western Blot Analysis
4.5. Immunofluorescence Assay
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pappas, G.; Papadimitriou, P.; Akritidis, N.; Christou, L.; Tsianos, E.V. The new global map of human brucellosis. Lancet Infect. Dis. 2006, 6, 91–99. [Google Scholar] [CrossRef]
- Byndloss, M.X.; Tsolis, R.M. Brucella spp. Virulence Factors and Immunity. Annu. Rev. Anim. Biosci. 2016, 4, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Samartino, L.E.; Enright, F.M. Pathogenesis of abortion of bovine brucellosis. Comp. Immunol. Microbiol. Infect. Dis. 1993, 16, 95–101. [Google Scholar] [CrossRef]
- Celli, J. The changing nature of the Brucella-containing vacuole. Cell. Microbiol. 2015, 17, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Zheng, K.; Liu, Z.F. Establishment of Chronic Infection: Brucella’s Stealth Strategy. Front. Cell. Infect. Microbiol. 2016, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Pascual, D.W.; Yang, X.; Wang, H.; Goodwin, Z.; Hoffman, C.; Clapp, B. Alternative strategies for vaccination to brucellosis. Microbes Infect. 2018, 20, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Chauhan, R.S.; Rout, C. Common antigens prediction in bacterial bioweapons: A perspective for vaccine design. Infect. Genet. Evol. 2014, 21, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Caporale, V.; Bonfini, B.; Di Giannatale, E.; Di Provvido, A.; Forcella, S.; Giovannini, A.; Tittarelli, M.; Scacchia, M. Efficacy of Brucella abortus vaccine strain RB51 compared to the reference vaccine Brucella abortus strain 19 in water buffalo. Vet. Ital. 2010, 46, 5–11. [Google Scholar]
- Zhu, L.; Feng, Y.; Zhang, G.; Jiang, H.; Zhang, Z.; Wang, N.; Ding, J.; Suo, X. Brucella suis strain 2 vaccine is safe and protective against heterologous Brucella spp. infections. Vaccine 2016, 34, 395–400. [Google Scholar] [CrossRef]
- Myeni, S.; Child, R.; Ng, T.W.; Kupko, J.J., 3rd; Wehrly, T.D.; Porcella, S.F.; Knodler, L.A.; Celli, J. Brucella modulates secretory trafficking via multiple type IV secretion effector proteins. PLoS Pathog. 2013, 9, e1003556. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.F.; Sun, Y.H.; den Hartigh, A.B.; van Dijl, J.M.; Tsolis, R.M. Identification of VceA and VceC, two members of the VjbR regulon that are translocated into macrophages by the Brucella type IV secretion system. Mol. Microbiol. 2008, 70, 1378–1396. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.F.; Starr, T.; Winter, M.G.; den Hartigh, A.B.; Child, R.; Knodler, L.A.; van Dijl, J.M.; Celli, J.; Tsolis, R.M. Sensing of Bacterial Type IV Secretion via the Unfolded Protein Response. mBio 2013, 4, e00418-12. [Google Scholar] [CrossRef] [PubMed]
- Keestra-Gounder, A.M.; Byndloss, M.X.; Seyffert, N.; Young, B.M.; Chavez-Arroyo, A.; Tsai, A.Y.; Cevallos, S.A.; Winter, M.G.; Pham, O.H.; Tiffany, C.R.; et al. NOD1 and NOD2 signalling links ER stress with inflammation. Nature 2016, 532, 394. [Google Scholar] [CrossRef] [PubMed]
- Von Bargen, K.; Gorvel, J.P.; Salcedo, S.P. Internal affairs: Investigating the Brucella intracellular lifestyle. FEMS Microbiol. Rev. 2012, 36, 533–562. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.; de Chastellier, C.; Franchini, D.M.; Pizarro-Cerda, J.; Moreno, E.; Gorvel, J.P. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 2003, 198, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Khan, M.; Magnani, D.D.; Harms, J.S.; Durward, M.; Radhakrishnan, G.K.; Liu, Y.P.; Splitter, G.A. Brucella induces an unfolded protein response via TcpB that supports intracellular replication in macrophages. PLoS Pathog. 2013, 9, e1003785. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, P.; Li, Y.; Xiang, C.; Yin, Y.; Chen, Z.; Du, Y.; Zhou, D.; Jin, Y.; Wang, A. Brucella suis Vaccine Strain 2 Induces Endoplasmic Reticulum Stress that Affects Intracellular Replication in Goat Trophoblast Cells In vitro. Front. Cell. Infect. Microbiol. 2016, 6, 19. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Unfolded protein response. Curr. Biol. 2012, 22, R622-6. [Google Scholar] [CrossRef]
- Lee, W.-S.; Yoo, W.-H.; Chae, H.-J. ER stress and autophagy. Curr. Mol. Med. 2015, 15, 735–745. [Google Scholar] [CrossRef]
- Szegezdi, E.; Logue, S.E.; Gorman, A.M.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef]
- Atluri, V.L.; Xavier, M.N.; de Jong, M.F.; den Hartigh, A.B.; Tsolis, R.M. Interactions of the human pathogenic Brucella species with their hosts. Annu. Rev. Microbiol. 2011, 65, 523–541. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Terraza, A.; Ouahrani-Bettache, S.; Liautard, J.P.; Dornand, J. In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect. Immun. 2000, 68, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, M.; Di Carlo, P.; Abbadessa, V.; Titone, L.; Miceli, S.; Barbusca, E.; Cannizzo, G.; Mancuso, S.; Arista, S.; Scarlata, F. Monocyte and lymphocyte apoptosis resistance in acute and chronic brucellosis and its possible implications in clinical management. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2003, 36, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wang, Y.; Qiao, F.; Wang, Z.; Du, X.; Xu, J.; Zhao, J.; Qu, Q.; Dong, S.; Sun, Y.; et al. Cytotoxicity of Brucella smooth strains for macrophages is mediated by increased secretion of the type IV secretion system. Microbiology 2009, 155 Pt 10, 3392–3402. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Lu, Q.; Hu, Z.; Yu, Y.; Chen, Q.; Wang, Q.K. A non-canonical pathway regulates ER stress signaling and blocks ER stress-induced apoptosis and heart failure. Nat. Commun. 2017, 8, 133. [Google Scholar] [CrossRef]
- Masciarelli, S.; Fra, A.M.; Pengo, N.; Bertolotti, M.; Cenci, S.; Fagioli, C.; Ron, D.; Hendershot, L.M.; Sitia, R. CHOP-independent apoptosis and pathway-selective induction of the UPR in developing plasma cells. Mol. Immunol. 2010, 47, 1356–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinon, F.; Chen, X.; Lee, A.H.; Glimcher, L.H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 2010, 11, 411–418. [Google Scholar] [CrossRef]
- Taguchi, Y.; Imaoka, K.; Kataoka, M.; Uda, A.; Nakatsu, D.; Horii-Okazaki, S.; Kunishige, R.; Kano, F.; Murata, M. Yip1A, a novel host factor for the activation of the IRE1 pathway of the unfolded protein response during Brucella infection. PLoS Pathog. 2015, 11, e1004747. [Google Scholar] [CrossRef]
- Zhou, D.; Zhi, F.J.; Qi, M.Z.; Bai, F.R.; Zhang, G.D.; Li, J.M.; Liu, H.; Chen, H.T.; Lin, P.F.; Tang, K.Q.; et al. Brucella induces unfolded protein response and inflammatory response via GntR in alveolar macrophages. Oncotarget 2018, 9, 5184–5196. [Google Scholar] [CrossRef]





| Group | Normal Cells | The Early Apoptotic Cells (%) | The Late Apoptotic Cells (%) |
|---|---|---|---|
| Control | 85.05 ± 0.63 | 1.77 ± 0.23 | 3.59 ± 0.25 |
| B. suis S2-infected | 84.45 ± 4.03 | 1.23 ± 0.34 | 3.96 ± 0.49 |
| ΔVceC-infected | 84.55 ± 1.06 | 3.04 ± 0.10 * | 4.08 ± 0.74 |
| Group | Normal Cells | The Early Apoptotic Cells (%) | The Late Apoptotic Cells (%) |
|---|---|---|---|
| Control | 75.20 ± 1.41 | 5.15 ± 2.46 | 8.01 ± 4.51 |
| B. suis S2-infected | 79.50 ± 3.68 | 5.25 ± 0.71 | 6.18 ± 0.51 |
| ΔVceC-infected | 72.85 ± 2.47 | 8.96 ± 0.99 * | 6.37 ± 0.98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, F.; Zhou, D.; Bai, F.; Li, J.; Xiang, C.; Zhang, G.; Jin, Y.; Wang, A. VceC Mediated IRE1 Pathway and Inhibited CHOP-induced Apoptosis to Support Brucella Replication in Goat Trophoblast Cells. Int. J. Mol. Sci. 2019, 20, 4104. https://doi.org/10.3390/ijms20174104
Zhi F, Zhou D, Bai F, Li J, Xiang C, Zhang G, Jin Y, Wang A. VceC Mediated IRE1 Pathway and Inhibited CHOP-induced Apoptosis to Support Brucella Replication in Goat Trophoblast Cells. International Journal of Molecular Sciences. 2019; 20(17):4104. https://doi.org/10.3390/ijms20174104
Chicago/Turabian StyleZhi, Feijie, Dong Zhou, Furong Bai, Junmei Li, Caixia Xiang, Guangdong Zhang, Yaping Jin, and Aihua Wang. 2019. "VceC Mediated IRE1 Pathway and Inhibited CHOP-induced Apoptosis to Support Brucella Replication in Goat Trophoblast Cells" International Journal of Molecular Sciences 20, no. 17: 4104. https://doi.org/10.3390/ijms20174104
APA StyleZhi, F., Zhou, D., Bai, F., Li, J., Xiang, C., Zhang, G., Jin, Y., & Wang, A. (2019). VceC Mediated IRE1 Pathway and Inhibited CHOP-induced Apoptosis to Support Brucella Replication in Goat Trophoblast Cells. International Journal of Molecular Sciences, 20(17), 4104. https://doi.org/10.3390/ijms20174104