Scaffold-Free 3-D Cell Sheet Technique Bridges the Gap between 2-D Cell Culture and Animal Models
Abstract
1. Introduction
2. Scaffold-Based Strategies
3. Scaffold-Free Strategy
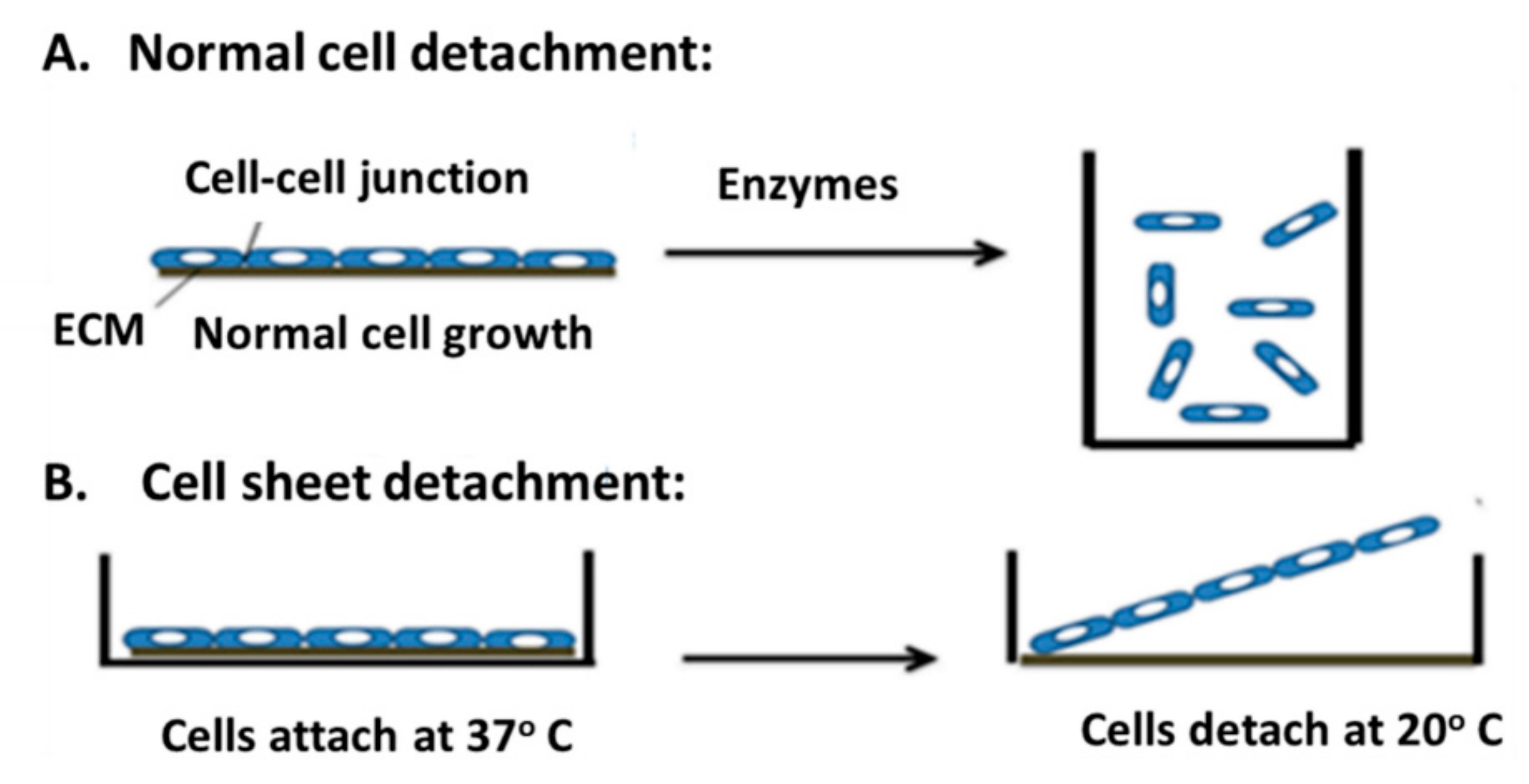
3.1. Cell Sheet Technique
3.2. Modeling Solid Tumor Using the Cell Sheet Technique
4. Scaffold-Based Bioprinting or Scaffold-Free Bioprinting
5. Vascularization in 3-D Tissues
6. Challenges in Scaffold Fabrication
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. ASSAY Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 1–19. [Google Scholar] [CrossRef]
- Luo, Z.; Pan, J.; Sun, Y.; Zhang, S.; Yang, Y.; Liu, H.; Li, Y.; Xu, X.; Sui, Y.; Wei, S. Injectable 3D Porous Micro-Scaffolds with a Bio-Engine for Cell Transplantation and Tissue Regeneration. Adv. Funct. Mater. 2018, 28, 1804335. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Khademhosseini, A.; Mironov, V. The Synergy of Scaffold-Based and Scaffold-Free Tissue Engineering Strategies. Trends Biotechnol. 2018, 36, 348–357. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Eswaramoorthy, R.; Hadidi, P.; Hu, J.C. Self-Organization and the Self-Assembling Process in Tissue Engineering. Annu. Rev. Biomed. Eng. 2013, 15, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Miyauchi, S.; Matsuzaka, S.; Yamagishi, C.; Kobayashi, K. Formation of Proteoglycan and Collagen-Rich Scaffold-Free Stiff Cartilaginous Tissue Using Two-Step Culture Methods with Combinations of Growth Factors. Tissue Eng. Part A 2010, 16, 1575–1584. [Google Scholar] [CrossRef]
- Oliva, J.; Florentino, A.; Bardag-Gorce, F.; Niihara, Y. Engineering, differentiation and harvesting of human adipose-derived stem cell multilayer cell sheets. Regen. Med. 2019, 14, 151–163. [Google Scholar] [CrossRef]
- Matsuda, N.; Shimizu, T.; Yamato, M.; Okano, T. Tissue Engineering Based on Cell Sheet Technology. Adv. Mater. 2007, 19, 3089–3099. [Google Scholar] [CrossRef]
- Elloumi-Hannachi, I.; Yamato, M.; Okano, T. Cell sheet engineering: a unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. J. Intern. Med. 2010, 267, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, H.; Planat-Benard, V.; Bel, A.; Puymirat, E.; Geha, R.; Pidial, L.; Nematalla, H.; Bellamy, V.; Bouaziz, P.; Peyrard, S.; et al. Epicardial adipose stem cell sheets results in greater post-infarction survival than intramyocardial injections. Cardiovasc. Res. 2011, 91, 483–491. [Google Scholar] [CrossRef]
- Hofmann, M.; Wollert, K.C.; Meyer, G.P.; Menke, A.; Arseniev, L.; Hertenstein, B.; Ganser, A.; Knapp, W.H.; Drexler, H. Monitoring of Bone Marrow Cell Homing Into the Infarcted Human Myocardium. Circle 2005, 111, 2198–2202. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, S.; Sawa, Y.; Sakakida, S.; Taketani, S.; Kondoh, H.; Memon, I.A.; Imanishi, Y.; Shimizu, T.; Okano, T.; Matsuda, H. Tissue cardiomyoplasty using bioengineered contractile cardiomyocyte sheets to repair damaged myocardium: Their integration with recipient myocardium. Transplant 2005, 80, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Nagamoto, Y.; Takayama, K.; Ohashi, K.; Okamoto, R.; Sakurai, F.; Tachibana, M.; Kawabata, K.; Mizuguchi, H. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J. Hepatol. 2016, 64, 1068–1075. [Google Scholar] [CrossRef]
- Ohashi, K.; Yokoyama, T.; Yamato, M.; Kuge, H.; Kanehiro, H.; Tsutsumi, M.; Amanuma, T.; Iwata, H.; Yang, J.; Okano, T.; et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat. Med. 2007, 13, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal Reconstruction with Tissue-Engineered Cell Sheets Composed of Autologous Oral Mucosal Epithelium. New Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef]
- Nuzzi, R.; Marolo, P.; Tridico, F. From DMEK to Corneal Endothelial Cell Therapy: Technical and Biological Aspects. J. Ophthalmol. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Sowden, J.; Beebe, D.; Powell, C.; Bird, A.; Cepko, C.; Clegg, D. Restoring vision to the blind: Stem cells and transplantation. Transl. Vis. Sci. Technol. 2014, 33, 33–41. [Google Scholar]
- Labrador-Velandia, S.; Alonso-Alonso, M.L.; Alvarez-Sanchez, S.; González-Zamora, J.; Carretero-Barrio, I.; Pastor, J.C.; Fernandez-Bueno, I.; Srivastava, G.K. Mesenchymal stem cell therapy in retinal and optic nerve diseases: An update of clinical trials. World J. Stem. Cells 2016, 8, 376–383. [Google Scholar] [CrossRef]
- Hata, H.; Matsumiya, G.; Miyagawa, S.; Kondoh, H.; Kawaguchi, N.; Matsuura, N.; Shimizu, T.; Okano, T.; Matsuda, H.; Sawa, Y. Grafted skeletal myoblast sheets attenuate myocardial remodeling in pacing-induced canine heart failure model. J. Thorac. Cardiovasc. Surg. 2006, 132, 918–924. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sekine, H.; Shimizu, T.; Hobo, K.; Sekiya, S.; Yang, J.; Yamato, M.; Kurosawa, H.; Kobayashi, E.; Okano, T. Endothelial Cell Coculture Within Tissue-Engineered Cardiomyocyte Sheets Enhances Neovascularization and Improves Cardiac Function of Ischemic Hearts. Circle 2008, 118, S145–S152. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Møller, B. Predicting the future burden of cancer. Nat. Rev. Cancer 2006, 6, 63. [Google Scholar] [CrossRef]
- Shimizu, T. Cell Sheet-Based Tissue Engineering for Fabricating 3-Dimensional Heart Tissues. Circ. J. 2014, 78, 2594–2603. [Google Scholar] [CrossRef] [PubMed]
- Bellis, S.L. Variant glycosylation: An underappreciated regulatory mechanism for β1 integrins. Biochim. Biophys. Acta (BBA) Biomembr. 2004, 1663, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Aruga, A.; Kobayashi, H.; Yamato, M.; Yamamoto, M. Development of a novel in vivo cancer model using cell sheet engineering. Anticancer Res. 2014, 34, 4747–4754. [Google Scholar]
- Collins, A.T.; Lang, S.H. A systematic review of the validity of patient derived xenograft (PDX) models: the implications for translational research and personalised medicine. Peer J. 2018, 6, e5981. [Google Scholar] [CrossRef] [PubMed]
- Holen, I.; Speirs, V.; Morrissey, B.; Blyth, K. In vivo models in breast cancer research: progress, challenges and future directions. Dis. Model. Mech. 2017, 10, 359–371. [Google Scholar] [CrossRef]
- Alshareeda, A.T.; Alsowayan, B.; Almubarak, A.; Alghuwainem, A.; Alshawakir, Y.; Alahmed, M. Exploring the Potential of Mesenchymal Stem Cell Sheet on The Development of Hepatocellular Carcinoma In Vivo. J. Vis. Exp. 2018, 139, e57805. [Google Scholar] [CrossRef]
- Alshareeda, A.T.; Sakaguchi, K.; Abumaree, M.; Zin, N.K.M.; Shimizu, T. The potential of cell sheet technique on the development of hepatocellular carcinoma in rat models. PLOS ONE 2017, 12, e0184004. [Google Scholar] [CrossRef]
- Reiberger, T.; Chen, Y.; Ramjiawan, R.R.; Hato, T.; Fan, C.; Samuel, R.; Roberge, S.; Huang, P.; Lauwers, G.Y.; Zhu, A.X.; et al. An orthotopic mouse model of hepatocellular carcinoma with underlying liver cirrhosis. Nat. Protoc. 2015, 10, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Verissimo, A.R.; Nakayama, K. Scaffold-Free Biofabrication. 3D Print. Biofabrication 2018, 431–450. [Google Scholar]
- Lee, A.Y.; Mahler, N.; Best, C.; Lee, Y.-U.; Breuer, C.K. Regenerative implants for cardiovascular tissue engineering. Transl. Res. 2014, 163, 321–341. [Google Scholar] [CrossRef]
- Kachouie, N.N.; Du, Y.; Bae, H.; Khabiry, M.; Ahari, A.F.; Zamanian, B.; Fukuda, J.; Khademhosseini, A. Directed assembly of cell-laden hydrogels for engineering functional tissues. Organogenesis 2010, 6, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.W.; Cao, T.; Zou, X.H.; Heng, B.C.; Wang, L.L.; Song, X.H.; Huang, H.F. Mesenchymal Stem Cell Sheets Revitalize Nonviable Dense Grafts: Implications for Repair of Large-Bone and Tendon Defects. Transplant 2006, 82, 170–174. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, Y.; Barnabas, S.T.; Woodruff, M.A.; Hutmacher, D.W. Engineering tubular bone constructs. J. Biomech. 2007, 40, S73–S79. [Google Scholar] [CrossRef]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.-W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.A.; Mironov, V.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 013001. [Google Scholar] [CrossRef]
- Moroni, L.; Boland, t.; Burdick, J.A.; Maria, C.D.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A guide to technology and terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef]
- Ozbolat, I.T. Scaffold-Based or Scaffold-Free Bioprinting: Competing or Complementing Approaches? J. Nanotechnol. Eng. Med. 2015, 6, 024701. [Google Scholar] [CrossRef]
- Sekiya, S.; Shimizu, T.; Okano, T. Vascularization in 3D tissue using cell sheet technology. Regen. Med. 2013, 8, 371–377. [Google Scholar] [CrossRef]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization Strategies for Tissue Engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Sekine, H.; Yang, J.; Isoi, Y.; Yamato, M.; Kikuchi, A.; Kobayashi, E.; Okano, T. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006, 20, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.-K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, R.; Giese, C. An overview on bioreactor design, prototyping and process control for reproducible three-dimensional tissue culture. Cult. Cells Tissue Eng. 2007, 53–78. [Google Scholar]
- Stephenson, M.; Grayson, W. Recent advances in bioreactors for cell-based therapies. F1000 Res. 2018, 7, 517. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, K.; Okano, T. Hepatocyte Transplantation: Cell Sheet Technology for Liver Cell Transplantation. Curr. Transplant. Rep. 2017, 4, 184–192. [Google Scholar] [CrossRef]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Die Makromol. Chemie Rapid Commun. 1990, 11, 571–576. [Google Scholar] [CrossRef]
- Matsuura, K.; Utoh, R.; Nagase, K.; Okano, T. Cell sheet approach for tissue engineering and regenerative medicine. J. Control. Release 2014, 190, 228–239. [Google Scholar] [CrossRef]
- Egami, M.; Haraguchi, Y.; Shimizu, T.; Yamato, M.; Okano, T. Latest status of the clinical and industrial applications of cell sheet engineering and regenerative medicine. Arch. Pharmacal Res. 2014, 37, 96–106. [Google Scholar] [CrossRef]
- Moschouris, K.; Firoozi, N.; Kang, Y. The application of cell sheet engineering in the vascularization of tissue regeneration. Regen. Med. 2016, 11, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wu, M.; Guo, F.; Li, P.; Chan, C.Y.; Mao, Z.; Li, S.; Ren, L.; Zhang, R.; Huang, T.J. Rapid formation of size-controllable multicellular spheroids via 3D acoustic tweezers. Lab A Chip 2016, 16, 2636–2643. [Google Scholar] [CrossRef]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The Role of Macrophage Phenotype in Vascularization of Tissue Engineering Scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef] [PubMed]
- Kamperman, T.; Henke, S.; van den Berg, A.; Shin, S.R.; Tamayol, A.; Khademhosseini, A.; Karperien, M.; Leijten, J. Single Cell Microgel Based Modular Bioinks for Uncoupled Cellular Micro-and Macroenvironments. Adv. Healthc. Mater. 2017, 6, 1600913. [Google Scholar] [CrossRef]
- Laib, A.M.; Bartol, A.; Alajati, A.; Korff, T.; Weber, H.; Augustin, H.G. Spheroid-based human endothelial cell microvessel formation in vivo. Nat. Protoc. 2009, 4, 1202–1215. [Google Scholar] [CrossRef]
- Go, G.; Han, J.; Zhen, J.; Yoo, A.; Jeon, M.; Park, J.; Zheng, S. A Magnetically Actuated Microscaffold Containing Mesenchymal Stem Cells for Articular Cartilage Repair. Adv. Heal. Mater. 2017, 6, 1601378. [Google Scholar] [CrossRef] [PubMed]
| Application | Cell Sheet | Direct Classic Injection of Dissociated Cells | Ref. | ||
|---|---|---|---|---|---|
| Effect | Cell Type | Effect | Cell Type | ||
| Infarcted Myocardium |
| Cardiomyocyte Adipose-derived stromal cell | Poor survival of the cells | BM-MSCs as sources for cardiac muscle cells Adipose-derived stromal cell | [12,13,14] |
| Heart Disease | Improvement in the cardiac function. | Myoblast cell | Hard to control the form, dimensions, or positions of implanted cells. | Skeletal myoblasts | [13,14,21,22] |
| Cardiomyocytes + Endothelial cells | Poor survival and engraftment. | Cardiomyocytes + Endothelial cells | ||
| Ocular Trauma |
| Oral mucosal epithelium sheet | Not tested | [17,18,19,20] | |
| Retinitis Pigmentosa |
| Photoreceptor precursor cells |
| Photoreceptor precursor cells | |
| Blindness and Visions |
| Fetal human retina sheets |
| MSCs | |
| Liver Disease |
| Hepatic sheets |
| Injecting hepatocytes into spleens or portal veins | [16] |
| iPS-HLC sheets |
| Intrasplenic injection of iPS-HLC | [15] | |
| Target Organ | Cell Sheet | Classic Cell Suspension Injection | Ref. | ||
|---|---|---|---|---|---|
| Advantages | Disadvantages | Advantages | Disadvantages | ||
| Any Tumor |
|
|
|
| [28] |
| Hepatocellular Carcinoma |
|
|
|
| [29,30,31] |
| Colon Cancer |
| Not discussed |
|
| [26] |
| Pancreas Cancer |
| Not discussed | Not discussed |
| [26] |
| Gastric Cancer |
| Not discussed | Not discussed |
| [26] |
| Features | Scaffold-Based Bioprinting | Scaffold-Free Bioprinting |
|---|---|---|
| Bioprinting Modes | Extrusion, droplet, or laser-based bioprinting | Extrusion-based bioprinting |
| Resolution and Accuracy | High | Low |
| Process Time | Short | Medium-long |
| Bio-Ink | Essentially soft biomaterials;Hydrogel, micro-carriers | Cells produce optimal matrix; Cell pellet, tissue spheroids, and tissue strands. |
| Intercellular Interaction | Limited communication | Natural interaction/high |
| Cell Viability | Variable | Higher efficiency |
| Tissue Bio-Mimicry | Low-medium | High |
| Preference for Application | Good for large, cell-homogenous, matrix-rich tissue | Best for smaller, cell-heterogeneous, matrix-poor tissues |
| Affordability | Low-high | High |
| Commercial Availability | Available | Available |
| Feature | Technique Type | ||
|---|---|---|---|
| Scaffold-Free Models | Scaffold-Based Models | ||
| Cell-Sheet | Classic Cell Suspension Injection | ||
| Preparation Time | Fast | Fast | Medium-long |
| Tissue Regeneration Time | Short | Short | Considerably long |
| Rate of Cell Survival $ | High ^ | Low | Low |
| Similarity of Composition to Natural Tissue | Yes | Sometimes * | Sometimes ** |
| Scaffold Degradation | No | Sometimes * | Yes |
| Proteolytic Enzyme Treatment | No | Yes | Sometimes |
| Level of Uniform Distribution of the Seeded Cells | High | Low | Low |
| Immunological Interference from the Scaffold | No | Sometimes * | High |
| Damage the Surrounded Tissue | No | Possible | Possible ^^ |
| Mechanical/Structural Integrity | Fragile and thin sheet | Injectable cells/weak | High |
| Requirement of a Supportive Sutures | No | No | Yes + |
| Affordability | High cost | Low cost | Low-high cost |
| Commercial Availability | Available | Available | Available |
| Tissue Biomimicry | High | High | Low-medium |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghuwainem, A.; Alshareeda, A.T.; Alsowayan, B. Scaffold-Free 3-D Cell Sheet Technique Bridges the Gap between 2-D Cell Culture and Animal Models. Int. J. Mol. Sci. 2019, 20, 4926. https://doi.org/10.3390/ijms20194926
Alghuwainem A, Alshareeda AT, Alsowayan B. Scaffold-Free 3-D Cell Sheet Technique Bridges the Gap between 2-D Cell Culture and Animal Models. International Journal of Molecular Sciences. 2019; 20(19):4926. https://doi.org/10.3390/ijms20194926
Chicago/Turabian StyleAlghuwainem, Ayidah, Alaa T. Alshareeda, and Batla Alsowayan. 2019. "Scaffold-Free 3-D Cell Sheet Technique Bridges the Gap between 2-D Cell Culture and Animal Models" International Journal of Molecular Sciences 20, no. 19: 4926. https://doi.org/10.3390/ijms20194926
APA StyleAlghuwainem, A., Alshareeda, A. T., & Alsowayan, B. (2019). Scaffold-Free 3-D Cell Sheet Technique Bridges the Gap between 2-D Cell Culture and Animal Models. International Journal of Molecular Sciences, 20(19), 4926. https://doi.org/10.3390/ijms20194926





