Bone Metastasis Pain, from the Bench to the Bedside
Abstract
1. The Healthy Bone Tissue
The Virtuous Cycle in the Physiology of Bone
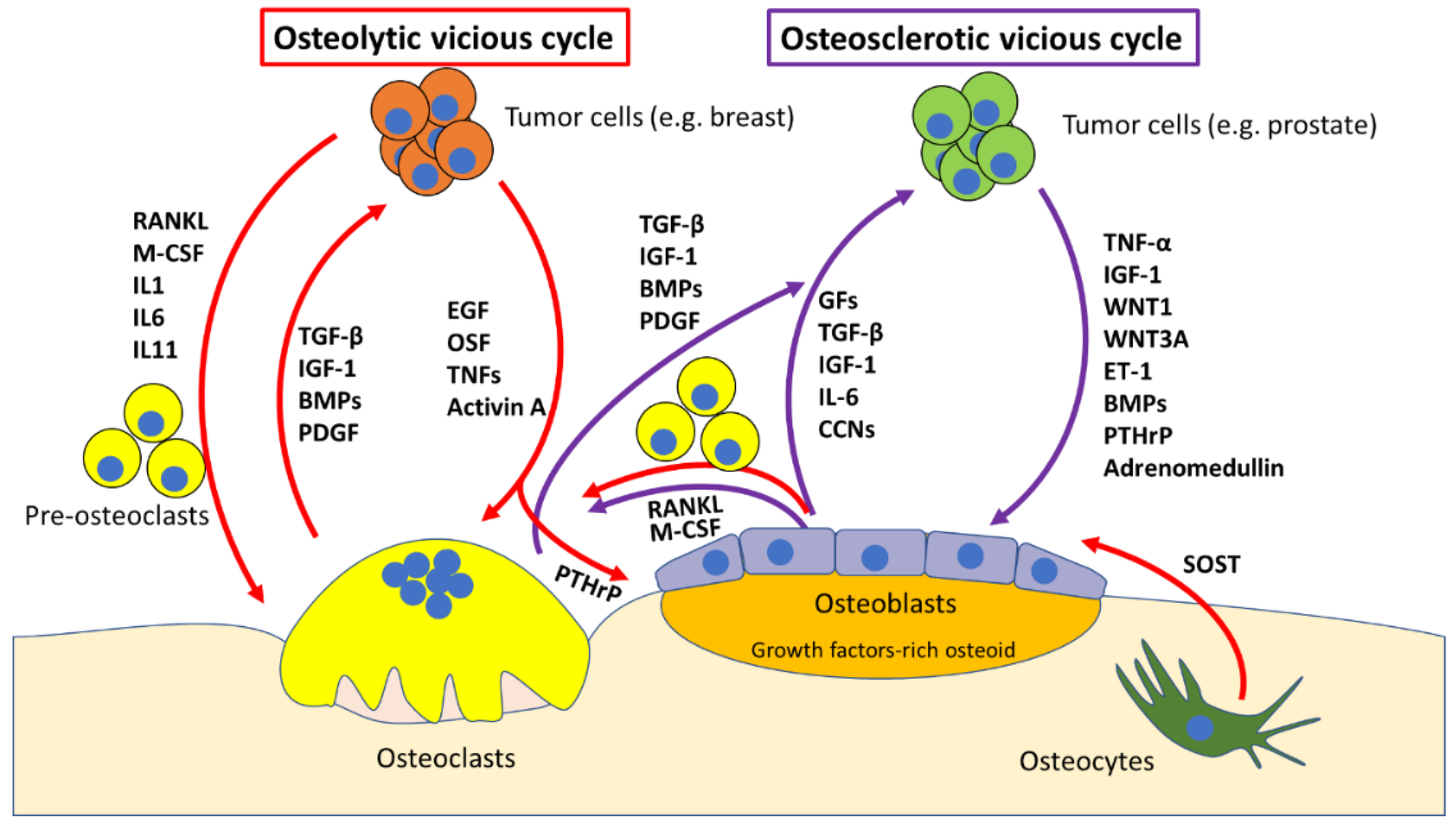
2. Molecular Mechanisms of Bone Metastases
The “Vicious Cycle”
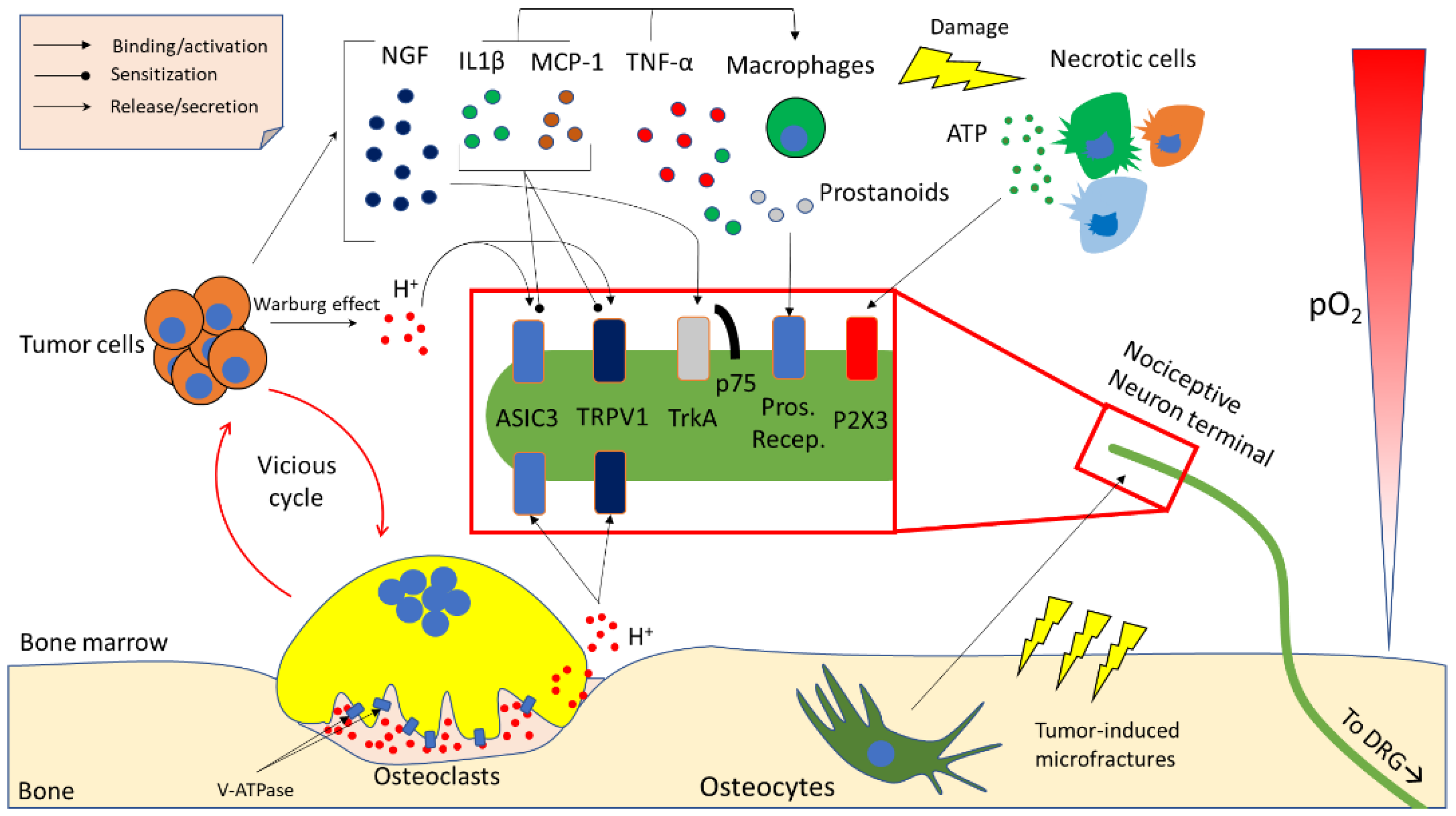
3. Molecular Determinants of Cancer-Induced Bone Pain (CIBP)
3.1. Acidity
3.2. Neurotrophins
3.3. Inflammatory Cytokines and Chemokines
3.4. Other Microenvironment- and Tumour-Derived Factors
4. In Vivo Models of Bone Pain
5. Current Treatments for CIBP
5.1. Surgical Intervention
5.2. Radiotherapy
5.3. Drug Treatment
5.3.1. Nonsteroidal Anti-Inflammatory Drugs
5.3.2. Opioid Treatment
5.3.3. Anti-Resorptive Agents
5.3.4. Endothelin-A (ET-A) Receptor Antagonists: a New Avenue
Author Contributions
Funding
Conflicts of Interest
References
- Rucci, N.; Teti, A. The “love-hate” relationship between osteoclasts and bone matrix. Matrix Biol. 2016, 54, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Capulli, M.; Paone, R.; Rucci, N. Osteoblast and osteocyte: Games without frontiers. Arch. Biochem. Biophys. 2014, 561, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Cappariello, A.; Ponzetti, M.; Rucci, N. The “soft” side of the bone: Unveiling its endocrine functions. Horm. Biol. Clin. Investig. 2016, 28, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Jähn, K.; Kelkar, S.; Zhao, H.; Xie, Y.; Tiede-Lewis, L.M.; Dusevich, V.; Dallas, S.L.; Bonewald, L.F. Osteocytes acidify their microenvironment in response to PTHrP in vitro and in lactating mice in vivo. J. Bone Miner. Res. 2017, 32, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Uda, Y.; Azab, E.; Sun, N.; Shi, C.; Pajevic, P.D. Osteocyte mechanobiology. Curr. Osteoporos. Rep. 2017, 15, 318–325. [Google Scholar] [CrossRef]
- Gong, Y.; Slee, R.B.; Fukai, N.; Rawadi, G.; Roman-Roman, S.; Reginato, A.M.; Wang, H.; Cundy, T.; Glorieux, F.H.; Lev, D.; et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 2001, 107, 513–523. [Google Scholar] [CrossRef]
- Boyden, L.M.; Mao, J.; Belsky, J.; Mitzner, L.; Farhi, A.; Mitnick, M.A.; Wu, D.; Insogna, K.; Lifton, R.P. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 2002, 346, 1513–1521. [Google Scholar] [CrossRef]
- Little, R.D.; Carulli, J.P.; Del Mastro, R.G.; Dupuis, J.; Osborne, M.; Folz, C.; Manning, S.P.; Swain, P.M.; Zhao, S.C.; Eustace, B.; et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet. 2002, 70, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/β-Catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Zhang, R.; Geoffry, V.; Ridall, A.I.; Karsenty, G. Osf/2Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Lee, B.; Thirunavukkarasu, K.; Zhou, L.; Pastore, L.; Baldini, A.; Hecht, J.; Geoffroy, V.; Ducy, P.; Karsenty, G. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat. Genet. 1997, 16, 307–310. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; Palmieri, F.; Tatullo, M. CD146 expression influences periapical cyst mesenchymal stem cell properties. Stem Cell Rev. 2016, 12, 592–603. [Google Scholar] [CrossRef]
- Aulino, P.; Costa, A.; Chiaravalloti, E.; Perniconi, B.; Adamo, S.; Coletti, D.; Marrelli, M.; Tatullo, M.; Teodori, L. Muscle extracellular matrix scaffold is a multipotent environment. Int. J. Med. Sci. 2015, 12, 336–340. [Google Scholar] [CrossRef]
- Anderson, H.C. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 2003, 5, 222–226. [Google Scholar] [CrossRef]
- Brown, H.K.; Schiavone, K.; Gouin, F.; Heymann, M.F.; Heymann, D. Biology of bone sarcomas and new therapeutic developments. Calcif. Tissue Int. 2018, 102, 174–195. [Google Scholar] [CrossRef]
- Ottewell, P.D. The role of osteoblasts in bone metastasis. J. Bone Oncol. 2016, 5, 124–127. [Google Scholar] [CrossRef]
- Feng, X.; Teitelbaum, S. Osteoclasts: New insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef]
- Tondravi, M.M.; McKercher, S.R.; Anderson, K.; Erdmann, J.M.; Quiroz, M.; Maki, R.; Teitelbaum, S.L. Osteopetrosis in mice lacking hematopoietic transcription factor PU.1. Nature 1997, 386, 81–84. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Noda, M. Mitf is expressed in osteoclast progenitors in vitro. Exp. Cell Res. 2000, 260, 284–291. [Google Scholar] [CrossRef]
- Biskobing, D.M.; Fan, X.; Rubin, J. Characterization of MCS-induced proliferation and subsequent osteoclast formation in murine marrow culture. J. Bone Miner. Res. 1995, 10, 1025–1032. [Google Scholar] [CrossRef]
- Arai, F.; Myamoto, T.; Ohneda, O.; Inada, T.; Sudo, T.; Brasel, K.; Miyata, T.; Anderson, D.M.; Susa, T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J. Exp. Med. 1999, 190, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Bodmer, J.L.; Schneider, P.; Tschopp, J. The molecular architecture of the TNF superfamily. Trends Biochem. Sci. 2002, 27, 19–26. [Google Scholar] [CrossRef]
- Franzoso, G.; Carlson, L.; Xing, L.; Poljak, L.; Shores, E.W.; Brown, K.D.; Leonardi, A.; Tran, T.; Boyce, B.F.; Siebenlist, U. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997, 11, 3482–3496. [Google Scholar] [CrossRef]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.R.; Besser, D.; Kim, N.; Arron, J.R.; Vologodskaia, M.; Hanafusa, H.; Choy, Y. TRANCE, a TNF family member, activates Akt/PKB through a signalling complex involving TRAF6 and c-Src. Mol. Cell 1999, 4, 1041–1049. [Google Scholar] [CrossRef]
- Kudo, O.; Sabokbar, A.; Pocock, A.; Itonaga, I.; Fujikawa, Y.; Athanasou, N.A. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone 2003, 32, 1–7. [Google Scholar] [CrossRef]
- Lam, J.; Takeshita, S.; Barker, J.E.; Kanagawa, O.; Ross, F.P.; Teitelbaum, S.L. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Investig. 2000, 106, 1481–1488. [Google Scholar] [CrossRef]
- Kim, J.H.; Jin, H.M.; Kim, K.; Song, I.; Youn, B.U.; Matsuo, K.; Kim, N. The mechanism of osteoclast differentiation induced by IL-1. J. Immunol. 2009, 183, 1862–1870. [Google Scholar] [CrossRef]
- Cappariello, A.; Maurizi, A.; Veeriah, V.; Teti, A. The great beauty of the osteoclast. Arch. Biochem. Biophys. 2014, 558, 70–78. [Google Scholar] [CrossRef]
- Harada, S.I.; Rodan, G.A. Control of osteoblast function and regulation of bone mass. Nature 2003, 423, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.; Jagdev, S.; McPartland, I.; Clark, K.; Gregory, W.; Newsham, A.; Rogerson, S.; Hayward, K.; Selby, P.; Brown, J. Skeletal complications and survival in renal cancer patients with bone metastases. Bone 2011, 48, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [PubMed]
- Van Moos, R.; Body, J.J.; Egerdie, B.; Stopeck, A.; Brown, J.; Fallowfield, I.; Patrick, D.L.; Cleeland, C.; Damyanov, D.; Palazzo, F.S.; et al. Pain and analgesic use associated with skeletal-related events in patients with advanced cancer and bone metastases. Support. Care Cancer 2016, 24, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Brodowicz, T.; Hadji, P.; Niepel, D.; Diel, I. Early identification and intervention matters: A comprehensive review of current evidence and recommendations for the monitoring of bone health in patients with cancer. Cancer Treat. Rev. 2017, 61, 23–34. [Google Scholar] [CrossRef]
- Coleman, R.E. Bone cancer 2011: Prevention and treatment of bone metastases. Nat. Rev. Clin. Oncol. 2011, 9, 76–78. [Google Scholar] [CrossRef]
- Rucci, N.; Teti, A. Osteomimicry: How the seed growth in the soil. Calcif. Tissue Int. 2018, 102, 131–140. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. Cancer Metast. Rev. 1889, 8, 98–101. [Google Scholar] [CrossRef]
- Maurizi, A.; Rucci, N. The osteoclast in bone metastasis: Player and target. Cancers 2018, 10, 218. [Google Scholar] [CrossRef]
- Ibrahim, T.; Flamini, E.; Mercatali, L.; Sacanna, E.; Serra, P.; Amadori, D. Pathogenesis of osteoblastic bone metastases from prostate cancer. Cancer 2010, 116, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Guise, T.A.; Mohammad, K.S.; Clines, G.; Stebbins, E.G.; Wong, D.H.; Higgins, L.S.; Vessella, R.; Corey, E.; Padalecki, S.; Suva, L.; et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin. Cancer Res. 2006, 12 (Suppl. 20), 6213s–6246s. [Google Scholar] [CrossRef]
- Keller, E.T.; Brown, J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J. Cell Biochem. 2004, 91, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Roudier, M.P.; Morrissey, C.; True, L.D.; Higano, C.S.; Vessella, R.L.; Ott, S.M. Histopathologic assessment of prostate cancer bone “osteoblastic” metastases. J. Urol. 2008, 180, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Zheng, M.; et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J. Natl. Cancer Inst. 2004, 96, 879–882. [Google Scholar] [CrossRef]
- Deftosa, L.J.; Barkend, I.; Burtona, D.W.; Hoffmanb, R.M.; Gellere, J. Direct evidence that PTHrP expression promotes prostate cancer progression in bone. Biochem. Biophys. Res. Commun. 2005, 327, 468–472. [Google Scholar] [CrossRef]
- Iwamura, M.; di Santagnese, P.A.; Wu, G.; Benning, C.M.; Cockett, A.T.; Deftosa, L.J.; Abrahamsson, P.A. Immunohistochemical localization of parathyroid hormone-related protein in human prostate cancer. Cancer Res. 2001, 61, 2572–2578. [Google Scholar]
- Sohail, A.; Sherin, L.; Butt, S.I.; Javed, S.; Li, Z.; Iqbal, S.; Be’g, O.A. Role of key players in paradigm shifts of prostate cancer bone metastasis. Cancer Manag. Res. 2018, 10, 1619–1626. [Google Scholar] [CrossRef]
- Nandana, S.; Tripathi, M.; Duan, P.; Chu, C.Y.; Mishra, R.; Liu, C.; Jin, R.; Yamashita, H.; Zayzafoon, M.; Bhowmick, N.A.; et al. Bone metastasis of prostate cancer can be therapeutically targeted at the TBX2–WNT signaling axis. Cancer Res. 2017, 77, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.J.; Mohammad, K.S.; Käkönen, S.M.; Harris, S.; Wu-Wong, J.R.; Wessale, J.L.; Padley, R.J.; Garrett, I.R.; Chirgwin, J.M.; Guise, T.A. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc. Natl. Acad. Sci. USA 2003, 100, 10954–10959. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.M.; Shay, G.; Aruajo, A.; Lynch, C.C. Integrating new discoveries into the “vicious cycle” paradigm of prostate to bone metastases. Cancer Metast. Rev. 2014, 33, 511–525. [Google Scholar] [CrossRef]
- Hernandez, R.K.; Wade, S.W.; Reich, A.; Pirolli, M.; Liede, A.; Lyman, G.H. Incidence of bone metastases in patients with solid tumors: Analysis of oncology electronic medical records in the United States. BMC Cancer 2018, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Guise, T.A.; Yin, J.J.; Taylor, S.D.; Kumagai, Y.; Dallas, M.; Boyce, B.F.; Yoneda, T.; Mundy, G.R. Evidence for a causal role of parathyroid hormone–related protein in the pathogenesis of human breast cancer–mediated osteolysis. J. Clin. Investig. 1996, 98, 1544–1549. [Google Scholar] [CrossRef] [PubMed]
- Wein, M.N. Parathyroid hormone signaling in osteocytes. JBMR Plus 2018, 2, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Rana, T.; Chakrabarti, A.; Freeman, M.; Biswasbirra, S. Doxorubicin-mediated bone loss in breast cancer bone metastases is driven by an interplay between oxidative stress and induction of TGFβ. PLoS ONE 2013, 8, e78043. [Google Scholar] [CrossRef]
- Tatullo, M.; Simone, G.M.; Tarullo, F.; Irlandese, G.; De Vito, D.; Marrelli, M.; Santacroce, L.; Cocco, T.; Ballini, A.; Scacco, S. Antioxidant and Antitumor Activity of a Bioactive Polyphenolic Fraction Isolated from the Brewing Process. Sci. Rep. 2016, 6, 36042. [Google Scholar] [CrossRef]
- Van den Beuken-van Everdingen, M.H.; Hochstenbach, L.M.; Joosten, E.A.; Tjan-Heijnen, V.C.; Janssen, D.J. Update on prevalence of pain in patients with cancer: Systematic review and meta-analysis. J. Pain Symptom Manag. 2016, 51, 1070–1090. [Google Scholar] [CrossRef]
- Middlemiss, T.; Laird, B.J.A.; Fallon, M.T. Mechanisms of cancer-induced bone pain. Clin. Oncol. 2011, 23, 387–392. [Google Scholar] [CrossRef]
- Lozano-Ondoua, A.N.; Symons-Liguori, A.M.; Vanderah, T.W. Cancer-induced bone pain: Mechanisms and models. Neurosci. Lett. 2013, 557, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Eber, M.R.; Widner, D.B.; Shiozawa, Y. Role of the bone microenvironment in the development of painful complications of skeletal metastases. Cancers 2018, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Levy, D. The sensory innervation of the calvarial periosteum is nociceptive and contributes to headache-like behavior. Pain 2014, 155, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Mahns, D.A.; Ivanusic, J.J.; Sahai, V.; Rowe, M.J. An intact peripheral nerve preparation for monitoring the activity of single, periosteal afferent nerve fibres. J. Neurosci. Methods 2006, 156, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Ivanusic, J. The size, neurochemistry and segmental distribution of sensory neurons innervating the rat tibia. J. Comp. Neurol. 2009, 517, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Tanaka, S.; Sekiguchi, T.; Sugiyama, D.; Kawamata, M. Spinal nociceptive transmission by mechanical stimulation of bone marrow. Mol. Pain 2016, 12. [Google Scholar] [CrossRef] [PubMed]
- Fock, S.; Mense, S. Excitatory effects of 5-hydroxytryptamine, histamine and potassium ions on muscular group IV afferent units: A comparison with bradykinin. Brain Res. 1976, 105, 459–469. [Google Scholar] [CrossRef]
- Inman, V.; Saunders, J. Referred pain from skeletal structures. J. Nerv. Ment. Dis. 1944, 99, 660–667. [Google Scholar] [CrossRef]
- Brjussowa, S.S.; Lebedenko, W.W. Zur Schmerzleitungsfähigkeit der Gefäße. Z. Gesamte Exp. Med. 1930, 69, 29–40. [Google Scholar] [CrossRef]
- Lemperg, R.K.; Arnoldi, C.C. The significance of intraosseous pressure in normal and diseased states with special reference to the intraosseous engorgement-pain syndrome. Clin. Orthop. Relat. Res. 1978, 136, 143–156. [Google Scholar]
- Arnoldi, C.C.; Djurhuus, J.C.; Heerfordt, J.; Karle, A. Intraosseous phlebography, intraosseous pressure measurements and 99mTC-polyphosphate scintigraphy in patients with various painful conditions in the hip and knee. Acta Orthop. Scand. 1980, 51, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Sottnik, J.L.; Dai, J.; Zhang, H.; Campbell, B.; Keller, E.T. Tumor-induced pressure in the bone microenvironment causes osteocytes to promote the growth of prostate cancer bone metastases. Cancer Res. 2015, 75, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Teti, A.; Zallone, A. Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone 2009, 44, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, P.; Jonsson, J.I. The hematopoietic stem cell niche: Low in oxygen but a nice place to be. J. Cell. Physiol. 2010, 222, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, J.R.; Röhrich, H.; Lindsay, T.H.; Sevcik, M.A.; Schwei, M.J.; Kubota, K.; Halvorson, K.G.; Poblete, J.; Chaplan, S.R.; Dubin, A.E.; et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J. Neurosci. 2005, 25, 3126–3131. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Wakisaka, S.; Hiraga, T.; Sakurai, T.; Tominaga, M.; Yoneda, T. Role of acid-sensing TRPV1 in bone pain associated with cancer colonization in bone. J. Bone Miner. Res. 2005, 20 (Suppl. 1), S32. [Google Scholar]
- Nagae, M.; Hiraga, T.; Yoneda, T. Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J. Bone Miner. Metab. 2007, 25, 99–104. [Google Scholar] [CrossRef]
- Pan, H.L.; Zhang, Y.Q.; Zhao, Z.Q. Involvement of lysophosphatidic acid in bone cancer pain by potentiation of TRPV1 via PKCepsilon pathway in dorsal root ganglion neurons. Mol. Pain 2010, 6, 85. [Google Scholar] [CrossRef]
- Lautner, M.A.; Ruparel, S.B.; Patil, M.J.; Hargreaves, K.M. In vitro sarcoma cells release a lipophilic substance that activates the pain transduction system via TRPV1. Ann. Surg. Oncol. 2011, 18, 866–871. [Google Scholar] [CrossRef]
- Qiu, F.; Wei, X.L.; Zhang, S.Z.; Yuan, W.X.; Mi, W.D. Increased expression of acid sensing ion channel 3 within dorsal root ganglia in a rat model of bone cancer pain. Neuroreport 2014, 25, 887–893. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Hata, K.; Nagayama, T.; Sakurai, T.; Nishisho, T.; Wakabayashi, H.; Hiraga, T.; Ebisu, S.; Yoneda, T. Acid activation of Trpv1 leads to an up-regulation of calcitonin gene-related peptide expression in dorsal root ganglion neurons via the CaMK-CREB cascade: A potential mechanism of inflammatory pain. Mol. Biol. Cell 2010, 21, 2568–2577. [Google Scholar] [CrossRef] [PubMed]
- Niiyama, Y.; Kawamata, T.; Yamamoto, J.; Furuse, S.; Namiki, A. SB366791, a TRPV1 antagonist, potentiates analgesic effects of systemic morphine in a murine model of bone cancer pain. Br. J. Anaesth. 2009, 102, 251–258. [Google Scholar] [CrossRef]
- Deval, E.; Gasull, X.; Noël, J.; Salinas, M.; Baron, A.; Diochot, S.; Lingueglia, E. Acid-sensing ion channels (ASICs): Pharmacology and implication in pain. Pharmacol. Ther. 2010, 128, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-G.; Xu, T.-L. ASIC3 channels in multimodal sensory perception. ACS Chem. Neurosci. 2011, 2, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Hiasa, M.; Okui, T.; Allette, Y.M.; Ripsch, M.S.; Sun-Wada, G.H.; Wakabayashi, H.; Roodman, G.D.; White, F.A.; Yoneda, T. Bone pain induced by multiple myeloma is reduced by targeting V.-ATPase and ASIC3. Cancer Res. 2017, 77, 1283–1295. [Google Scholar] [CrossRef]
- Izumi, M.; Ikeuchi, M.; Ji, Q.; Tani, T. Local ASIC3 modulates pain and disease progression in a rat model of osteoarthritis. J. Biomed. Sci. 2012, 19, 77. [Google Scholar] [CrossRef]
- Yoneda, T.; Hiasa, M.; Nagata, Y.; Okui, T.; White, F.A. Acidic microenvironment and bone pain in cancer-colonized bone. Bonekey Rep. 2015, 4, 690. [Google Scholar] [CrossRef]
- Di Pompo, G.; Lemma, S.; Canti, L.; Rucci, N.; Ponzetti, M.; Errani, C.; Donati, D.M.; Russell, S.; Gillies, R.; Chano, T.; et al. Intratumoral acidosis fosters cancer-induced bone pain through the activation of the mesenchymal tumor-associated stroma in bone metastasis from breast carcinoma. Oncotarget 2017, 8, 54478–54496. [Google Scholar] [CrossRef]
- Skaper, S.D. The neurotrophin family of neurotrophic factors: An overview. Methods Mol. Biol. 2012, 846, 1–12. [Google Scholar] [CrossRef]
- Halvorson, K.G.; Kubota, K.; Sevcik, M.A.; Lindsay, T.H.; Sotillo, J.E.; Ghilardi, J.R.; Rosol, T.J.; Boustany, L.; Shelton, D.L.; Mantyh, P.W. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res. 2005, 65, 9426–9435. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Andrade, J.M.; Ghilardi, J.R.; Castañeda-Corral, G.; Kuskowski, M.A.; Mantyh, P.W. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain 2011, 152, 2564–2574. [Google Scholar] [CrossRef] [PubMed]
- Buehlmann, D.; Ielacqua, G.D.; Xandry, J.; Rudin, M. Prospective administration of anti-nerve growth factor treatment effectively suppresses functional connectivity alterations after cancer-induced bone pain in mice. Pain 2019, 160, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.N.; Yang, J.P.; Ji, F.H.; Zhan, Y.; Jin, X.H.; Xu, Q.N.; Wang, X.Y.; Zuo, J.L. Brain-derived neurotrophic factor modulates N-methyl-D-aspartate receptor activation in a rat model of cancer-induced bone pain. J. Neurosci. Res. 2012, 90, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, J.R.; Freeman, K.T.; Jimenez-Andrade, J.M.; Mantyh, W.G.; Bloom, A.P.; Kuskowski, M.A.; Mantyh, P.W. Administration of a tropomyosin receptor kinase inhibitor attenuates sarcoma-induced nerve sprouting, neuroma formation and bone cancer pain. Mol. Pain 2010, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Hondermarck, H. Neurotrophins and their receptors in breast cancer. Cytokine Growth Factor Rev. 2012, 23, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Molloy, N.H.; Read, D.E.; Gorman, A.E. Nerve growth factor in cancer cell death and survival. Cancers 2011, 3, 510–530. [Google Scholar] [CrossRef]
- Williams, K.S.; Killebrew, D.A.; Clary, G.P.; Seawell, J.A.; Meeker, R.B. Differential regulation of macrophage phenotype by mature and pro-nerve growth factor. J. Neuroimmunol. 2015, 285, 76–93. [Google Scholar] [CrossRef]
- Zhang, X.C.; Kainz, V.; Burstein, R.; Levy, D. Tumor necrosis factor-alpha induces sensitization of meningeal nociceptors mediated via local COX and P38 map kinase actions. Pain 2011, 152, 140–149. [Google Scholar] [CrossRef]
- Binshtok, A.M.; Wang, H.; Zimmermann, K.; Amaya, F.; Vardeh, D.; Shi, L.; Brenner, G.J.; Ji, R.R.; Bean, B.P.; Woolf, C.J.; et al. Nociceptors are interleukin-1beta sensors. J. Neurosci. 2008, 28, 14062–14073. [Google Scholar] [CrossRef]
- Mamet, J.; Baron, A.; Lazdunski, M.; Voilley, N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J. Neurosci. 2002, 22, 10662–10670. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Rodiles, M.; Chadee, K. Novel regulation of cyclooxygenase-2 expression and prostaglandin e2 production by IFN-gamma in human macrophages. J. Immunol. 1998, 161, 2441–2448. [Google Scholar] [PubMed]
- Sabino, M.A.; Ghilardi, J.R.; Jongen, J.L.; Keyser, C.P.; Luger, N.M.; Mach, D.B.; Peters, C.M.; Rogers, S.D.; Schwei, M.J.; de Felipe, C.; et al. Simultaneous reduction in cancer pain, bone destruction, and tumor growth by selective inhibition of cyclooxygenase-2. Cancer Res. 2002, 62, 7343–7349. [Google Scholar]
- Baamonde, A.; Curto-Reyes, V.; Juarez, L.; Meana, A.; Hidalgo, A.; Menendez, L. Antihyperalgesic effects induced by the IL-1 receptor antagonist anakinra and increased IL-1beta levels in inflamed and osteosarcoma-bearing mice. Life Sci. 2007, 81, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Constantin, C.E.; Mair, N.; Sailer, C.A.; Andratsch, M.; Xu, Z.Z.; Blumer, M.J.; Scherbakov, N.; Davis, J.B.; Bluethmann, H.; Ji, R.R.; et al. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J. Neurosci. 2008, 28, 5072–5081. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.H.; Zheng, X.Y.; Yang, J.P.; Wang, L.N.; Ji, F.H. Involvement of spinal monocyte chemoattractant protein-1 (MCP-1) in cancer-induced bone pain in rats. Neurosci. Lett. 2012, 517, 60–63. [Google Scholar] [CrossRef]
- Giuliani, A.L.; Sarti, A.C.; Di Virgilio, F. Extracellular nucleotides and nucleosides as signalling molecules. Immunol. Lett. 2018. [Google Scholar] [CrossRef]
- Kaan, T.K.; Yip, P.K.; Patel, S.; Davies, M.; Marchand, F.; Cockayne, D.A.; Nunn, P.A.; Dickenson, A.H.; Ford, A.P.; Zhong, Y.; et al. Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behaviour in rats. Brain 2010, 133, 2549–2564. [Google Scholar] [CrossRef]
- Hansen, R.R.; Nasser, A.; Falk, S.; Baldvinsson, S.B.; Ohlsson, P.H.; Bahl, J.M.; Jarvis, M.F.; Ding, M.; Heegaard, A.M. Chronic administration of the selective P2X3, P2X2/3 receptor antagonist, A-317491, transiently attenuates cancer-induced bone pain in mice. Eur. J. Pharmacol. 2012, 688, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Baron, R. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism; Favus, M.J., Ed.; ASBMR: Washington, DC, USA, 2003; pp. 1–8. [Google Scholar]
- Breuksch, I.; Weinert, M.; Brenner, W. The role of extracellular calcium in bone metastasis. J. Bone Oncol. 2016, 5, 143–145. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, X.M.; Duan, K.Z.; Gu, X.Y.; Han, M.; Liu, B.L.; Zhao, Z.Q.; Zhang, Y.Q. Peripheral TGF-β1 signaling is a critical event in bone cancer-induced hyperalgesia in rodents. J. Neurosci. 2013, 33, 19099–19111. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, J.; Han, Y.; Xiao, X.; Meng, X.L.; Su, L.; Liu, F.Y.; Xing, G.G.; Wan, Y. Enhanced function of TRPV1 via up-regulation by insulin-like growth factor-1 in a rat model of bone cancer pain. Eur. J. Pain 2014, 18, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Calle, J.; Anderson, J.; Cregor, M.D.; Condon, K.W.; Kuhstoss, S.A.; Plotkin, L.I.; Bellido, T.; Roodman, G.D. Genetic deletion of Sost or pharmacological inhibition of sclerostin prevent multiple myeloma-induced bone disease without affecting tumor growth. Leukemia 2017, 31, 2686–2694. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, W.G.; Yu, Y.; Xiao, X.; Cheng, J.; Zeng, W.Z.; Peng, Z.; Xi Zhu, M.; Xu, T.L. Serotonin facilitates peripheral pain sensitivity in a manner that depends on the nonproton ligand sensing domain of ASIC3 channel. J. Neurosci. 2013, 33, 4265–4279. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. Br. J. Pharmacol. 2008, 155, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Studer, M.; McNaughton, P.A. Modulation of single-channel properties of TRPV1 by phosphorylation. J. Physiol. 2010, 588, 3743–3756. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Jia, R.; Bertaa, T.; Nedergaardb, M. Glia and pain: Is chronic pain a gliopathy? Pain 2013, 154, S10–S28. [Google Scholar] [CrossRef]
- De Ciantis, P.D.; Yashpal, K.; Henry, J.; Singh, G. Characterization of a rat model of metastatic prostate cancer bone pain. J. Pain Res. 2010, 3, 213–221. [Google Scholar] [CrossRef]
- Hald, A.; Hansen, R.R.; Thomsen, M.W.; Ding, M.; Croucher, P.I.; Gallagher, O.; Ebetino, F.H.; Kassem, M.; Heegaard, A.M. Cancer-induced bone loss and associated pain-related behavior is reduced by risedronate but not its phosphonocarboxylate analog NE-10790. Int. J. Cancer 2009, 125, 1177–1185. [Google Scholar] [CrossRef]
- Zhu, X.C.; Ge, C.T.; Wang, P.; Zhang, J.L.; Yu, Y.Y.; Fu, C.Y. Analgesic effects of lappaconitine in leukemia bone pain in a mouse model. Peer J. 2015, 3, e936. [Google Scholar] [CrossRef] [PubMed]
- Majutaa, L.A.; Guedona, J.G.; Mitchella, S.A.T.; Kuskowskib, M.A.; Mantyh, P.W. Mice with cancer-induced bone pain show a marked decline in day/night activity. Pain Rep. 2017, 2, e614. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Randall, L.O.; Selitto, J.J. A method for measurement of analgesic activity on inflamed tissue. Arch. Int. Pharmacodyn. Ther. 1957, 111, 409–419. [Google Scholar] [PubMed]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Nakamura, A.; Ono, H.; Ando, A.; Hinata, M.; Niidome, K.; Omachi, S.; Sakaguchi, G.; Shinohara, S. Suppression of the acute upregulation of phosphorylated-extracellular regulated kinase in ventral tegmental area by a mu-opioid receptor agonist is related to resistance to rewarding effects in a mouse model of bone cancer. J. Pharmacol. Sci. 2017, 133, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, G.; Nagakura, Y.; Takeshita, N.; Shimizu, Y. Efficacy of drugs with different mechanisms of action in relieving spontaneous pain at rest and during movement in a rat model of osteoarthritis. Eur. J. Pharmacol. 2014, 738, 111–117. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, E.M.; Azevedo, R.; Vega, T.A.; Brodkin, J.; Akamatsu, W.; Okano, H.; Wagner, G.C.; Rasin, M.L. Prenatal deletion of the RNA-binding protein HuD disrupts postnatal cortical circuit maturation and behavior. J. Neurosci. 2014, 34, 3674–3686. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G. Frequency of pain in patients with cancer. Recent Res. Cancer Res. 1984, 89, 64–71. [Google Scholar]
- Coleman, R.E. Monitoring of bone metastases. Eur. J. Cancer 1998, 34, 252–259. [Google Scholar] [CrossRef]
- Zhu, X.C.; Zhang, J.L.; Ge, C.T.; Yun, Y.Y.; Wang, P.; Yuan, T.F.; Fu, C.Y. Advances in cancer pain from bone metastasis. Drug Des. Dev. Ther. 2015, 9, 4239–4245. [Google Scholar] [CrossRef]
- Jehn, C.F.; Diel, I.J.; Overkamp, F.; Kurth, A.; Schaefer, R.; Miller, K.; Luftner, D. Management of metastatic bone disease algorithms for diagnostics and treatment. Anticancer Res. 2016, 36, 2631–2638. [Google Scholar]
- Suva, L.J.; Washam, C.; Nicholas, R.W.; Griffin, R.J. Bone metastasis: Mechanisms and therapeutic opportunities. Nat. Rev. Endocrinol. 2011, 7, 208–218. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, M.M.; Ahsraf, M.F.; Naeem, A.; Tasleem, A.; Ahmed, M.; Farooqi, M.S. Pain management in metastatic bone disease: A literature review. Cureus 2018, 10, e3286. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Finlay, I.; Ray, A.; Simpson, B. Is there still a role for open cordotomy in cancer pain management? J. Pain Symptom Manag. 2003, 25, 179–184. [Google Scholar] [CrossRef]
- Chow, E. Update on radiation treatment for cancer pain. Curr. Opin. Support. Palliat. Care 2007, 1, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.; Gillick, L.; Hendrickson, F.R. The palliation of symptomatic osseous metastases: Final results of the study by the Radiation Therapy Oncology Group. Cancer 1982, 50, 893–899. [Google Scholar] [CrossRef]
- McQuay, H.J.; Collins, S.L.; Carroll, D.; Moore, R.A. Radiotherapy for the palliation of painful bone metastases. Cochrane Database Syst. Rev. 2000. [Google Scholar] [CrossRef]
- Nomiya, T.; Teruyama, K.; Wada, H.; Nemoto, K. Time course of pain relief in atients treated with radiotherapy for cancer pain: A prospective study. Clin. J. Pain 2010, 26, 38–42. [Google Scholar] [CrossRef]
- Liepe, K.; Kotzerke, J. A comparative study of 188Re-HEDP, 186Re-HEDP, 153Sm-EDTMP and 89Sr in the treatment of painful skeletal metastases. Nucl. Med. Commun. 2007, 28, 623–630. [Google Scholar] [CrossRef]
- Porter, A.T.; Davis, L.P. Systemic radionuclide therapy of bone metastases with strontium-89. Oncology 1994, 8, 93–96. [Google Scholar] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alsympca Investigators: Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef]
- Ma, X.; Yang, Q.; Wilson, K.T.; Kundu, N.; Meltzer, S.J.; Fulton, A.M. Promoter methylation regulates cyclooxygenase expression in breast cancer. Breast Cancer Res. 2004, 6, R316–R321. [Google Scholar] [CrossRef]
- Sabino, M.C.; Ghilardi, J.R.; Feia, K.J.; Jongen, J.L.; Keyser, C.P.; Luger, N.M.; Mach, D.B.; Peters, C.M.; Rogers, S.D.; Schwei, M.J.; et al. The involvement of prostaglandins in tumorigenesis, tumor-induced osteolysis and bone cancer pain. J. Musculoskelet. Neuronal Interact. 2002, 2, 561–562. [Google Scholar] [PubMed]
- Bombardier, C.; Laine, L.; Reicin, A.; Shapiro, D.; Burgos-Vargas, R.; Davis, B.; Day, R.; Ferraz, M.B.; Hawkey, C.J.; Hochberg, M.C.; et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. Vigor study group. N. Engl. J. Med. 2000, 343, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Otis, V.; Sarret, P.; Gendron, L. Spinal activation of delta opioid receptors alleviates cancerrelated bone pain. Neuroscience 2011, 183, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Ferrari, S.; Russel, R.G. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone 2011, 48, 677–692. [Google Scholar] [CrossRef]
- Hadji, P.; Ziller, M.; Maurer, T.; Autenrieth, M.; Muth, M.; Ruebel, M.; May, C.; Birkholz, K.; Diebel, E.; Gleissner, J.; et al. The ZOTEC study: Effect of zoledronic acid on bone metabolism in patients with bone metastases from prostate or breast cancer. J. Bone Oncol. 2012, 1, 88–94. [Google Scholar] [CrossRef]
- Coleman, R.E. Adjuvant bone-targeted therapy to prevent metastasis: Lessons from the AZURE study. Curr. Opin. Support. Palliat. Care 2012, 6, 322–329. [Google Scholar] [CrossRef]
- Nigro, C.; Donadio, M.; Ardine, M.; Beano, A.; Mistrangelo, M.; Coccorullo, Z.; Bertetto, O. Pain control with zoledronic acid in patients with breast cancer and metastatic bone disease. Am. J. Cancer 2004, 3, 257–263. [Google Scholar] [CrossRef]
- Clemons, M.; Dranitsaris, G.; Ooi, W.; Cole, D.E.C. A phase II trial evaluating the palliative benefit of secondline oral ibandronate in breast cancer patients with breast cancer: A systematic review and meta-analysis. PLoS ONE 2008, 108, 79–85. [Google Scholar] [CrossRef]
- Eleutherakis-Papaiakovou, E.; Barmias, A. Antiresorptive treatment-associated ONJ. Eur. J. Cancer Care (Engl.) 2017, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, K.H.; Wanyan, P.; Tian, J.H. Comparison of the efficacy and safety of denosumab versus bisphosphonates in breast cancer and metastases treatment: A meta-analysis of randomized controlled trials. Oncol. Lett. 2014, 7, 1997–2002. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.S.; Gordon, D.; Kaminski, M.; Howell, A.; Belch, A.; Mackey, J.; Apffelstaedt, J.; Hussein, M.A.; Coleman, R.E.; Reitsma, D.J.; et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: A randomized, double-blind, multicentre, comparative trial. Cancer 2003, 98, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Coleman, R.E.; Klotz, L.; Pittman, K.; Milecki, P.; Ng, S.; Chi, K.N.; Balakumaran, A.; Wei, R.; Wang, H.; et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: Comparison of skeletal-related events and symptomatic skeletal events. Ann. Oncol. 2015, 26, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Posta-Sales, J.; Garzon-Rodriguez, C.; Llorens-Torromé, S.; Brunelli, C.; Pigni, A.; Caraceni, A. Evidence of the analgesic role of bisphosphonates and denosumab in the treatment of pain due to bone metastases: A systematic review within the European Association for the Palliative Care guidelines project. Palliat. Med. 2017, 31, 5–25. [Google Scholar] [CrossRef]
- Khodorova, A.; Montmayeur, J.P.; Strichartz, G. Endothelin receptors and pain. J. Pain 2009, 10, 4–28. [Google Scholar] [CrossRef]
- Wacnik, P.W.; Eikmeier, L.J.; Ruggles, T.R.; Ramnaraine, M.L.; Walcheck, B.K.; Beitz, A.J.; Wilcox, G.L. Functional interactions between tumor and peripheral nerve: Morphology, algogen identification, and behavioural characterization of a new murine model of cancer pain. J. Neurosci. 2001, 21, 9355–9366. [Google Scholar] [CrossRef]
- Rove, K.O.; Crawford, E.D. Evolution of treatment options for patients with CRPC and bone metastases: Bone-target agents that go beyond palliation of symptoms to improve overall survival. Oncology 2011, 25, 1362–1370. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aielli, F.; Ponzetti, M.; Rucci, N. Bone Metastasis Pain, from the Bench to the Bedside. Int. J. Mol. Sci. 2019, 20, 280. https://doi.org/10.3390/ijms20020280
Aielli F, Ponzetti M, Rucci N. Bone Metastasis Pain, from the Bench to the Bedside. International Journal of Molecular Sciences. 2019; 20(2):280. https://doi.org/10.3390/ijms20020280
Chicago/Turabian StyleAielli, Federica, Marco Ponzetti, and Nadia Rucci. 2019. "Bone Metastasis Pain, from the Bench to the Bedside" International Journal of Molecular Sciences 20, no. 2: 280. https://doi.org/10.3390/ijms20020280
APA StyleAielli, F., Ponzetti, M., & Rucci, N. (2019). Bone Metastasis Pain, from the Bench to the Bedside. International Journal of Molecular Sciences, 20(2), 280. https://doi.org/10.3390/ijms20020280






