Diet-Induced Obese Mice and Leptin-Deficient Lepob/ob Mice Exhibit Increased Circulating GIP Levels Produced by Different Mechanisms
Abstract
1. Introduction
2. Results
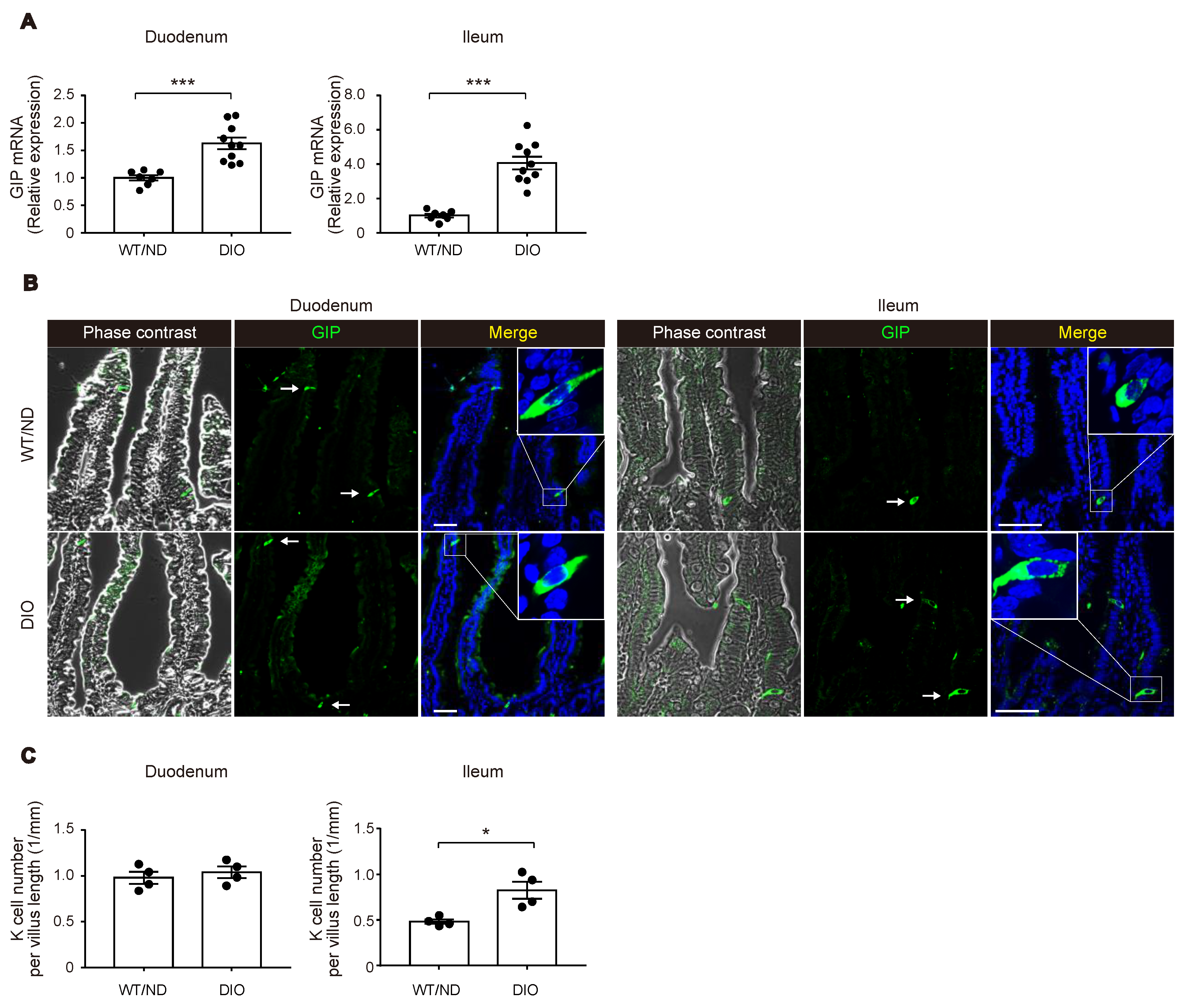
2.1. HFD Feeding Increased Plasma GIP Concentration along with an Increase of K Cell Number and GIP mRNA Expression in the Lower Small Intestine
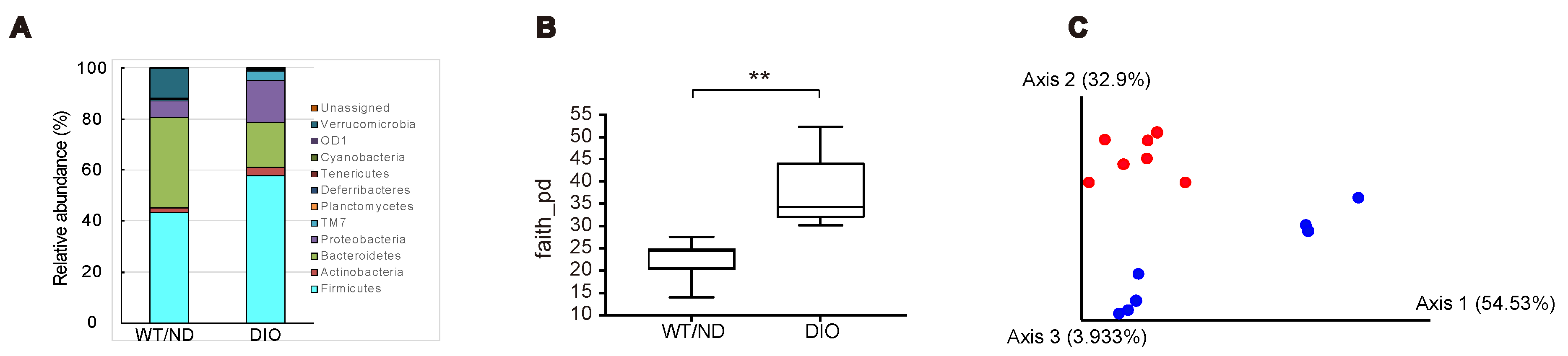
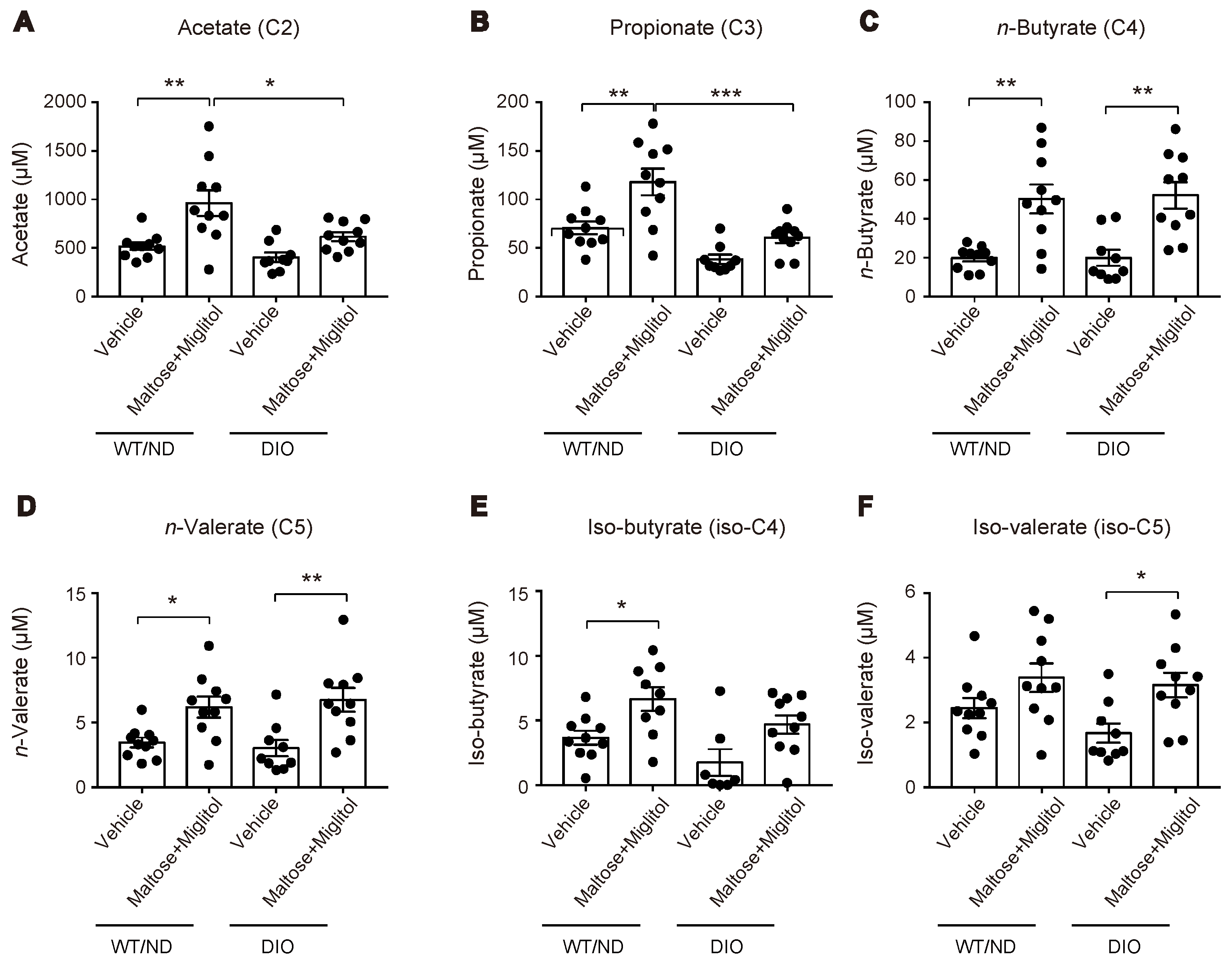
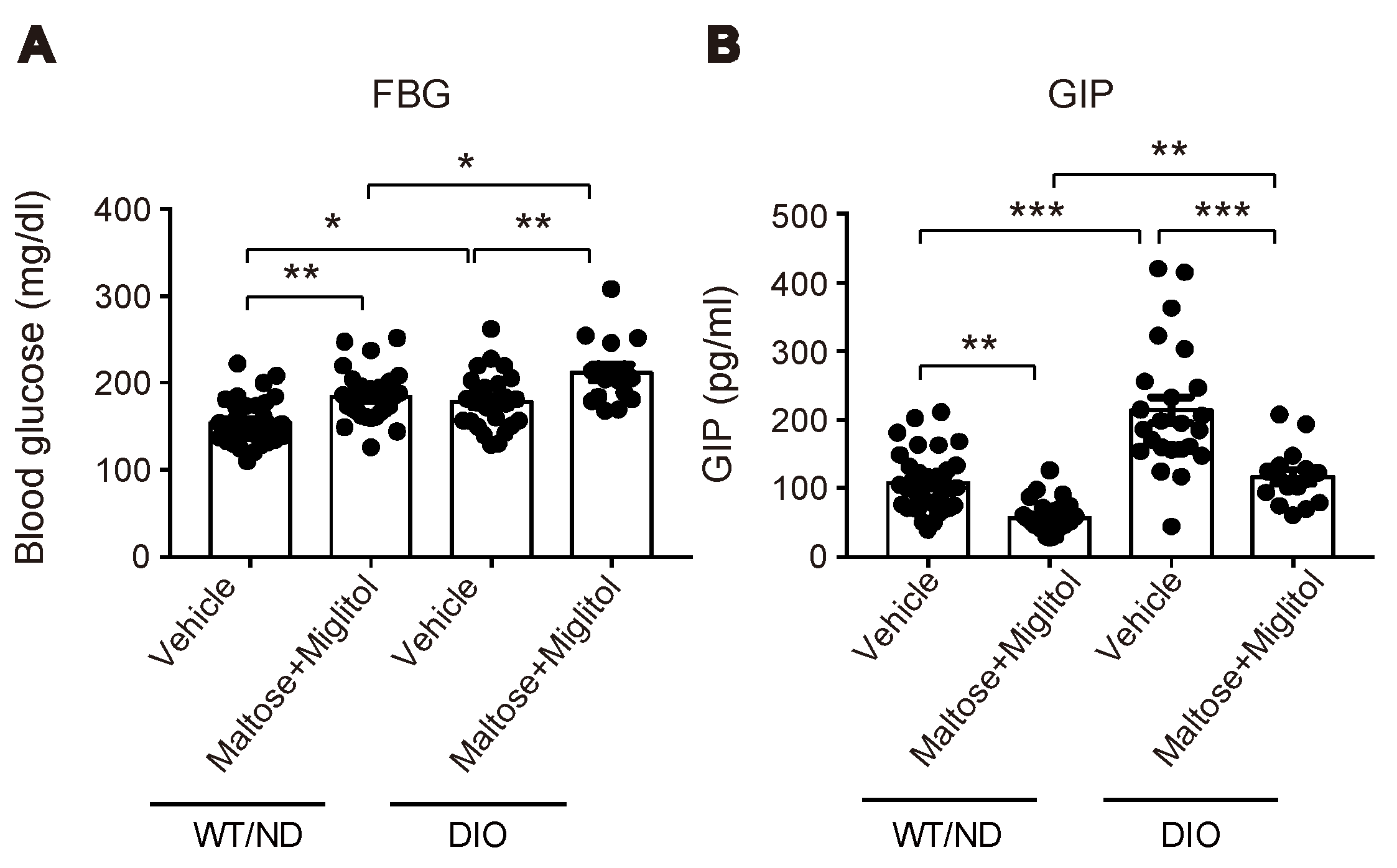
2.2. HFD Feeding Altered the Qualitative Composition of the Gut Microbiome, but Did not Affect the Maltose/Miglitol-Induced Increase of Plasma SCFAs or Maltose/Miglitol-Induced Suppression of Plasma GIP
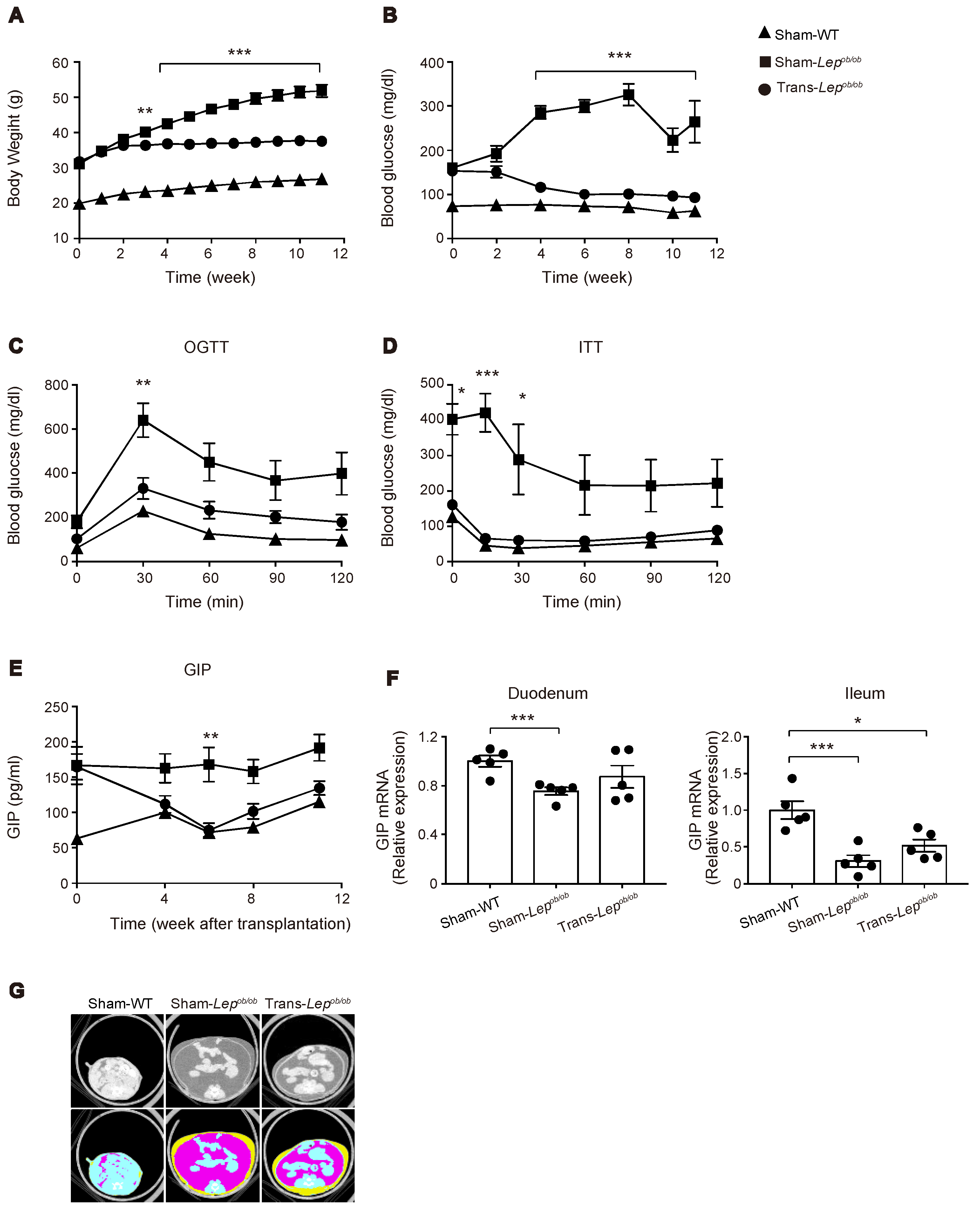
2.3. Transplantation of Wild-Type Subcutaneous Fat to Lepob/ob Mice Ameliorated Obesity, Glucose Intolerance, and Insulin Resistance
2.4. The Plasma GIP Concentration Was Increased in Lepob/ob Mice, but Was Significantly Suppressed by Fat Transplantation
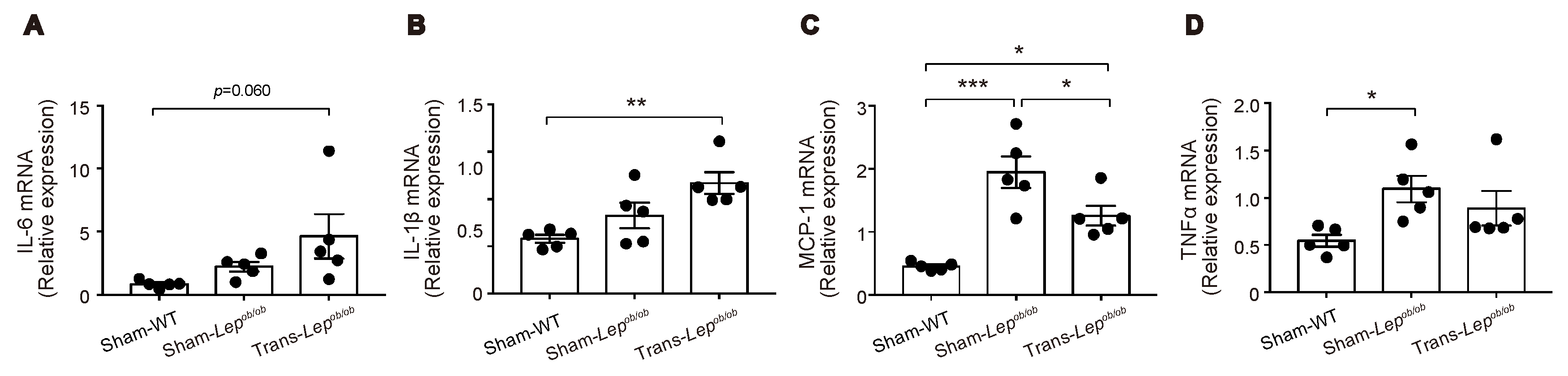
2.5. Expressions of Several Pro-Inflammatory Cytokines Were Increased in Epididymal Fat Tissues of Lepob/ob Mice, which Were Partially Ameliorated by Fat Transplantation
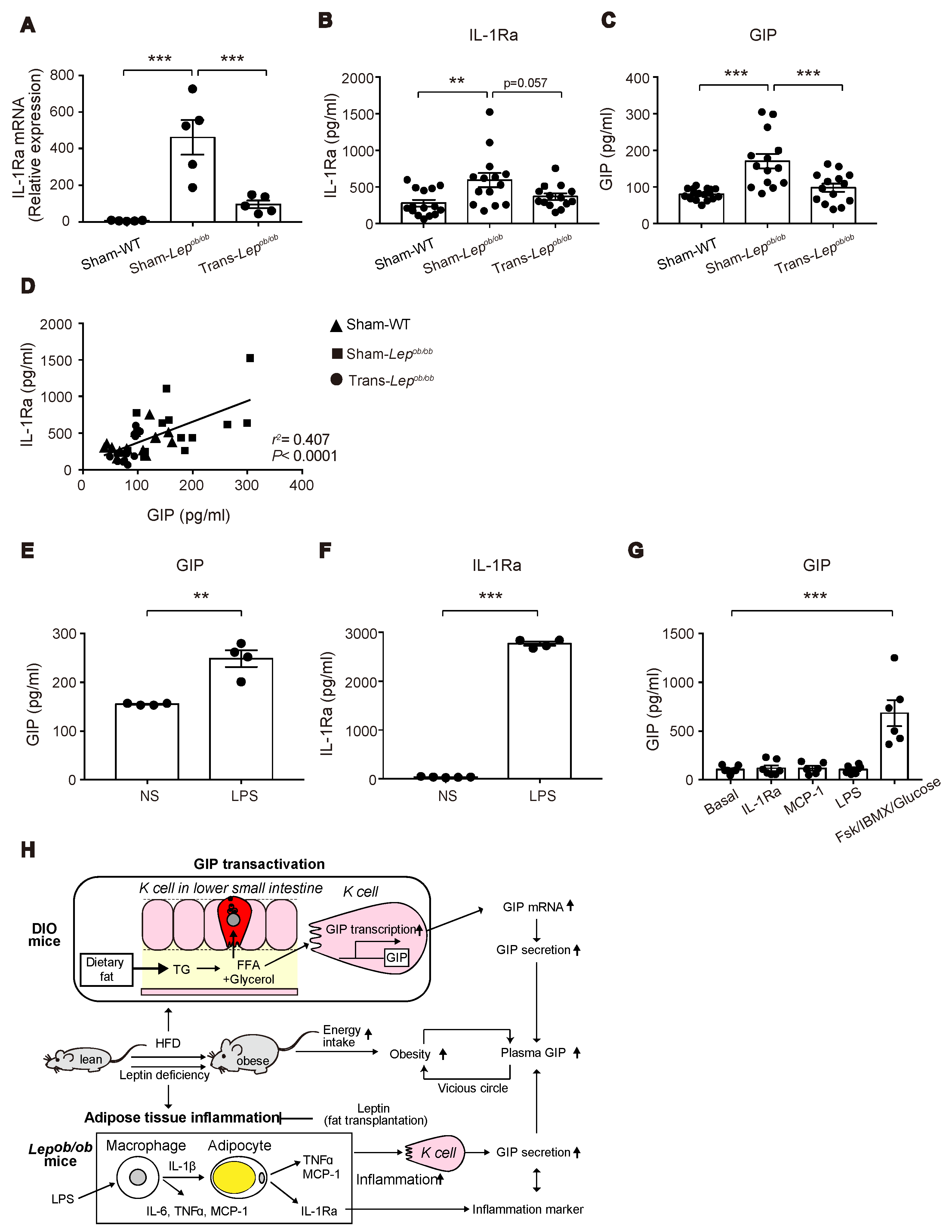
2.6. Messenger RNA and Protein Levels of Interleukin-1 Receptor Antagonist (IL-1Ra) in Lepob/ob Mice Were Elevated, but Were Suppressed by Fat Transplantation, and Its Protein Levels Were Positively Correlated with Plasma GIP Concentrations
2.7. Lipopolysaccharide (LPS) Administration to Mice Increased Plasma Levels of GIP and IL-1Ra
2.8. GIP Secretion Was Not Triggered in the Small Intestinal Organoids by LPS, MCP-1, or IL-1Ra
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experiments on DIO Mice
4.2.1. Measurement of Plasma GIP, K Cell Number, and GIP Expression of DIO Mice
4.2.2. Immuno-Histological Analysis of the Gut
4.2.3. 16S rRNA Gene Analysis
4.2.4. Measurement of Portal SCFAs and GIP after Maltose/Miglitol Administration
4.3. Experiments of Lepob/ob Mice
4.3.1. Fat Transplantation to Lepob/ob Mice
4.3.2. Oral Glucose Tolerance Test and Insulin Tolerance Test
4.4. Experiments of In Vivo GIP and IL-1Ra Secretion by LPS
4.5. Experiments of In Vitro GIP Secretion from the Gut Organoids
4.6. ELISA Assay
4.7. Quantitative Real-Time PCR
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GIP | glucose-dependent insulinotropic polypeptide |
| GLP-1 | glucagon-like peptide 1 |
| HFD | high-fat diet |
| ND | normal diet |
| DIO | diet-induced obesity |
| SCFA | short-chain fatty acid |
| IL-1Ra | interleukin-1 receptor antagonist |
| MCP-1 | monocyte chemoattractant protein-1 |
| IL-6 | interleukin-6 |
| TNF-α | tumor necrosis factor-α |
| IL-1β | interleukin-1β |
| IFN-β | interferon-β |
| LPS | lipopolysaccharide |
| PMA | phorbol myristate acetate |
| FABP5 | fatty acid binding protein 5 |
| α-GI | α-glucosidase inhibitor |
| PCoA | principal coordinate analysis |
| OTU | operational taxonomic unit |
References
- Holst, J.J.; Windelov, J.A.; Boer, G.A.; Pedersen, J.; Svendsen, B.; Christensen, M.; Torekov, S.; Asmar, M.; Hartmann, B.; Nissen, A. Searching for the physiological role of glucose-dependent insulinotropic polypeptide. J. Diabetes Investig. 2016, 7 (Suppl. 1), 8–12. [Google Scholar] [CrossRef]
- Mabilleau, G.; Perrot, R.; Mieczkowska, A.; Boni, S.; Flatt, P.R.; Irwin, N.; Chappard, D. Glucose-dependent insulinotropic polypeptide (GIP) dose-dependently reduces osteoclast differentiation and resorption. Bone 2016, 91, 102–112. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef]
- Nguyen, N.Q.; Debreceni, T.L.; Bambrick, J.E.; Chia, B.; Wishart, J.; Deane, A.M.; Rayner, C.K.; Horowitz, M.; Young, R.L. Accelerated intestinal glucose absorption in morbidly obese humans: Relationship to glucose transporters, incretin hormones, and glycemia. J. Clin. Endocrinol. Metab. 2015, 100, 968–976. [Google Scholar] [CrossRef]
- Miyawaki, K.; Yamada, Y.; Ban, N.; Ihara, Y.; Tsukiyama, K.; Zhou, H.; Fujimoto, S.; Oku, A.; Tsuda, K.; Toyokuni, S.; et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat. Med. 2002, 8, 738–742. [Google Scholar] [CrossRef]
- Yamane, S.; Harada, N.; Inagaki, N. Mechanisms of fat-induced gastric inhibitory polypeptide/glucose-dependent insulinotropic polypeptide secretion from K cells. J. Diabetes Investig. 2016, 7 (Suppl. 1), 20–26. [Google Scholar] [CrossRef]
- Shibue, K.; Yamane, S.; Harada, N.; Hamasaki, A.; Suzuki, K.; Joo, E.; Iwasaki, K.; Nasteska, D.; Harada, T.; Hayashi, Y.; et al. Fatty acid-binding protein 5 regulates diet-induced obesity via GIP secretion from enteroendocrine K cells in response to fat ingestion. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E583–E591. [Google Scholar] [CrossRef]
- Irwin, N.; Flatt, P.R. Enteroendocrine hormone mimetics for the treatment of obesity and diabetes. Curr. Opin. Pharmacol. 2013, 13, 989–995. [Google Scholar] [CrossRef]
- Gasbjerg, L.S.; Gabe, M.B.N.; Hartmann, B.; Christensen, M.B.; Knop, F.K.; Holst, J.J.; Rosenkilde, M.M. Glucose-dependent insulinotropic polypeptide (GIP) receptor antagonists as anti-diabetic agents. Peptides 2018, 100, 173–181. [Google Scholar] [CrossRef]
- Asmar, M.; Asmar, A.; Simonsen, L.; Gasbjerg, L.S.; Sparre-Ulrich, A.H.; Rosenkilde, M.M.; Hartmann, B.; Dela, F.; Holst, J.J.; Bulow, J. The Gluco- and Liporegulatory and Vasodilatory Effects of Glucose-Dependent Insulinotropic Polypeptide (GIP) Are Abolished by an Antagonist of the Human GIP Receptor. Diabetes 2017, 66, 2363–2371. [Google Scholar] [CrossRef]
- Lee, E.Y.; Kaneko, S.; Jutabha, P.; Zhang, X.; Seino, S.; Jomori, T.; Anzai, N.; Miki, T. Distinct action of the alpha-glucosidase inhibitor miglitol on SGLT3, enteroendocrine cells, and GLP1 secretion. J. Endocrinol. 2015, 224, 205–214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, E.Y.; Zhang, X.; Miyamoto, J.; Kimura, I.; Taknaka, T.; Furusawa, K.; Jomori, T.; Fujimoto, K.; Uematsu, S.; Miki, T. Gut carbohydrate inhibits GIP secretion via a microbiota/SCFA/FFAR3 pathway. J. Endocrinol. 2018, 239, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Husted, A.S.; Trauelsen, M.; Rudenko, O.; Hjorth, S.A.; Schwartz, T.W. GPCR-Mediated Signaling of Metabolites. Cell Metab. 2017, 25, 777–796. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Harada, N.; Sasaki, K.; Yamane, S.; Iida, K.; Suzuki, K.; Hamasaki, A.; Nasteska, D.; Shibue, K.; Joo, E.; et al. Free fatty acid receptor GPR120 is highly expressed in enteroendocrine K cells of the upper small intestine and has a critical role in GIP secretion after fat ingestion. Endocrinology 2015, 156, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.L.; Egerod, K.L.; Engelstoft, M.S.; Dmytriyeva, O.; Theodorsson, E.; Patel, B.A.; Schwartz, T.W. Enterochromaffin 5-HT cells—A major target for GLP-1 and gut microbial metabolites. Mol. Metab. 2018, 11, 70–83. [Google Scholar] [CrossRef]
- Mulders, R.J.; de Git, K.C.G.; Schele, E.; Dickson, S.L.; Sanz, Y.; Adan, R.A.H. Microbiota in obesity: Interactions with enteroendocrine, immune and central nervous systems. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2018, 19, 435–451. [Google Scholar] [CrossRef]
- Schulz, M.D.; Atay, C.; Heringer, J.; Romrig, F.K.; Schwitalla, S.; Aydin, B.; Ziegler, P.K.; Varga, J.; Reindl, W.; Pommerenke, C.; et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 2014, 514, 508–512. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation Evaluation and Phylogenetic Diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Bindels, L.B.; Dewulf, E.M.; Delzenne, N.M. GPR43/FFA2: Physiopathological relevance and therapeutic prospects. Trends Pharmacol. Sci. 2013, 34, 226–232. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Flatt, P.R.; Bailey, C.J.; Kwasowski, P.; Swanston-Flatt, S.K.; Marks, V. Abnormalities of GIP in spontaneous syndromes of obesity and diabetes in mice. Diabetes 1983, 32, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Shimazu-Kuwahara, S.; Harada, N.; Yamane, S.; Joo, E.; Sankoda, A.; Kieffer, T.J.; Inagaki, N. Attenuated secretion of glucose-dependent insulinotropic polypeptide (GIP) does not alleviate hyperphagic obesity and insulin resistance in ob/ob mice. Mol. Metab. 2017, 6, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Klebanov, S.; Astle, C.M.; DeSimone, O.; Ablamunits, V.; Harrison, D.E. Adipose tissue transplantation protects ob/ob mice from obesity, normalizes insulin sensitivity and restores fertility. J. Endocrinol. 2005, 186, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Juge-Aubry, C.E.; Somm, E.; Giusti, V.; Pernin, A.; Chicheportiche, R.; Verdumo, C.; Rohner-Jeanrenaud, F.; Burger, D.; Dayer, J.M.; Meier, C.A. Adipose tissue is a major source of interleukin-1 receptor antagonist: Upregulation in obesity and inflammation. Diabetes 2003, 52, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cai, X.; Yang, W.; Chen, Y.; Han, X.; Ji, L. Meta-analysis and critical review on the efficacy and safety of alpha-glucosidase inhibitors in Asian and non-Asian populations. J. Diabetes Investig. 2018, 9, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tong, Y.; Zhang, Y.; Huang, L.; Wu, T.; Tong, N. Acarbose monotherapy and weight loss in Eastern and Western populations with hyperglycaemia: An ethnicity-specific meta-analysis. Int. J. Clin. Pract. 2014, 68, 1318–1332. [Google Scholar] [CrossRef] [PubMed]
- Do, H.J.; Lee, Y.S.; Ha, M.J.; Cho, Y.; Yi, H.; Hwang, Y.J.; Hwang, G.S.; Shin, M.J. Beneficial effects of voglibose administration on body weight and lipid metabolism via gastrointestinal bile acid modification. Endocr. J. 2016, 63, 691–702. [Google Scholar] [CrossRef]
- Sugimoto, S.; Nakajima, H.; Kosaka, K.; Hosoi, H. Review: Miglitol has potential as a therapeutic drug against obesity. Nutr. Metab. 2015, 12, 51. [Google Scholar] [CrossRef]
- Chehab, F.F. 20 years of leptin: Leptin and reproduction: Past milestones, present undertakings, and future endeavors. J. Endocrinol. 2014, 223, T37–T48. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Obesity-induced inflammatory changes in adipose tissue. J. Clin. Investig. 2003, 112, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Osborn, O.; Olefsky, J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 2012, 18, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y.; Maron, R.; Tukpah, A.M.; Maioli, T.U.; Murugaiyan, G.; Yang, K.; Wu, H.Y.; Weiner, H.L. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc. Natl. Acad. Sci. USA 2010, 107, 9765–9770. [Google Scholar] [CrossRef] [PubMed]
- Kahles, F.; Meyer, C.; Diebold, S.; Foldenauer, A.C.; Stohr, R.; Mollmann, J.; Lebherz, C.; Findeisen, H.M.; Marx, N.; Lehrke, M. Glucose-dependent insulinotropic peptide secretion is induced by inflammatory stimuli in an interleukin-1-dependent manner in mice. Diabetes Obes. Metab. 2016, 18, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Stienstra, R.; Tack, C.J.; Kanneganti, T.D.; Joosten, L.A.; Netea, M.G. The inflammasome puts obesity in the danger zone. Cell Metab. 2012, 15, 10–18. [Google Scholar] [CrossRef]
- Ballak, D.B.; Stienstra, R.; Tack, C.J.; Dinarello, C.A.; van Diepen, J.A. IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance. Cytokine 2015, 75, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Juge-Aubry, C.E.; Somm, E.; Chicheportiche, R.; Burger, D.; Pernin, A.; Cuenod-Pittet, B.; Quinodoz, P.; Giusti, V.; Dayer, J.M.; Meier, C.A. Regulatory effects of interleukin (IL)-1, interferon-beta, and IL-4 on the production of IL-1 receptor antagonist by human adipose tissue. J. Clin. Endocrinol. Metab. 2004, 89, 2652–2658. [Google Scholar] [CrossRef]
- Meier, C.A.; Bobbioni, E.; Gabay, C.; Assimacopoulos-Jeannet, F.; Golay, A.; Dayer, J.M. IL-1 receptor antagonist serum levels are increased in human obesity: A possible link to the resistance to leptin? J. Clin. Endocrinol. Metab. 2002, 87, 1184–1188. [Google Scholar] [CrossRef]
- Charles, B.A.; Doumatey, A.; Huang, H.; Zhou, J.; Chen, G.; Shriner, D.; Adeyemo, A.; Rotimi, C.N. The roles of IL-6, IL-10, and IL-1RA in obesity and insulin resistance in African-Americans. J. Clin. Endocrinol. Metab. 2011, 96, E2018–E2022. [Google Scholar] [CrossRef]
- Nowak, C.; Sundstrom, J.; Gustafsson, S.; Giedraitis, V.; Lind, L.; Ingelsson, E.; Fall, T. Protein Biomarkers for Insulin Resistance and Type 2 Diabetes Risk in Two Large Community Cohorts. Diabetes 2016, 65, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lee, J.; Salazar Hernandez, M.A.; Mazitschek, R.; Ozcan, U. Treatment of obesity with celastrol. Cell 2015, 161, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Liu, J.; Feng, X.; Salazar Hernandez, M.A.; Mucka, P.; Ibi, D.; Choi, J.W.; Ozcan, U. Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nat. Med. 2016, 22, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Grisouard, J.; Sauter, N.S.; Herzog-Radimerski, T.; Dembinski, K.; Peterli, R.; Frey, D.M.; Zulewski, H.; Keller, U.; Muller, B.; et al. Glucose-dependent insulinotropic polypeptide induces cytokine expression, lipolysis, and insulin resistance in human adipocytes. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1–E13. [Google Scholar] [CrossRef] [PubMed]
- Usui, Y.; Kimura, Y.; Satoh, T.; Takemura, N.; Ouchi, Y.; Ohmiya, H.; Kobayashi, K.; Suzuki, H.; Koyama, S.; Hagiwara, S.; et al. Effects of long-term intake of a yogurt fermented with Lactobacillus delbrueckii subsp. bulgaricus 2038 and Streptococcus thermophilus 1131 on mice. Int. Immunol. 2018, 30, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Eri, T.; Kawahata, K.; Kanzaki, T.; Imamura, M.; Michishita, K.; Akahira, L.; Bannai, E.; Yoshikawa, N.; Kimura, Y.; Satoh, T.; et al. Intestinal microbiota link lymphopenia to murine autoimmunity via PD-1(+)CXCR5(-/dim) B-helper T cell induction. Sci. Rep. 2017, 7, 46037. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, T.; Sakurai, K.; Lee, E.Y.; Yokote, K.; Accili, D.; Miki, T. Distinct roles of systemic and local actions of insulin on pancreatic beta-cells. Metab. Clin. Exp. 2018, 82, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Kaga, N.; Nakanishi, Y.; Ohno, H.; Miyamoto, J.; Kimura, I.; Hori, S.; Sasaki, T.; Hiramatsu, K.; Okumura, K.; et al. Maternal High Fiber Diet during Pregnancy and Lactation Influences Regulatory T Cell Differentiation in Offspring in Mice. J. Immunol. 2017, 199, 3516–3524. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Sakurai, K.; Zhang, X.; Toda, C.; Tanaka, T.; Jiang, M.; Shirasawa, T.; Tachibana, K.; Yokote, K.; Vidal-Puig, A.; et al. Unsuppressed lipolysis in adipocytes is linked with enhanced gluconeogenesis and altered bile acid physiology in Insr(P1195L/+) mice fed high-fat-diet. Sci. Rep. 2015, 5, 17565. [Google Scholar] [CrossRef]
- Goldspink, D.A.; Lu, V.B.; Billing, L.J.; Larraufie, P.; Tolhurst, G.; Gribble, F.M.; Reimann, F. Mechanistic insights into the detection of free fatty and bile acids by ileal glucagon-like peptide-1 secreting cells. Mol. Metab. 2018, 7, 90–101. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.; Miedzybrodzka, E.L.; Zhang, X.; Hatano, R.; Miyamoto, J.; Kimura, I.; Fujimoto, K.; Uematsu, S.; Rodriguez-Cuenca, S.; Vidal-Puig, A.; et al. Diet-Induced Obese Mice and Leptin-Deficient Lepob/ob Mice Exhibit Increased Circulating GIP Levels Produced by Different Mechanisms. Int. J. Mol. Sci. 2019, 20, 4448. https://doi.org/10.3390/ijms20184448
Lee E, Miedzybrodzka EL, Zhang X, Hatano R, Miyamoto J, Kimura I, Fujimoto K, Uematsu S, Rodriguez-Cuenca S, Vidal-Puig A, et al. Diet-Induced Obese Mice and Leptin-Deficient Lepob/ob Mice Exhibit Increased Circulating GIP Levels Produced by Different Mechanisms. International Journal of Molecular Sciences. 2019; 20(18):4448. https://doi.org/10.3390/ijms20184448
Chicago/Turabian StyleLee, Eunyoung, Emily L. Miedzybrodzka, Xilin Zhang, Ryo Hatano, Junki Miyamoto, Ikuo Kimura, Kosuke Fujimoto, Satoshi Uematsu, Sergio Rodriguez-Cuenca, Antonio Vidal-Puig, and et al. 2019. "Diet-Induced Obese Mice and Leptin-Deficient Lepob/ob Mice Exhibit Increased Circulating GIP Levels Produced by Different Mechanisms" International Journal of Molecular Sciences 20, no. 18: 4448. https://doi.org/10.3390/ijms20184448
APA StyleLee, E., Miedzybrodzka, E. L., Zhang, X., Hatano, R., Miyamoto, J., Kimura, I., Fujimoto, K., Uematsu, S., Rodriguez-Cuenca, S., Vidal-Puig, A., Gribble, F. M., Reimann, F., & Miki, T. (2019). Diet-Induced Obese Mice and Leptin-Deficient Lepob/ob Mice Exhibit Increased Circulating GIP Levels Produced by Different Mechanisms. International Journal of Molecular Sciences, 20(18), 4448. https://doi.org/10.3390/ijms20184448





