Hydrogen Indirectly Suppresses Increases in Hydrogen Peroxide in Cytoplasmic Hydroxyl Radical-Induced Cells and Suppresses Cellular Senescence
Abstract
:1. Introduction
2. Results
2.1. H2 Suppressed p16 and p21 Expression and Cellular Senescence
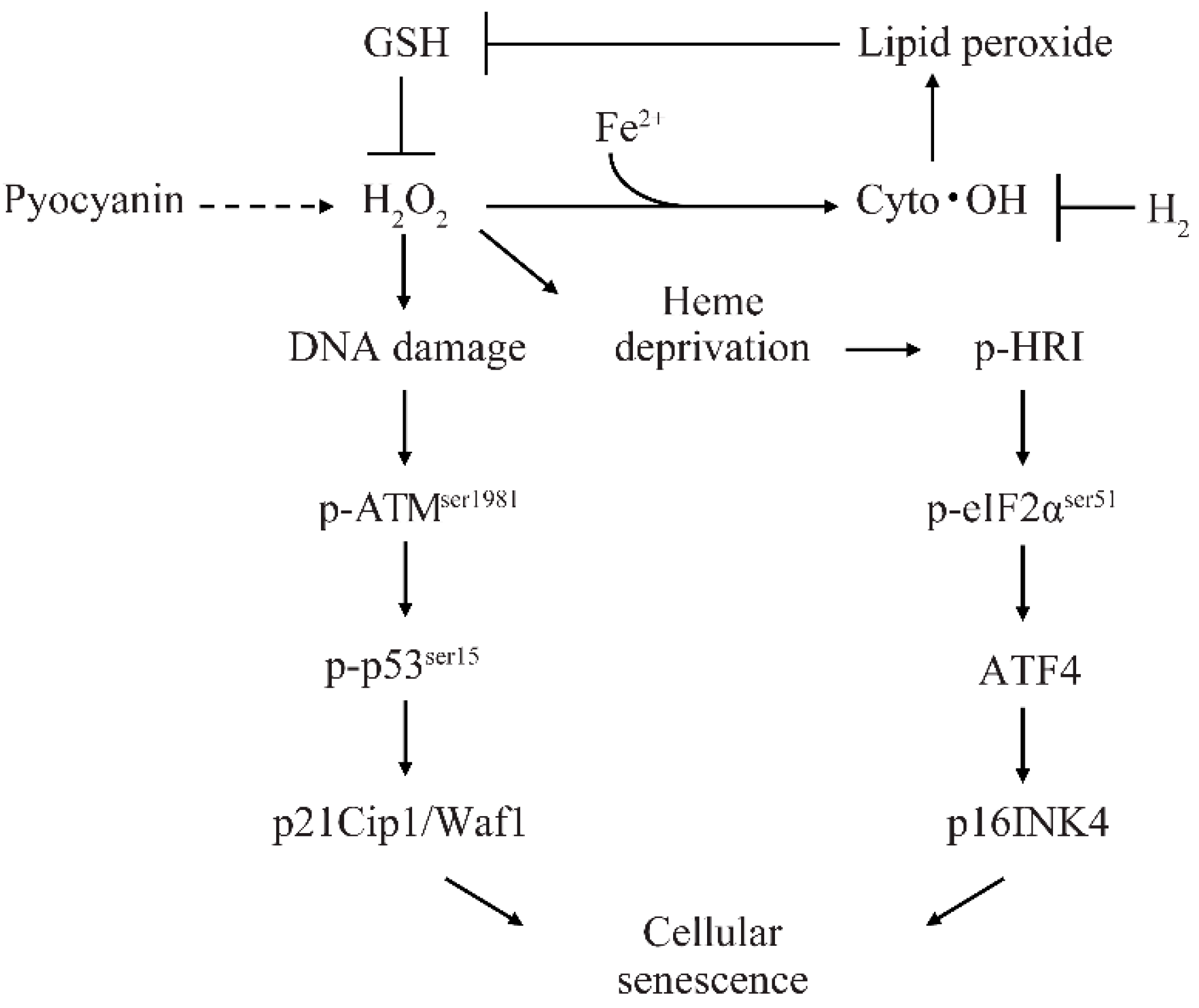
2.2. H2 Indirectly Suppressed Heme Depletion and Cellular Senescence via the p-HRI/p-eIF2α/ATF4/p16 Pathway
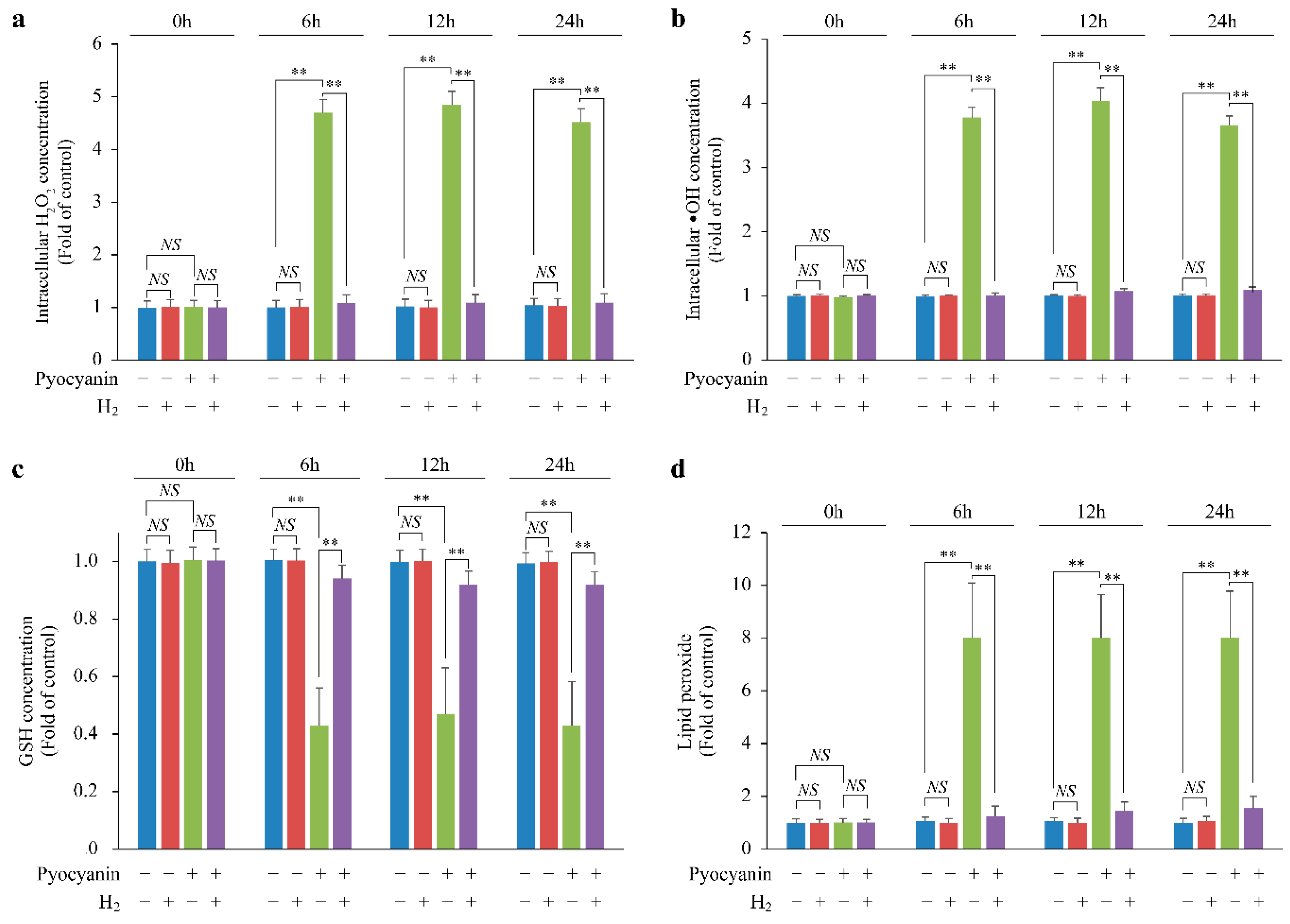
2.3. H2 Suppressed Lipid Peroxide Production and Increased H2O2 by Suppressing Glutathione (GSH) Depletion
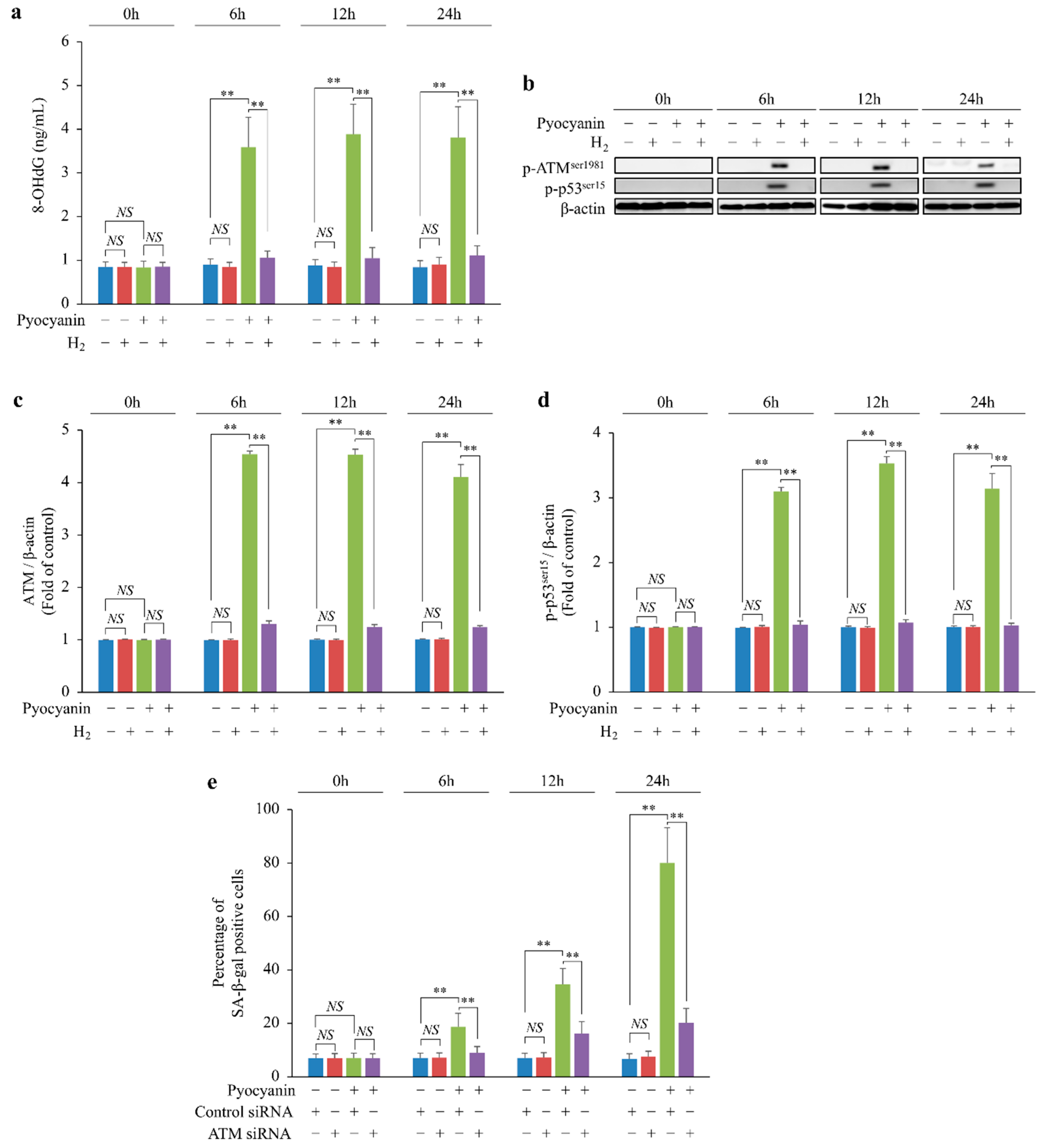
2.4. H2 Indirectly Suppressed Intracellular H2O2 Concentration Increase and DNA Oxidative Damage and Suppressed Cellular Senescence via p-ATMser1981/p-p53ser15/p21 Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Production of H2 Super-Rich Medium and Measurement of H2 Content in Medium
4.3. Primary Mouse Embryonic Fibroblast (MEF) Cell Culture and ROS Induction
4.4. Measurement of Senescence-Associated β-galactosidase (SA-β-gal) Activity
4.5. Western Blotting
4.6. Quantitation of Intracellular Heme
4.7. Measurement of Lipid Peroxide
4.8. Knockdown of HRI and ATM in Cells
4.9. Measurement of Intracellular H2O2
4.10. Measurement of Oxidative DNA Damage
4.11. In Vitro Electron Spin Resonance (ESR) Measurements
4.12. Quantitation of Intracellular GSH
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 8-OHdG | 8-Hydroxy-2-deoxyguanosine |
| ATF4 | Activating transcription factor 4 |
| ATM | Ataxia telangiectasia mutated kinase |
| cyto •OH | Cytoplasmic hydroxyl radical |
| DMEM | Dulbecco’s modified Eagle’s medium |
| ESR | Electron spin resonance |
| GSH | Glutathione |
| HRI | Heme-regulated inhibitor |
| MAC | Microbiota-accessible carbohydrates |
| MEF | Mouse embryonic fibroblast |
| •OH | Hydroxyl radical |
| p-eIF2αser51 | Phospho-eukaryotic translation initiation factor 2 subunit alpha serine 51 |
| ROS | Reactive oxygen species |
| SA-β-gal | Senescence-associated β-galactosidase |
References
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 6, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Imai, J.; Ito, T.; Takagaki, H.; Ui, M.; Hatta, S. The novel antioxidant TA293 reveals the role of cytoplasmic hydroxyl radicals in oxidative stress-induced senescence and inflammation. Biochem. Biophys. Res. Commun. 2017, 482, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, J.R.; Chen, X.M.; Cai, G.Y.; Lin, L.R.; He, Y.N. Impact of ER stress-regulated ATF4/p16 signaling on the premature senescence of renal tubular epithelial cells in diabetic nephropathy. Am. J. Physiol. Cell Physiol. 2015, 308, C621–C630. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, N.; Gorman, A.M.; Gupta, S.; Samali, A. The eIF2α kinases: Their structures and functions. Cell Mol. Life Sci. 2013, 70, 3493–3511. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Yu, C.; Lu, L.; Fujiwara, Y.; Browne, C.; Chin, G.; Fleming, M.; Leboulch, P.; Orkin, S.H.; Chen, J.J. Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J. 2001, 20, 6909–6918. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, J.; Murase, M.; Iizuka, A.; Pichierri, F.; Martinkova, M.; Shimizu, T. Elucidation of the heme binding site of heme-regulated eukaryotic initiation factor 2alpha kinase and the role of the regulatory motif in heme sensing by spectroscopic and catalytic studies of mutant proteins. J. Biol. Chem. 2008, 283, 18782–18791. [Google Scholar] [CrossRef]
- Lipinski, B. Hydroxyl radical and its scavengers in health and disease. Oxid. Med. Cell Longev. 2011, 2011, 809696. [Google Scholar] [CrossRef]
- Kajiya, M.; Sato, K.; Silva, M.J.; Ouhara, K.; Do, P.M.; Shanmugam, K.T.; Kawai, T. Hydrogen from intestinal bacteria is protective for Concanavalin A-induced hepatitis. Biochem. Biophys. Res. Commun. 2009, 386, 316–321. [Google Scholar] [CrossRef]
- Semchyshyn, H.M. Reactive carbonyl species in vivo: Generation and dual biological effects. Sci. World J. 2014, 2014, 417842. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.E.; Bren, K.L. The chemistry and biochemistry of heme c: Functional bases for covalent attachment. Nat. Prod. Rep. 2008, 6, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Iketani, M.; Sekimoto, K.; Igarashi, T.; Takahashi, M.; Komatsu, M.; Sakane, I.; Takahashi, H.; Kawaguchi, H.; Ohtani-Kaneko, R.; Ohsawa, I. Administration of hydrogen-rich water prevents vascular aging of the aorta in LDL receptor-deficient mice. Sci. Rep. 2018, 8, 16822. [Google Scholar] [CrossRef] [PubMed]
- Hara, F.; Tanabe, J.; Watanabe, I.; Yamazaki, J.; Ikeda, T.; Morita, T. Molecular hydrogen alleviates cellular senescence in endothelial cells. Circ. J. 2016, 80, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sato, T.; Sugimoto, M.; Baskoro, H.; Karasutani, K.; Mitsuti, A.; Nurwidya, F.; Arano, N.; Kodama, Y.; Hirano, S.; et al. Hydrogen-rich water prevents cigarette smoke-induced pulmonary emphysema in SMP30 knockout mice. Biochem. Biophys. Res. Commun. 2017, 492, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, C.; Sun, A.; Qiao, L.; Zhang, X.; Huang, J.; Sun, X.; Yang, X.; Sun, S. Hydrogen alleviates cellular senescence via regulation of ROS/p53/p21 pathway in bone marrow-derived mesenchymal stem cells in vivo. Biomed. Pharmacother 2018, 106, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Hsu, W.L.; Tsai, M.H.; Liang, J.L.; Lu, J.H.; Yen, C.J.; Yu, H.S.; Noda, M.; Lu, C.Y.; Chen, C.H.; et al. Hydrogen gas protects IP3Rs by reducing disulfide bridges in human keratinocytes under oxidative stress. Sci. Rep. 2017, 7, 3606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todaro, M.; Di Gaudio, F.; Lavitrano, M.; Stassi, G.; Papaccio, G. Islet beta-cell apoptosis triggered in vivo by interleukin-1beta is not related to the inducible nitric oxide synthase pathway: Evidence for mitochondrial function impairment and lipoperoxidation. Endocrinology 2003, 10, 4264–4271. [Google Scholar] [CrossRef] [PubMed]
- Barbisan, F.; Azzolin, V.F.; Ribeiro, E.E.; Duarte, M.M.M.F.; da Cruz, I.B.M. The in vitro influence of a genetic superoxide-hydrogen peroxide imbalance on immunosenescence. Rejuvenation Res. 2017, 20, 334–345. [Google Scholar] [CrossRef]
- Das, K.C.; Lewis-Molock, Y.; White, C.W. Thiol modulation of TNF alpha and IL-1 induced MnSOD gene expression and activation of NF-kappa B. Mol. Cell. Biochem. 1995, 148, 45–57. [Google Scholar] [CrossRef]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Shidoji, Y.; Komura, S.; Ohishi, N.; Yagi, K. Interaction between cytochrome c and oxidized mitochondrial lipids. In Phospholipid Metabolism in Apoptosis, 1st ed.; Peter, J.Q., Valerian, E.K., Eds.; Springer: Boston, MA, USA, 2002; Volume 36, pp. 19–37. [Google Scholar]
- Gu, Y.; Huang, C.S.; Inoue, T.; Yamashita, T.; Ishida, T.; Kang, K.M.; Nakao, A. Drinking hydrogen water ameliorated cognitive impairment in senescence-accelerated mice. J. Clin. Biochem. Nutr. 2010, 46, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ito, M.; Fujita, Y.; Ito, M.; Ichihara, M.; Masuda, A.; Suzuki, Y.; Maesawa, S.; Kajita, Y.; Hirayama, M.; et al. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson’s disease. Neurosci. Lett. 2009, 453, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Hirayama, M.; Yamai, K.; Goto, S.; Ito, M.; Ichihara, M.; Ohno, K. Drinking hydrogen water and intermittent hydrogen gas exposure, but not lactulose or continuous hydrogen gas exposure, prevent 6-hydorxydopamine-induced Parkinson’s disease in rats. Med. Gas. Res. 2012, 2, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohsawa, I.; Nishimaki, K.; Yamagata, K.; Ishikawa, M.; Ohta, S. Consumption of hydrogen water prevents atherosclerosis in apolipoprotein E knockout mice. Biochem. Biophys. Res. Commun. 2008, 377, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Nishimaki, K.; Asada, T.; Ohsawa, I.; Nakajima, E.; Ikejima, C.; Yokota, T.; Kamimura, N.; Ohta, S. Effects of molecular hydrogen assessed by an animal model and a randomized clinical study on mild cognitive impairment. Curr. Alzheimer Res. 2018, 15, 482–492. [Google Scholar] [CrossRef] [PubMed]
- So, M.J.; Cho, E.J. Phloroglucinol attenuates free radical-induced oxidative stress. Prev. Nutr. Food Sci. 2014, 19, 129–135. [Google Scholar] [CrossRef]
- Kurokawa, R.; Seo, T.; Sato, B.; Hirano, S.; Sato, F. Convenient methods for ingestion of molecular hydrogen: Drinking, injection, and inhalation. Med. Gas. Res. 2015, 5, 13. [Google Scholar] [CrossRef]
- Seo, T.; Kurokawa, R.; Sato, B. A convenient method for determining the concentration of hydrogen in water: Use of methylene blue with colloidal platinum. Med. Gas. Res. 2012, 2, 1. [Google Scholar] [CrossRef]
- Hogan, B.; Beddington, R.; Costantini, F.; Lacy, E. Manipulating the mouse embryo: A laboratory manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1994; pp. 127–188. [Google Scholar]
- Zhang, Y.; Zhao, L.; Wang, C.; Lei, B. Isolation and culture of mouse embryonic fibroblast. Sichuan Da Xue Xue Bao Yi Xue Ban 2003, 34, 344–346. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakai, T.; Kurokawa, R.; Hirano, S.-i.; Imai, J. Hydrogen Indirectly Suppresses Increases in Hydrogen Peroxide in Cytoplasmic Hydroxyl Radical-Induced Cells and Suppresses Cellular Senescence. Int. J. Mol. Sci. 2019, 20, 456. https://doi.org/10.3390/ijms20020456
Sakai T, Kurokawa R, Hirano S-i, Imai J. Hydrogen Indirectly Suppresses Increases in Hydrogen Peroxide in Cytoplasmic Hydroxyl Radical-Induced Cells and Suppresses Cellular Senescence. International Journal of Molecular Sciences. 2019; 20(2):456. https://doi.org/10.3390/ijms20020456
Chicago/Turabian StyleSakai, Takahiro, Ryosuke Kurokawa, Shin-ichi Hirano, and Jun Imai. 2019. "Hydrogen Indirectly Suppresses Increases in Hydrogen Peroxide in Cytoplasmic Hydroxyl Radical-Induced Cells and Suppresses Cellular Senescence" International Journal of Molecular Sciences 20, no. 2: 456. https://doi.org/10.3390/ijms20020456
APA StyleSakai, T., Kurokawa, R., Hirano, S.-i., & Imai, J. (2019). Hydrogen Indirectly Suppresses Increases in Hydrogen Peroxide in Cytoplasmic Hydroxyl Radical-Induced Cells and Suppresses Cellular Senescence. International Journal of Molecular Sciences, 20(2), 456. https://doi.org/10.3390/ijms20020456





