From Osteoclast Differentiation to Osteonecrosis of the Jaw: Molecular and Clinical Insights
Abstract
1. Osteoclast Differentiation
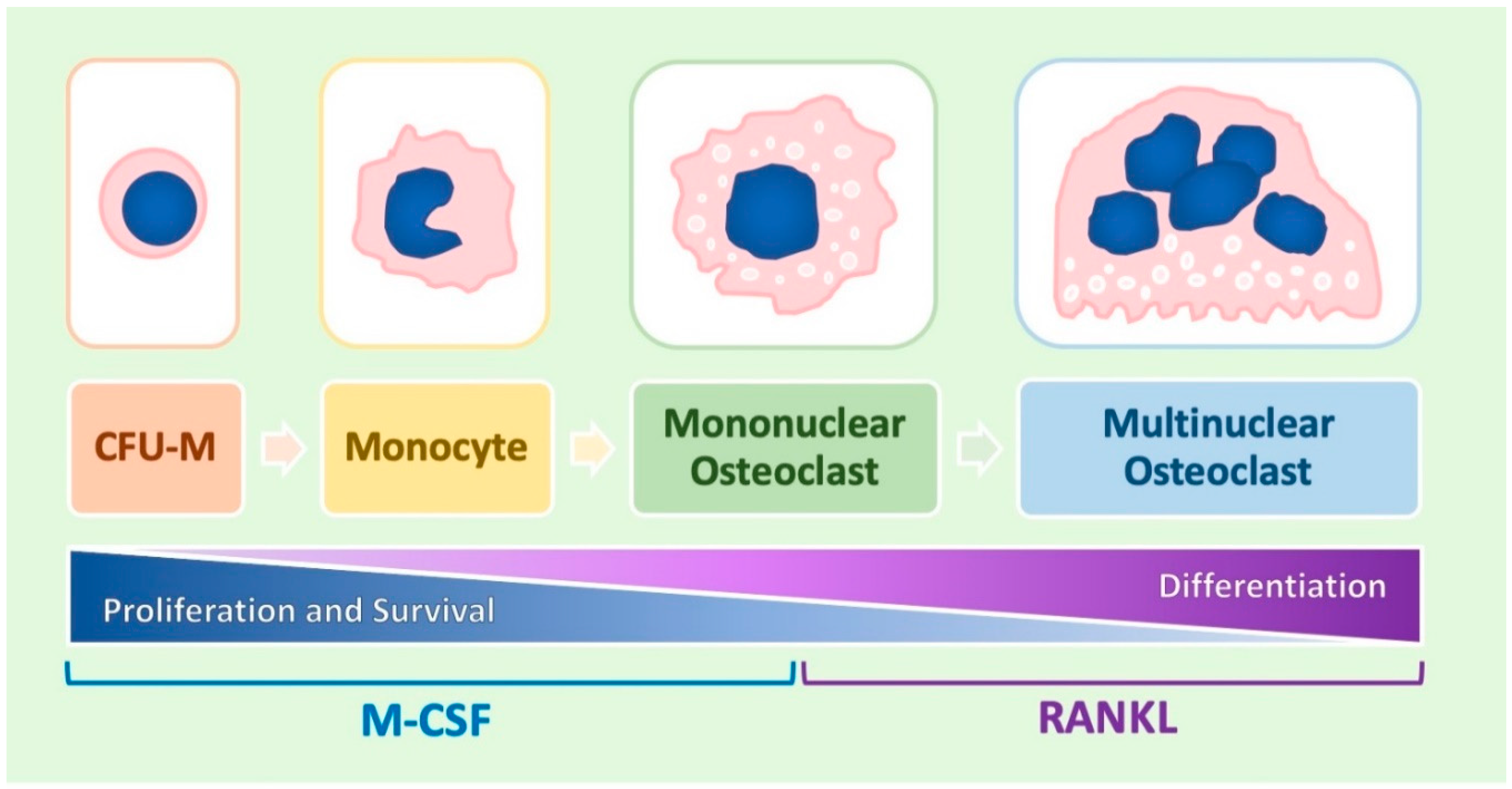
1.1. A General Overview of Osteoclast Differentiation
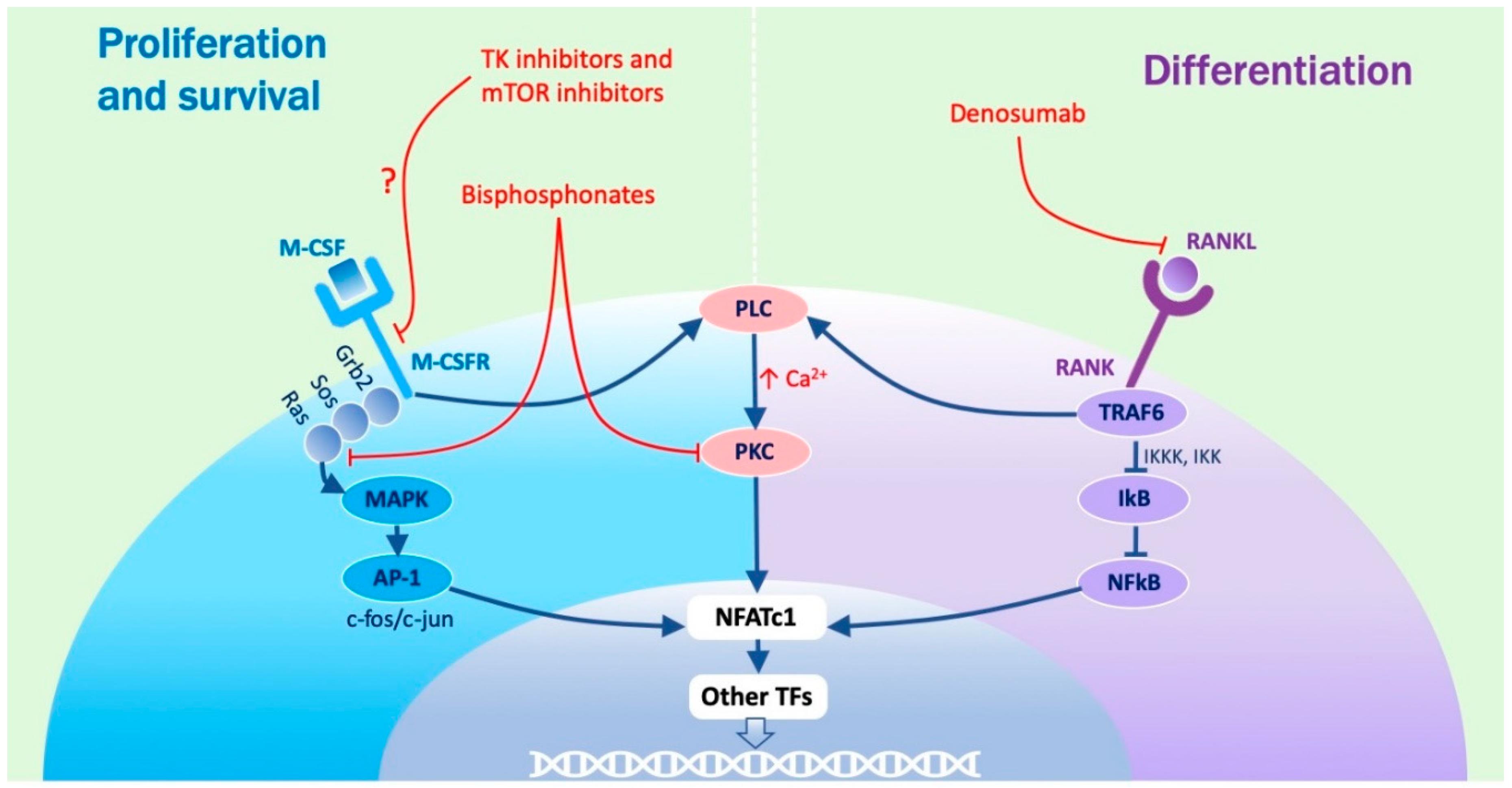
1.2. Molecular Regulation of the Process
1.3. Role Played by M-CSF
1.4. Role Played by RANKL
1.5. Role of Osteoclast Differentiation Markers
1.6. Transcription Factors Regulating NFATc1 Activity
1.7. Role Played by PKC
1.8. Role Played by Vitamin D3
1.9. Role of Cytokines, Macrophage Polarization, and Chemical Factors
1.10. The Cross-Talk between Osteoblasts and Osteoclasts
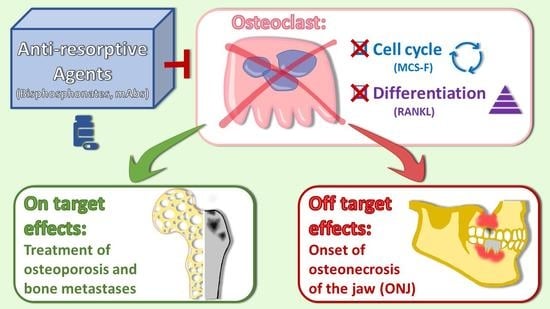
1.11. Osteoclasts as Therapeutic Targets in Human Diseases
1.12. Bisphosphonates
1.13. Denosumab
2. A Pathological Condition Related to Anti-Resorptive Agents: Osteonecrosis of the Jaw
2.1. Osteoclasts Dependent Factors
2.2. Osteoclast-Independent Factors
2.2.1. Invasive Procedures and Possibly Delayed Healing
2.2.2. Angiogenesis
3. Clinical Definition, Epidemiology, and Risk Factors for Osteonecrosis of the JAW
- (1)
- Exposed bone in the maxillofacial region which does not heal within 8 weeks after identification by a health care provider;
- (2)
- Exposure to an anti-resorptive agent (BPs or DMAb);
- (3)
- No history of radiation therapy to the craniofacial region.
- (1)
- dental extraction (61.7%)
- (2)
- spontaneous occurrence (14.8%)
- (3)
- oral surgery (7.2%)
- (4)
- prosthodontic trauma (7.4%)
- (5)
- periodontitis (5.0%)
- (6)
- dental implant treatment (3.9%)
Drug Holiday and Treatment
4. Conclusions and Hypothesis on Pathogenesis of Osteonecrosis of the JAW
Author Contributions
Funding
Conflicts of Interest
References
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.R.; Mundy, G.R. Advances in osteoclast biology: Old findings and new insights from mouse models. Nat. Rev. Rheumatol. 2011, 7, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, N. Signaling Pathways in Osteoclast Differentiation. Chonnam. Med. J. 2016, 52, 12. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xiu, Y.; Li, J.; Xing, L.; Yao, Z. NF-κB-Mediated Regulation of Osteoclastogenesis. Endocrinol. Metab. 2015, 30, 35. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Gohda, J.; Akiyama, T.; Koga, T.; Takayanagi, H.; Tanaka, S.; Inoue, J. RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J. 2005, 24, 790–799. [Google Scholar] [CrossRef]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef]
- Wagner, E.F.; Eferl, R. Fos/AP-1 proteins in bone and the immune system. Immunol. Rev. 2005, 208, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, K.; Yashiro, T.; Nakano, N.; Kasakura, K.; Miura, R.; Hara, M.; Kawai, F.; Maeda, K.; Tamura, N.; Okumura, K.; et al. Involvement of PU.1 in NFATc1 promoter function in osteoclast development. Allergol. Int. 2015, 64, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Weilbaecher, K.N.; Motyckova, G.; Huber, W.E.; Takemoto, C.M.; Hemesath, T.J.; Xu, Y.; Hershey, C.L.; Dowland, N.R.; Wells, A.G.; Fisher, D.E. Linkage of M-CSF signaling to Mitf, TFE3, and the osteoclast defect in Mitf(mi/mi) mice. Mol. Cell 2001, 8, 749–758. [Google Scholar] [CrossRef]
- Zanocco-Marani, T.; Vignudelli, T.; Gemelli, C.; Pirondi, S.; Testa, A.; Montanari, M.; Parenti, S.; Tenedini, E.; Grande, A.; Ferrari, S. Tfe3 expression is closely associated to macrophage terminal differentiation of human hematopoietic myeloid precursors. Exp. Cell Res. 2006, 312, 4079–4089. [Google Scholar] [CrossRef] [PubMed]
- Zanocco-Marani, T.; Vignudelli, T.; Parenti, S.; Gemelli, C.; Condorelli, F.; Martello, A.; Selmi, T.; Grande, A.; Ferrari, S. TFE3 transcription factor regulates the expression of MAFB during macrophage differentiation. Exp. Cell Res. 2009, 315, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.H.; Lee, J.; Jin, H.M.; Kook, H.; Kim, K.K.; Lee, S.Y.; Kim, N. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood 2007, 109, 3253–3259. [Google Scholar] [CrossRef] [PubMed]
- Mammoli, F.; Castiglioni, S.; Parenti, S.; Cappadone, C.; Farruggia, G.; Iotti, S.; Davalli, P.; Maier, J.A.M.; Grande, A.; Frassineti, C. Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D3. Int. J. Mol. Sci. 2019, 20, 385. [Google Scholar] [CrossRef]
- Gemelli, C.; Montanari, M.; Tenedini, E.; Zanocco Marani, T.; Vignudelli, T.; Siena, M.; Zini, R.; Salati, S.; Tagliafico, E.; Manfredini, R.; et al. Virally mediated MafB transduction induces the monocyte commitment of human CD34+ hematopoietic stem/progenitor cells. Cell Death Differ. 2006, 13, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Gemelli, C.; Zanocco Marani, T.; Bicciato, S.; Mazza, E.M.C.; Boraschi, D.; Salsi, V.; Zappavigna, V.; Parenti, S.; Selmi, T.; Tagliafico, E.; et al. MafB is a downstream target of the IL-10/STAT3 signaling pathway, involved in the regulation of macrophage de-activation. Biochim. Biophys. Acta 2014, 1843, 955–964. [Google Scholar] [CrossRef]
- Italiani, P.; Mazza, E.M.C.; Lucchesi, D.; Cifola, I.; Gemelli, C.; Grande, A.; Battaglia, C.; Bicciato, S.; Boraschi, D. Transcriptomic profiling of the development of the inflammatory response in human monocytes in vitro. PLoS ONE 2014, 9, e87680. [Google Scholar] [CrossRef]
- Miyamoto, T. Regulators of osteoclast differentiation and cell-cell fusion. Keio J. Med. 2011, 60, 101–105. [Google Scholar] [CrossRef]
- Hwang, S.-Y.; Putney, J.W. Calcium signaling in osteoclasts. Biochim. Biophys. Acta 2011, 1813, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kwak, H.B.; Chung, W.J.; Cheong, H.; Kim, H.-H.; Lee, Z.H. Participation of protein kinase C beta in osteoclast differentiation and function. Bone 2003, 32, 217–227. [Google Scholar] [CrossRef]
- Li, A.; Cong, Q.; Xia, X.; Leong, W.F.; Yeh, J.; Miao, D.; Mishina, Y.; Liu, H.; Li, B. Pharmacologic Calcitriol Inhibits Osteoclast Lineage Commitment via the BMP-Smad1 and IκB-NF-κB Pathways. J. Bone Miner. Res. 2017, 32, 1406–1420. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Udagawa, N.; Udagawa, N.; Suda, T. Vitamin D endocrine system and osteoclasts. Bonekey Rep. 2014, 3, 495. [Google Scholar] [CrossRef] [PubMed]
- Grande, A.; Manfredini, R.; Pizzanelli, M.; Tagliafico, E.; Balestri, R.; Trevisan, F.; Barbieri, D.; Franceschi, C.; Battini, R.; Ferrari, S.; et al. Presence of a functional vitamin D receptor does not correlate with vitamin D3 phenotypic effects in myeloid differentiation. Cell Death Differ. 1997, 4, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Grande, A.; Montanari, M.; Tagliafico, E.; Manfredini, R.; Zanocco Marani, T.; Siena, M.; Tenedini, E.; Gallinelli, A.; Ferrari, S. Physiological levels of 1alpha, 25 dihydroxyvitamin D3 induce the monocytic commitment of CD34+ hematopoietic progenitors. J. Leukoc. Biol. 2002, 71, 641–651. [Google Scholar]
- Montanari, M.; Gemelli, C.; Tenedini, E.; Zanocco Marani, T.; Vignudelli, T.; Siena, M.; Zini, R.; Salati, S.; Chiossi, G.; Tagliafico, E.; et al. Correlation between differentiation plasticity and mRNA expression profiling of CD34+-derived CD14- and CD14+ human normal myeloid precursors. Cell Death Differ. 2005, 12, 1588–1600. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gemelli, C.; Orlandi, C.; Zanocco Marani, T.; Martello, A.; Vignudelli, T.; Ferrari, F.; Montanari, M.; Parenti, S.; Testa, A.; Grande, A.; et al. The vitamin D3/Hox-A10 pathway supports MafB function during the monocyte differentiation of human CD34+ hemopoietic progenitors. J. Immunol. 2008, 181, 5660–5672. [Google Scholar] [CrossRef]
- Gemelli, C.; Martello, A.; Montanari, M.; Zanocco Marani, T.; Salsi, V.; Zappavigna, V.; Parenti, S.; Vignudelli, T.; Selmi, T.; Ferrari, S.; et al. The Orosomucoid 1 protein is involved in the vitamin D - mediated macrophage de-activation process. Exp. Cell Res. 2013, 319, 3201–3213. [Google Scholar] [CrossRef]
- Amoui, M.; Suhr, S.-M.; Baylink, D.J.; Lau, K.-H.W. An osteoclastic protein-tyrosine phosphatase may play a role in differentiation and activity of human monocytic U-937 cell-derived, osteoclast-like cells. Am. J. Physiol. Cell Physiol. 2004, 287, C874–C884. [Google Scholar] [CrossRef]
- Takeda, S.; Yoshizawa, T.; Nagai, Y.; Yamato, H.; Fukumoto, S.; Sekine, K.; Kato, S.; Matsumoto, T.; Fujita, T. Stimulation of osteoclast formation by 1,25-dihydroxyvitamin D requires its binding to vitamin D receptor (VDR) in osteoblastic cells: Studies using VDR knockout mice. Endocrinology 1999, 140, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Maeda, K.; Ishihara, A.; Uehara, S.; Kobayashi, Y. Regulatory mechanism of osteoclastogenesis by RANKL and Wnt signals. Front. Biosci. Landmark Ed. 2011, 16, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Prince, C.W.; Butler, W.T. 1,25-Dihydroxyvitamin D3 regulates the biosynthesis of osteopontin, a bone-derived cell attachment protein, in clonal osteoblast-like osteosarcoma cells. Coll. Relat. Res. 1987, 7, 305–313. [Google Scholar] [CrossRef]
- Reinholt, F.P.; Hultenby, K.; Oldberg, A.; Heinegard, D. Osteopontin—A possible anchor of osteoclasts to bone. Proc. Natl. Acad. Sci. USA 1990, 87, 4473–4475. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.; Jourdain, M.-L.; Braux, J.; Guillaume, C.; Gangloff, S.C.; Jacquot, J.; Velard, F. An Optimized Method to Generate Human Active Osteoclasts From Peripheral Blood Monocytes. Front. Immunol. 2018, 9, 632. [Google Scholar] [CrossRef]
- Zarei, A.; Morovat, A.; Javaid, K.; Brown, C.P. Vitamin D receptor expression in human bone tissue and dose-dependent activation in resorbing osteoclasts. Bone Res. 2016, 4, 16030. [Google Scholar] [CrossRef]
- Starczak, Y.; Reinke, D.C.; Barratt, K.R.; Ryan, J.W.; Russell, P.K.; Clarke, M.V.; St-Arnaud, R.; Morris, H.A.; Davey, R.A.; Atkins, G.J.; et al. Absence of vitamin D receptor in mature osteoclasts results in altered osteoclastic activity and bone loss. J. Steroid Biochem. Mol. Biol. 2018, 177, 77–82. [Google Scholar] [CrossRef]
- Ormsby, R.T.; Findlay, D.M.; Kogawa, M.; Anderson, P.H.; Morris, H.A.; Atkins, G.J. Analysis of vitamin D metabolism gene expression in human bone: Evidence for autocrine control of bone remodelling. J. Steroid Biochem. Mol. Biol. 2014, 144, 110–113. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw. 2018, 18. [Google Scholar] [CrossRef]
- Fukui, S.; Iwamoto, N.; Takatani, A.; Igawa, T.; Shimizu, T.; Umeda, M.; Nishino, A.; Horai, Y.; Hirai, Y.; Koga, T.; et al. M1 and M2 Monocytes in Rheumatoid Arthritis: A Contribution of Imbalance of M1/M2 Monocytes to Osteoclastogenesis. Front. Immunol. 2018, 8. [Google Scholar] [CrossRef]
- He, D.; Kou, X.; Luo, Q.; Yang, R.; Liu, D.; Wang, X.; Song, Y.; Cao, H.; Zeng, M.; Gan, Y.; et al. Enhanced M1/M2 macrophage ratio promotes orthodontic root resorption. J. Dent. Res. 2015, 94, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.-L.; Xu, M.-H.; Li, X.; Xinlong, H.; Fang, W.; Dong, J. The Roles of Acidosis in Osteoclast Biology. Front. Physiol. 2016, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Park, B.; Yoon, D.S.; Kwon, S.H.; Shin, D.M.; Lee, J.W.; Lee, H.G.; Shim, J.H.; Park, J.H.; Lee, J.M. Zinc inhibits osteoclast differentiation by suppression of Ca2+-Calcineurin-NFATc1 signaling pathway. Cell Commun. Signal. 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Kenkre, J.S.; Bassett, J.H.D. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Rodan, G.A. Bone mass homeostasis and bisphosphonate action. Bone 1997, 20, 1–4. [Google Scholar] [CrossRef]
- Gong, L.; Altman, R.B.; Klein, T.E. Bisphosphonates pathway. Pharmacogenet. Genomics 2011, 21, 50–53. [Google Scholar] [CrossRef]
- Kimmel, D.B. Mechanism of Action, Pharmacokinetic and Pharmacodynamic Profile, and Clinical Applications of Nitrogen-containing Bisphosphonates. J. Dent. Res. 2007, 86, 1022–1033. [Google Scholar] [CrossRef]
- Baron, R.; Ferrari, S.; Russell, R.G.G. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone 2011, 48, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Aghaloo, T.L.; Felsenfeld, A.L.; Tetradis, S. Osteonecrosis of the Jaw in a Patient on Denosumab; W.B. Saunders: Philadelphia, PA, USA, 2010; Volume 68, pp. 959–963. [Google Scholar]
- Pimolbutr, K.; Porter, S.; Fedele, S. Osteonecrosis of the Jaw Associated with Antiangiogenics in Antiresorptive-Naïve Patient: A Comprehensive Review of the Literature. Biomed Res. Int. 2018, 2018, 8071579. [Google Scholar] [CrossRef]
- Nicolatou-Galitis, O.; Kouri, M.; Papadopoulou, E.; Vardas, E.; Galiti, D.; Epstein, J.B.; Elad, S.; Campisi, G.; Tsoukalas, N.; Bektas-Kayhan, K.; et al. Osteonecrosis of the jaw related to non-antiresorptive medications: A systematic review. Support. Care Cancer 2019, 27, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Ismaila, N.; Flynn, P.J.; Halabi, S.; Jagannath, S.; Ogaily, M.S.; Omel, J.; Raje, N.; Roodman, G.D.; Yee, G.C.; et al. Role of Bone-Modifying Agents in Multiple Myeloma: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 812–818. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. National Comprehensive Cancer Network Guidelines. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 22 September 2019).
- Gross, C.; Weber, M.; Creutzburg, K.; Möbius, P.; Preidl, R.; Amann, K.; Wehrhan, F. Osteoclast profile of medication-related osteonecrosis of the jaw secondary to bisphosphonate therapy: A comparison with osteoradionecrosis and osteomyelitis. J. Transl. Med. 2017, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Wehrhan, F.; Gross, C.; Creutzburg, K.; Amann, K.; Ries, J.; Kesting, M.; Geppert, C.-I.; Weber, M. Osteoclastic expression of higher-level regulators NFATc1 and BCL6 in medication-related osteonecrosis of the jaw secondary to bisphosphonate therapy: A comparison with osteoradionecrosis and osteomyelitis. J. Transl. Med. 2019, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.W.; Lee, C.; Kim, T.; Yagita, H.; Wu, H.; Park, S.; Yang, P.; Liu, H.; Shi, S.; Shin, K.-H.; et al. Impaired bone resorption and woven bone formation are associated with development of osteonecrosis of the jaw-like lesions by bisphosphonate and anti-receptor activator of NF-κB ligand antibody in mice. Am. J. Pathol. 2014, 184, 3084–3093. [Google Scholar] [CrossRef]
- Kim, J.-W.; Alfafara, A.M.D.; Kim, H.-Y.; Kim, S.-Y.; Kim, S.-J. Effects of pH alteration on the pathogenesis of medication-related osteonecrosis of the jaw. Bone 2019, 122, 45–51. [Google Scholar] [CrossRef]
- Gong, X.; Yu, W.; Zhao, H.; Su, J.; Sheng, Q. Skeletal Site-specific Effects of Zoledronate on in vivo Bone Remodeling and in vitro BMSCs Osteogenic Activity. Sci. Rep. 2017, 7, 36129. [Google Scholar] [CrossRef]
- Cremers, S.; Drake, M.T.; Ebetino, F.H.; Bilezikian, J.P.; Russell, R.G.G. Pharmacology of bisphosphonates. Br. J. Clin. Pharmacol. 2019, 85, 1052–1062. [Google Scholar] [CrossRef]
- Wen, D.; Qing, L.; Harrison, G.; Golub, E.; Akintoye, S.O. Anatomic site variability in rat skeletal uptake and desorption of fluorescently labeled bisphosphonate. Oral. Dis. 2011, 17, 427–432. [Google Scholar] [CrossRef]
- Yamazaki, T.; Yamori, M.; Ishizaki, T.; Asai, K.; Goto, K.; Takahashi, K.; Nakayama, T.; Bessho, K. Increased incidence of osteonecrosis of the jaw after tooth extraction in patients treated with bisphosphonates: A cohort study. Int. J. Oral Maxillofac. Surg. 2012, 41, 1397–1403. [Google Scholar] [CrossRef]
- Chang, J.; Hakam, A.E.; McCauley, L.K. Current Understanding of the Pathophysiology of Osteonecrosis of the Jaw. Curr. Osteoporos. Rep. 2018, 16, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xu, R.; Du, J.; Fu, Y.; Li, S.; Zhang, P.; Liu, L.; Jiang, H. Zoledronic acid promotes TLR-4-mediated M1 macrophage polarization in bisphosphonate-related osteonecrosis of the jaw. FASEB J. 2019, 33, 5208–5219. [Google Scholar] [CrossRef] [PubMed]
- Patntirapong, S.; Poolgesorn, M. Alteration of macrophage viability, differentiation, and function by bisphosphonates. Oral. Dis. 2018, 24, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; Bonjean, K.; Ruetz, S.; Bellahcène, A.; Devy, L.; Foidart, J.M.; Castronovo, V.; Green, J.R. Novel Antiangiogenic Effects of the Bisphosphonate Compound Zoledronic Acid. J. Pharmacol. Exp. Ther. 2002, 302, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Innao, V.; Pulvirenti, N.; Musolino, C. Antiresorptive Agents and Anti-Angiogenesis Drugs in the Development of Osteonecrosis of the Jaw. Tohoku J. Exp. Med. 2019, 248, 27–29. [Google Scholar] [CrossRef]
- Kün-Darbois, J.-D.; Libouban, H.; Mabilleau, G.; Pascaretti-Grizon, F.; Chappard, D. Bone mineralization and vascularization in bisphosphonate-related osteonecrosis of the jaw: An experimental study in the rat. Clin. Oral. Investig. 2018, 22, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.Q.S.; Van Dessel, J.; Jacobs, R.; da Silva Santos, P.S.; Cestari, T.M.; Garlet, G.P.; Duarte, M.A.H.; Imada, T.S.N.; Lambrichts, I.; Rubira-Bullen, I.R.F. Zoledronic Acid Induces Site-Specific Structural Changes and Decreases Vascular Area in the Alveolar Bone. J. Oral Maxillofac. Surg. 2018, 76, 1893–1901. [Google Scholar] [CrossRef]
- Oteri, G.; Allegra, A.; Bellomo, G.; Alonci, A.; Nastro, E.; Penna, G.; Catalfamo, L.; Cicciù, D.; De Ponte, F.S.; Musolino, C. Reduced serum levels of Interleukin 17 in patients with osteonecrosis of the jaw and in multiple myeloma subjects after bisphosphonates administration. Cytokine 2008, 43, 103–104. [Google Scholar] [CrossRef]
- Troeltzsch, M.; Woodlock, T.; Kriegelstein, S.; Steiner, T.; Messlinger, K.; Troeltzsch, M. Physiology and pharmacology of nonbisphosphonate drugs implicated in osteonecrosis of the jaw. J. Can. Dent. Assoc. 2012, 78, c85. [Google Scholar]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw—2014 Update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and Management of Osteonecrosis of the Jaw: A Systematic Review and International Consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Fedele, S.; Bedogni, G.; Scoletta, M.; Favia, G.; Colella, G.; Agrillo, A.; Bettini, G.; Di Fede, O.; Oteri, G.; Fusco, V.; et al. Up to a quarter of patients with osteonecrosis of the jaw associated with antiresorptive agents remain undiagnosed. Br. J. Oral Maxillofac. Surg. 2015, 53, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Morrison, A.; Cheung, A.; Hashem, W.; Compston, J. Osteonecrosis of the jaw (ONJ): Diagnosis and management in 2015. Osteoporos. Int. 2016, 27, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Fliefel, R.; Tröltzsch, M.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 568–585. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Tröltzsch, M.; Jambrovic, V.; Panya, S.; Probst, F.; Ristow, O.; Ehrenfeld, M.; Pautke, C. Tooth extraction in patients receiving oral or intravenous bisphosphonate administration: A trigger for BRONJ development? J. Cranio-Maxillofacial Surg. 2015, 43, 847–854. [Google Scholar] [CrossRef]
- Panya, S.; Fliefel, R.; Probst, F.; Tröltzsch, M.; Ehrenfeld, M.; Schubert, S.; Otto, S. Role of microbiological culture and polymerase chain reaction (PCR) of actinomyces in medication-related osteonecrosis of the jaw (MRONJ). J. Cranio-Maxillofacial Surg. 2017, 45, 357–363. [Google Scholar] [CrossRef]
- Schiodt, M.; Otto, S.; Fedele, S.; Bedogni, A.; Nicolatou-Galitis, O.; Guggenberger, R.; Herlofson, B.B.; Ristow, O.; Kofod, T. Workshop of European task force on medication-related osteonecrosis of the jaw—Current challenges. Oral Dis. 2019. [Google Scholar] [CrossRef]
- Schiodt, M.; Reibel, J.; Oturai, P.; Kofod, T. Comparison of nonexposed and exposed bisphosphonate-induced osteonecrosis of the jaws: A retrospective analysis from the Copenhagen cohort and a proposal for an updated classification system. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 204–213. [Google Scholar] [CrossRef]
- Bedogni, A.; Blandamura, S.; Lokmic, Z.; Palumbo, C.; Ragazzo, M.; Ferrari, F.; Tregnaghi, A.; Pietrogrande, F.; Procopio, O.; Saia, G.; et al. Bisphosphonate-associated jawbone osteonecrosis: A correlation between imaging techniques and histopathology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105, 358–364. [Google Scholar] [CrossRef]
- Fedele, S.; Porter, S.R.; D’Aiuto, F.; Aljohani, S.; Vescovi, P.; Manfredi, M.; Arduino, P.G.; Broccoletti, R.; Musciotto, A.; Di Fede, O.; et al. Nonexposed Variant of Bisphosphonate-associated Osteonecrosis of the Jaw: A Case Series. Am. J. Med. 2010, 123, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, A.; Saia, G.; Bettini, G.; Tronchet, A.; Totola, A.; Bedogni, G.; Tregnago, P.; Valenti, M.T.; Bertoldo, F.; Ferronato, G.; et al. Osteomalacia: The missing link in the pathogenesis of bisphosphonate-related osteonecrosis of the jaws? Oncologist 2012, 17, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Priemel, M.; von Domarus, C.; Klatte, T.O.; Kessler, S.; Schlie, J.; Meier, S.; Proksch, N.; Pastor, F.; Netter, C.; Streichert, T.; et al. Bone mineralization defects and vitamin D deficiency: Histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J. Bone Miner. Res. 2010, 25, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, A.; Bettini, G.; Bedogni, G.; Basso, D.; Gatti, D.; Valisena, S.; Brunello, A.; Sorio, M.; Berno, T.; Giannini, S.; et al. Is vitamin D deficiency a risk factor for osteonecrosis of the jaw in patients with cancer? A matched case–control study. J. Cranio-Maxillofacial Surg. 2019. [Google Scholar] [CrossRef]
- De Molon, R.S.; Shimamoto, H.; Bezouglaia, O.; Pirih, F.Q.; Dry, S.M.; Kostenuik, P.; Boyce, R.W.; Dwyer, D.; Aghaloo, T.L.; Tetradis, S. OPG-Fc but Not Zoledronic Acid Discontinuation Reverses Osteonecrosis of the Jaws (ONJ) in Mice. J. Bone Miner. Res. 2015, 30, 1627–1640. [Google Scholar] [CrossRef]
- Otto, S.; Baumann, S.; Ehrenfeld, M.; Pautke, C. Successful surgical management of osteonecrosis of the jaw due to RANK-ligand inhibitor treatment using fluorescence guided bone resection. J. Cranio-Maxillofacial Surg. 2013, 41, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Rupel, K.; Ottaviani, G.; Gobbo, M.; Contardo, L.; Tirelli, G.; Vescovi, P.; Di Lenarda, R.; Biasotto, M. A systematic review of therapeutical approaches in bisphosphonates-related osteonecrosis of the jaw (BRONJ). Oral Oncol. 2014, 50, 1049–1057. [Google Scholar] [CrossRef]
- Hayashida, S.; Soutome, S.; Yanamoto, S.; Fujita, S.; Hasegawa, T.; Komori, T.; Kojima, Y.; Miyamoto, H.; Shibuya, Y.; Ueda, N.; et al. Evaluation of the Treatment Strategies for Medication-Related Osteonecrosis of the Jaws (MRONJ) and the Factors Affecting Treatment Outcome: A Multicenter Retrospective Study with Propensity Score Matching Analysis. J. Bone Miner. Res. 2017, 32, 2022–2029. [Google Scholar] [CrossRef]
- Bedogni, A.; Saia, G.; Bettini, G.; Tronchet, A.; Totola, A.; Bedogni, G.; Ferronato, G.; Nocini, P.F.; Blandamura, S. Long-term outcomes of surgical resection of the jaws in cancer patients with bisphosphonate-related osteonecrosis. Oral Oncol. 2011, 47, 420–424. [Google Scholar] [CrossRef]
- Otto, S.; Ristow, O.; Pache, C.; Troeltzsch, M.; Fliefel, R.; Ehrenfeld, M.; Pautke, C. Fluorescence-guided surgery for the treatment of medication-related osteonecrosis of the jaw: A prospective cohort study. J. Cranio-Maxillofacial Surg. 2016, 44, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, P.; Hinkmann, F.M.; Lell, M.M.; Fenner, M.; Vairaktaris, E.; Neukam, F.-W.; Nkenke, E. Panoramic radiograph, computed tomography or magnetic resonance imaging. Which imaging technique should be preferred in bisphosphonate-associated osteonecrosis of the jaw? A prospective clinical study. Clin. Oral Investig. 2010, 14, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.; O’Ryan, F.; Chavez, V.; Lathon, P.V.; Sanchez, G.; Hatcher, D.C.; Indresano, A.T.; Lo, J.C. Radiographic Findings in Bisphosphonate-Treated Patients With Stage 0 Disease in the Absence of Bone Exposure. J. Oral Maxillofac. Surg. 2010, 68, 2232–2240. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, A.; Fedele, S.; Bedogni, G.; Scoletta, M.; Favia, G.; Colella, G.; Agrillo, A.; Bettini, G.; Di Fede, O.; Oteri, G.; et al. Staging of osteonecrosis of the jaw requires computed tomography for accurate definition of the extent of bony disease. Br. J. Oral Maxillofac. Surg. 2014, 52, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Anesi, A.; Ferretti, M.; Cavani, F.; Salvatori, R.; Bianchi, M.; Russo, A.; Chiarini, L.; Palumbo, C. Structural and ultrastructural analyses of bone regeneration in rabbit cranial osteotomy: Piezosurgery versus traditional osteotomes. J. Cranio-Maxillofacial Surg. 2018, 46, 107–118. [Google Scholar] [CrossRef]
- Pozzi, S.; Anesi, A.; Generali, L.; Bari, A.; Consolo, U.; Chiarini, L. Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ). In Complications in Endodontic Surgery; Tsesis, I., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2014; pp. 153–165. [Google Scholar]
- Salgarelli, A.C.; Bellini, P.; Magnoni, C.; Anesi, A.; Collini, M. Synergistic use of local flaps for total lower lip reconstruction. Dermatologic Surg. 2011, 37, 1666–1670. [Google Scholar] [CrossRef] [PubMed]
- Anesi, A.; Negrello, S.; Chiarini, L. Evolution in Indication; Springer International Publishing: Cham, Switzerland, 2019; pp. 69–79. [Google Scholar]
- Chiarini, L.; Anesi, A.; Negrello, S. Mandible: Lateral, Hemimandibular, Anterior; Springer International Publishing: Cham, Switzerland, 2019; pp. 27–38. [Google Scholar]
- Bedogni, A.; Anesi, A.; Fior, A.; Bettini, G.; Nocini, P. Microsurgical reconstruction of the mandible in a patient with Evans Syndrome: A case report and review of the literature. J. Reconstr. Microsurg. 2013, 29, 545–550. [Google Scholar]
- Bellucci, D.; Salvatori, R.; Cannio, M.; Luginina, M.; Orrù, R.; Montinaro, S.; Anesi, A.; Chiarini, L.; Cao, G.; Cannillo, V. Bioglass and bioceramic composites processed by Spark Plasma Sintering (SPS): Biological evaluation Versus SBF test. Biomed. Glas. 2018, 4, 21–31. [Google Scholar] [CrossRef][Green Version]
- Bellucci, D.; Salvatori, R.; Anesi, A.; Chiarini, L.; Cannillo, V. SBF assays, direct and indirect cell culture tests to evaluate the biological performance of bioglasses and bioglass-based composites: Three paradigmatic cases. Mater. Sci. Eng. C 2019, 96, 757–764. [Google Scholar] [CrossRef]
- Nocini, P.F.; Anesi, A.; Fior, A. Bone Augmentation. In Atlas of Mandibular and Maxillary Reconstruction with the Fibula Flap; Springer International Publishing: Cham, Switzerland, 2019; pp. 53–65. [Google Scholar]
- Bellucci, D.; Anesi, A.; Salvatori, R.; Chiarini, L.; Cannillo, V. A comparative in vivo evaluation of bioactive glasses and bioactive glass-based composites for bone tissue repair. Mater. Sci. Eng. C 2017, 79, 286–295. [Google Scholar] [CrossRef]
- Bellucci, D.; Cannillo, V.; Anesi, A.; Salvatori, R.; Chiarini, L.; Manfredini, T.; Zaffe, D. Bone Regeneration by Novel Bioactive Glasses Containing Strontium and/or Magnesium: A Preliminary In-Vivo Study. Materials 2018, 11, 2223. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Salvatori, R.; Giannatiempo, J.; Anesi, A.; Bortolini, S.; Cannillo, V. A New Bioactive Glass/Collagen Hybrid Composite for Applications in Dentistry. Materials 2019, 12, 2079. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, G.; Salvatori, R.; Zambon, A.; Lusvardi, G.; Rigamonti, L.; Chiarini, L.; Anesi, A. Cytocompatibility of potential bioactive cerium-doped glasses based on 45S5. Materials 2019, 12, 594. [Google Scholar] [CrossRef]
- Giannone, N.; Anesi, A.; Ciavarella, D.; La Torretta, G.; Di Liberto, C. Cyanoacrylate in oral surgery | I cianoacrilati in chirurgia orale. Ital. Oral Surg. 2008, 7, 23–27. [Google Scholar]
- Di Nisio, C.; Zizzari, V.L.; Zara, S.; Falconi, M.; Teti, G.; Tetè, G.; Nori, A.; Zavaglia, V.; Cataldi, A. RANK/RANKL/OPG signaling pathways in necrotic jaw bone from bisphosphonate-treated subjects. Eur. J. Histochem. 2015, 59, 2455. [Google Scholar] [CrossRef]


| Regulators | Activators | Inhibitors |
|---|---|---|
| Main Regulators | M-CSF, RANKL | OPG |
| Transcription Factors | NFATc-1; PU-1, C-FOS, MITF, TFE3 | BCL-6 |
| Modulating Signals | VD3, TNFα, IL-1, IL-6, IL-8, IL-7, IL-11, IL-15, IL-17, IL-23, IL-34 | IFNα, IFNβ, IFNγ, IL-3, IL-4, IL-10, IL-12, IL-27, IL-33 |
| Chemical factors | H+, Mg2+ | Zn2+ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anesi, A.; Generali, L.; Sandoni, L.; Pozzi, S.; Grande, A. From Osteoclast Differentiation to Osteonecrosis of the Jaw: Molecular and Clinical Insights. Int. J. Mol. Sci. 2019, 20, 4925. https://doi.org/10.3390/ijms20194925
Anesi A, Generali L, Sandoni L, Pozzi S, Grande A. From Osteoclast Differentiation to Osteonecrosis of the Jaw: Molecular and Clinical Insights. International Journal of Molecular Sciences. 2019; 20(19):4925. https://doi.org/10.3390/ijms20194925
Chicago/Turabian StyleAnesi, Alexandre, Luigi Generali, Laura Sandoni, Samantha Pozzi, and Alexis Grande. 2019. "From Osteoclast Differentiation to Osteonecrosis of the Jaw: Molecular and Clinical Insights" International Journal of Molecular Sciences 20, no. 19: 4925. https://doi.org/10.3390/ijms20194925
APA StyleAnesi, A., Generali, L., Sandoni, L., Pozzi, S., & Grande, A. (2019). From Osteoclast Differentiation to Osteonecrosis of the Jaw: Molecular and Clinical Insights. International Journal of Molecular Sciences, 20(19), 4925. https://doi.org/10.3390/ijms20194925