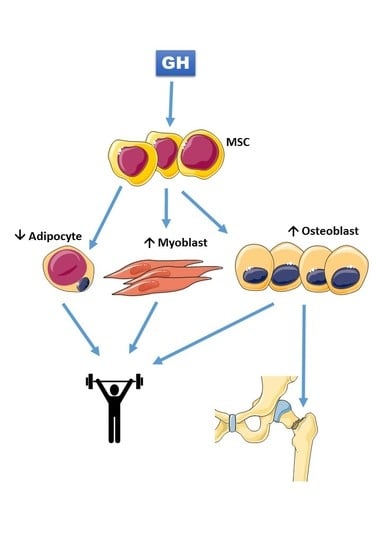
The Role of Growth Hormone in Mesenchymal Stem Cell Commitment
Abstract
1. Introduction
2. Growth Hormone Synthesis and Secretion
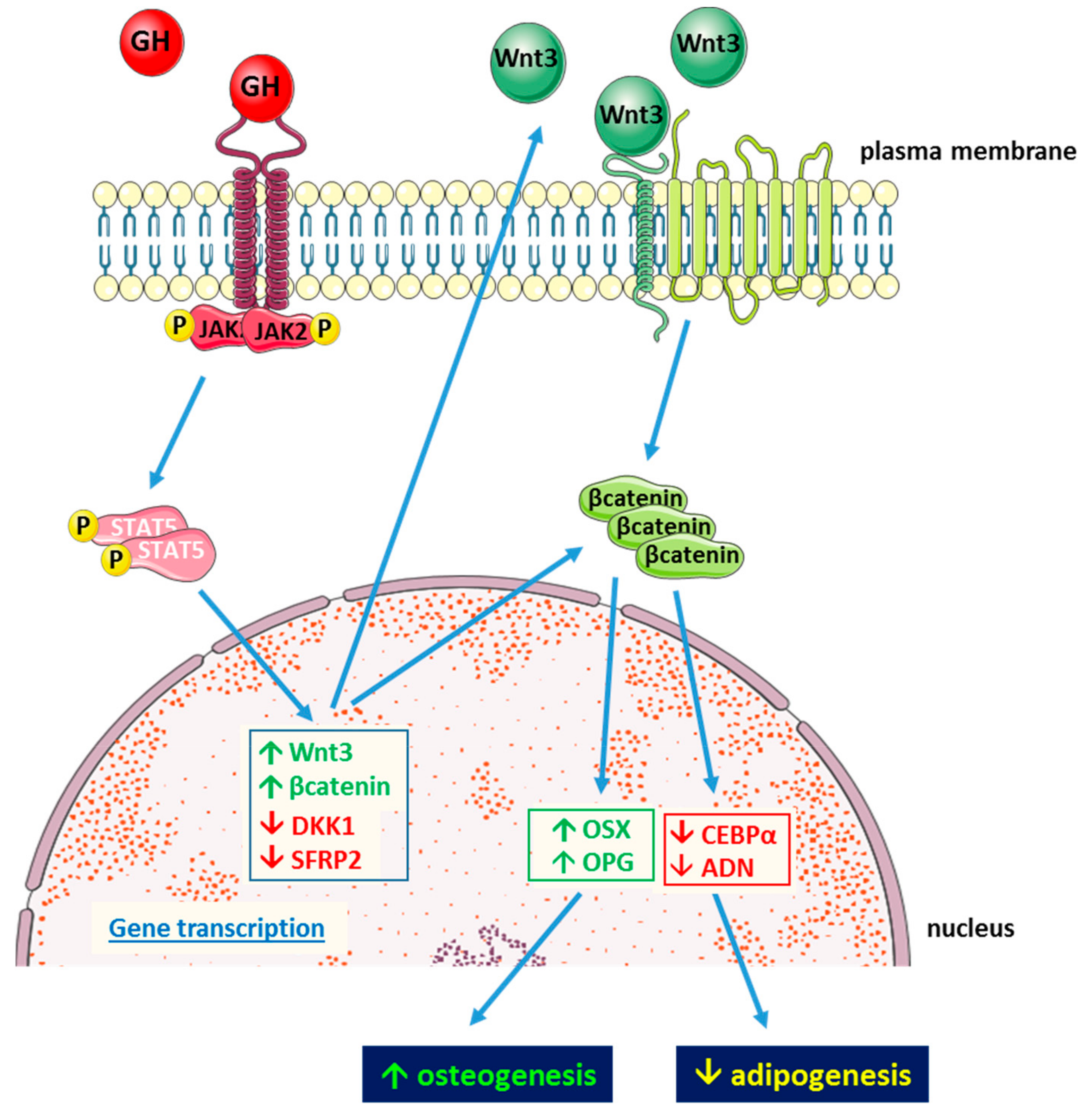
3. Growth Hormone Intracellular Signaling
4. Growth Hormone Activities
5. Mesenchymal Stem Cells: A Brief Overview
6. Growth Hormone and Mesenchymal Stem Cells
7. Growth Hormone and Mesenchymal Stem Cells in Bone Regenerative Medicine
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AT-MSC | adipose tissue mesenchymal stem cells |
| bGH | transgenic mice expressing constitutively bovine growth hormone |
| BM-MSC | bone marrow mesenchymal stem cells |
| CEBPα | CCAAT enhancer binding protein |
| ERK | extracellular signal regulated kinase |
| FABP4 | fatty acid-binding protein 4 |
| FAK | focal adhesion kinase |
| GH | growth hormone |
| GHBP | growth hormone-binding protein |
| GHD | growth hormone deficiency |
| GHR | growth hormone receptor |
| GHRH | growth hormone releasing hormone |
| GHRKO | mouse knockout for the growth hormone receptor |
| IGF I | insulin-like growth factor I |
| IRS | insulin receptor substrate |
| JAK2 | Janus kinase 2 |
| MSC | mesenchymal stem cell |
| PLGA | poly(lactic-co-glycolic acid) |
| PPARγ | peroxisome proliferator-activated receptor γ |
| PTP | phosphotyrosine phosphatases |
| rhGH | recombinant human growth hormone |
| SOCS | suppressors of cytokine signaling |
| STAT | signal transducers and activators of transcription |
References
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Veldhuis, J.D. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr. Rev. 1998, 19, 717–797. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.L.; Scanes, C.G. Nanobiology and physiology of growth hormone secretion. Exp. Biol. Med. (Maywood) 2012, 237, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.L.; Jeftinija, S.; Scanes, C.G. Growth hormone secretion: Molecular and cellular mechanisms and in vivo approaches. Exp. Biol. Med. (Maywood) 2004, 229, 291–302. [Google Scholar] [CrossRef]
- Cruz, C.R.; Smith, R.G. The growth hormone secretagogue receptor. Vitam. Horm. 2008, 77, 47–88. [Google Scholar]
- Wehrenberg, W.B.; Giustina, A. Basic counterpoint: Mechanisms and pathways of gonadal steroid modulation of growth hormone secretion. Endocr. Rev. 1992, 13, 299–308. [Google Scholar]
- Giustina, A.; Wehrenberg, W.B. The role of glucocorticoids in the regulation of growth hormone secretion. Trends Endocrinol. Metab. 1992, 3, 306–311. [Google Scholar] [CrossRef]
- Harvey, S. Extrapituitary growth hormone. Endocrine 2010, 38, 335–359. [Google Scholar] [CrossRef]
- Pérez-Ibave, D.C.; Rodríguez-Sánchez, I.P.; Garza-Rodríguez, M.d.L.; Barrera-Saldaña, H.A. Extrapituitary growth hormone synthesis in humans. Growth Horm. IGF Res. 2014, 24, 47–53. [Google Scholar] [CrossRef]
- Leung, D.W.; Spencer, S.A.; Cachianes, G.; Hammonds, R.G.; Collins, C.; Henzel, W.J.; Barnard, R.; Waters, M.J.; Wood, W.I. Growth hormone receptor and serum binding protein: Purification, cloning and expression. Nature 1987, 330, 537–543. [Google Scholar] [CrossRef]
- Clark, R.G.; Mortensen, D.L.; Carlsson, L.M.; Spencer, S.A.; McKay, P.; Mulkerrin, M.; Moore, J.; Cunningham, B.C. Recombinant human growth hormone (GH)-binding protein enhances the growthpromoting activity of human GH in the rat. Endocrinology 1996, 137, 4308–4315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amit, T.; Youdim, M.B.; Hochberg, Z. Does serum growth hormone (GH) binding protein reflect human GH receptor function? J. Clin. Endocrinol. Metab. 2000, 85, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Baumann, G. Growth hormone binding protein. J. Pediatr. Endocrinol. Metab. 2001, 14, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Fisker, S.; Vahl, N.; Jorgensen, J.O.; Christiansen, J.S.; Orskov, H. Abdominal fat determines growth hormone-binding protein levels in healthy non obese adults. J. Clin. Endocrinol. Metab. 1997, 82, 123–128. [Google Scholar] [PubMed]
- Ballesteros, M.; Leung, K.C.; Ross, R.J.; Iismaa, T.P.; Ho, K.K. Distribution and abundance of messenger ribonucleic acid for growth hormone receptor isoforms in human tissues. J. Clin. Endocrinol. Metab. 2000, 85, 2865–2871. [Google Scholar] [CrossRef] [PubMed]
- Bergan-Roller, H.E.; Sheridan, M.A. The growth hormone signaling system: Insights into coordinating the anabolic and catabolic actions of growth hormone. Gen. Comp. Endocrinol. 2018, 258, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Landin-Wilhelmens, K.; Wilhelmens, L.; Lappas, G. Serum IGF-I in a random population sample of men, and women: Relation to age, sex, smoking habits, coffee consumption and physical activity, blood pressure and concentrations of plasma lipids, fibrinogen, PTH and osteocalcin. Clin. Endocrinol. (Oxf) 1994, 41, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Hermann, M.; Berger, P. Hormonal changes in aging men: A therapeutic indication? Exp. Gerontol. 2001, 36, 1075–1082. [Google Scholar] [CrossRef]
- Waters, M.J. The growth hormone receptor. Growth Horm. IGF Res. 2016, 28, 6–10. [Google Scholar] [CrossRef]
- Brown, R.J.; Adams, J.J.; Pelekanos, R.A.; Wan, Y.; McKinstry, W.J.; Palethorpe, K.; Seeber, R.M.; Monks, T.A.; Eidne, K.A.; Parker, M.W.; et al. Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat. Struct. Mol. Biol. 2005, 12, 814–821. [Google Scholar] [CrossRef]
- De Vos, A.M.; Ultsch, M.; Kossiakoff, A.A. Human growth hormone and extracellular domain of its receptor: Crystal structure of the complex. Science 1992, 255, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.A. Binding in the growth hormone receptor complex. Proc. Natl. Acad. Sci. USA 1996, 93, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Birzniece, V.; Sata, A.; Ho, K.K.Y. Growth hormone receptor modulators. Rev. Endocr. Metab. Dis. 2009, 10, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Farquharson, C. The effect of GH and IGFI on linear growth and skeletal development and their modulation by SOCS proteins. J. Endocrinol. 2010, 206, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Bolamperti, S.; Mrak, E.; Moro, G.L.; Sirtori, P.; Fraschini, G.; Guidobono, F.; Rubinacci, A.; Villa, I. 17β-Estradiol positively modulates growth hormone signaling through the reduction of SOCS2 negative feedback in human osteoblasts. Bone 2013, 55, 84–92. [Google Scholar] [CrossRef]
- Strous, G.J.; van Kerkhof, P.; Govers, R.; Ciechanover, A.; Schwartz, A.L. The ubiquitin conjugation system is required for ligand-induced endocytosis and degradation of the growth hormone receptor. Embo J. 1996, 15, 3806–3812. [Google Scholar] [CrossRef]
- Strous, G.J.; van Kerkhof, P.; Govers, R.; Rotwein, P.; Schwartz, A.L. Growth hormone-induced signal transduction depends on an intact ubiquitin system. J. Biol. Chem. 1997, 272, 40–43. [Google Scholar] [CrossRef][Green Version]
- Zhu, T.; Goh, E.L.; Graichen, R.; Ling, L.; Lobie, P.E. Signal transduction via the growth hormone receptor. Cell. Signal. 2001, 13, 599–616. [Google Scholar] [CrossRef]
- Vanderkuur, J.A.; Butch, E.R.; Waters, S.B.; Pessin, J.E.; Guan, K.L.; Carter-Su, C. Signaling molecules involved in coupling growth hormone receptor to MAP kinase activity. Endocrinol. 1997, 138, 4301–4307. [Google Scholar] [CrossRef]
- Piwien-Pilipuk, G.; MacDougald, O.; Schwartz, J. Dual regulation of phosphorylation and dephosphorylation of C/EBPbeta modulate its transcriptional activation and DNA binding in response to growth hormone. J. Biol. Chem. 2002, 277, 44557–44565. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kaburagi, Y.; Ueki, K.; Tsuji, Y.; Stark, G.R.; Kerr, I.M.; Tsushima, T.; Akanuma, Y.; Komuro, I.; Tobe, K.; et al. Growth hormone and prolactin stimulate tyrosine phosphorylation of insulin receptor substrate-1, -2, and -3, their association with p85 phosphatidylinositol 3-kinase (PI3-kinase), and concomitantly PI3-kinase activation via JAK2 kinase. J. Biol. Chem. 1998, 273, 15719–15726. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Goh, E.L.; Lobie, P.E. Growth hormone stimulates the tyrosine phosphorylation and association of p125 focal adhesion kinase (FAK) with JAK2. FAK is not required for Stat-mediated transcription. J. Biol. Chem. 1998, 273, 10682–10689. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Lee, J.H.; Kim, K.S.; Jeong, S.M.; Kim, P.H.; Chung, H.T. Regulation of neutrophil adhesion by pituitary growth hormone accompanies tyrosine phosphorylation of Jak2, p125FAK, and paxillin. J. Immunol. 2000, 165, 2116–2123. [Google Scholar] [CrossRef]
- Takahashi, M.O.; Takahashi, Y.; Iida, K.; Okimura, Y.; Kaji, H.; Abe, H.; Chihara, K. Growth hormone stimulates tyrosine phosphorylation of focal adhesion kinase (p125(FAK)) and actin stress fiber formation in human osteoblast-like cells, Saos2. Biochem. Biophys. Res. Commun. 1999, 263, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, D.D.; Hunter, T. Integrin signaling and tyrosine phosphorylation: Just the FAKs? Trends Cell. Biol. 1998, 8, 151–157. [Google Scholar] [CrossRef]
- Herrington, J.; Smit, L.S.; Schwartz, J.; Carter-Su, C. The role of STAT proteins in growth hormone signaling. Oncogene Res. 2000, 19, 2585–2597. [Google Scholar] [CrossRef] [PubMed]
- Waxman, D.J.; O’Connor, C. Growth hormone regulation of sex dependent liver gene expression. Mol. Endocrinol. 2006, 20, 2613–2629. [Google Scholar] [CrossRef]
- Chia, D.J.; Ono, M.; Woelfle, J.; Schlesinger-Massart, M.; Jiang, H.; Rotwein, P. Characterisation of distinct Stat5b binding sites that mediate growth hormone-stimulated IGF-I gene transcription. J. Biol. Chem. 2006, 281, 3190–3197. [Google Scholar] [CrossRef]
- Rowlinson, S.W.; Yoshizato, H.; Barclay, J.L.; Brooks, A.J.; Behncken, S.N.; Kerr, L.M.; Millard, K.; Palethorpe, K.; Nielsen, K.; Clyde-Smith, J.; et al. An agonist-induced conformational change in the growth hormone receptor determines the choice of signalling pathway. Nat. Cell Biol. 2008, 10, 740–747. [Google Scholar] [CrossRef]
- Lincoln, D.T.; Sinowatz, F.; Kölle, S.; Takahashi, H.; Parsons, P.; Waters, M.J. Up-regulation of growth hormone receptor immunoreactivity in human melanoma. Anticancer Res. 1999, 19, 1919–1931. [Google Scholar]
- García-Caballero, T.; Mertani, H.M.; Lambert, A.; Gallego, R.; Fraga, M.; Pintos, E.; Forteza, J.; Chevallier, M.; Lobie, P.E.; Vonderhaar, B.K.; et al. Increased expression of growth hormone and prolactin receptors in hepatocellular carcinoma. Endocrine 2000, 12, 265–271. [Google Scholar] [CrossRef]
- Conway-Campbell, B.L.; Wooh, J.W.; Brooks, A.J.; Gordon, D.; Brown, R.J.; Lichanska, A.M.; Chin, H.S.; Barton, C.L.; Boyle, G.M.; Parsons, P.G.; et al. Nuclear targeting of the growth hormone receptor results in dysregulation of cell proliferation and tumorigenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 13331–13336. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Mazziotti, G.; Canalis, E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr. Rev. 2008, 29, 535–559. [Google Scholar] [CrossRef] [PubMed]
- Mrak, E.; Villa, I.; Lanzi, R.; Losa, M.; Guidobono, F.; Rubinacci, A. Growth hormone stimulates osteoprotegerin expression and secretion in human osteoblast-like cells. J. Endocrinol. 2007, 192, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Menagh, P.J.; Turner, R.T.; Jump, D.B.; Wong, C.P.; Lowry, M.B.; Yakar, S.; Rosen, C.J.; Iwaniec, U.T. Growth hormone regulates the balance between bone formation and bone marrow adiposity. J. Bone Min. Res. 2010, 25, 757–768. [Google Scholar] [CrossRef]
- Olney, R.C. Regulation of bone mass by growth hormone. Med. Pediatr. Oncol. 2003, 41, 228–234. [Google Scholar] [CrossRef]
- Ohlsson, C.; Bengtsson, B.A.; Isaksson, O.G.; Andreassen, T.T.; Slootweg, M.C. Growth hormone and bone. Endocr. Rev. 1998, 19, 55–79. [Google Scholar]
- Murray, P.G.; Clayton, P.E. Disorders of Growth Hormone in Childhood. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000; Available online: www.endotext.org (accessed on 16 November 2016).
- Richmond, E.; Rogol, A.D. Treatment of growth hormone deficiency in children, adolescents and at the transitional age. Best Pr. Res. Clin. Endocrinol. Metab. 2016, 30, 749–755. [Google Scholar] [CrossRef]
- Shi, J.; Sekhar, R.V.; Balasubramanyam, A.; Ellis, K.; Reeds, P.J.; Jahoor, F.; Sharma, M.D. Short- and long-term effects of growth hormone (GH) replacement on protein metabolism in GH-deficient adults. J. Clin. Endocrinol. Metab. 2003, 88, 5827–5833. [Google Scholar] [CrossRef]
- Chikani, V.; Ho, K.K.Y. Action of GH on skeletal muscle function: Molecular and metabolic mechanisms. J. Mol. Endocrinol. 2014, 52, R107–R123. [Google Scholar]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulos, A.; Ohanna, M.; Kedzia, C.; Menon, R.K.; Kopchick, J.J.; Kelly, P.A.; Pende, M. Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation. Proc. Natl. Acad. Sci. USA 2006, 103, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, J.; Cheng, C.M.; Kopchick, J.J.; Bondy, C.A. Evidence supporting dual, IGF-1 independent and IGF-1 dependent, roles for GH in promoting longitudinal bone growth. J. Endocrinol. 2004, 180, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Freda, P.U.; Shen, W.; Heymsfield, S.B.; Reyes-Vidal, C.M.; Geer, E.B.; Bruce, J.N.; Gallagher, D. Lower visceral and subcutaneous but higher intermuscular adipose tissue depots in patients with growth hormone and insulin-like growth factor I excess due to acromegaly. J. Clin. Endocrinol. Metab. 2008, 93, 2334–2343. [Google Scholar] [CrossRef] [PubMed]
- Benencia, F.; Harshman, S.; Duran-Ortiz, S.; Lubbers, E.R.; List, E.O.; Householder, L.; Alnaeeli, M.; Liang, X.; Welch, L.; Kopchick, J.J.; et al. Male bovine GH transgenic mice have decreased adiposity with an adipose depot-specific increase in immune cell populations. Endocrinology 2015, 156, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Berryman, D.E.; List, E.O.; Kohn, D.T.; Coschigano, K.T.; Seeley, R.J.; Kopchick, J.J. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology 2006, 147, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Chaves, V.E.; Mesquita Junior, F.; Bertolini, G.L. The metabolic effects of growth hormone in adipose tissue. Endocrine 2013, 44, 293–302. [Google Scholar] [CrossRef]
- Bredella, M.A.; Karastergiou, K.; Bos, S.A.; Gerweck, A.V.; Torriani, M.; Fried, S.K.; Miller, K.K. GH administration decreases subcutaneous abdominal adipocyte size in men with abdominal obesity. Growth Horm IGF Res. 2017, 35, 17–20. [Google Scholar] [CrossRef]
- Rasmussen, M.H. Obesity, growth hormone and weight loss. Mol. Cell. Endocrinol. 2010, 316, 147–153. [Google Scholar] [CrossRef]
- Lam, K.S.; Xu, A.; Tan, K.C.; Wong, L.C.; Tiu, S.C.; Tam, S. Serum adiponectin is reduced in acromegaly and normalized after correction of growth hormone excess. J. Clin. Endocrinol. Metab. 2004, 89, 5448–5453. [Google Scholar] [CrossRef]
- Silha, J.V.; Krsek, M.; Hana, V.; Marek, J.; Jezkova, J.; Weiss, V.; Murphy, L.J. Perturbations in adiponectin, leptin and resistin levels in acromegaly: Lack of correlation with insulin resistance. Clin. Endocrinol. 2003, 58, 736–742. [Google Scholar] [CrossRef]
- Møller, N.; Jørgensen, J.O. Effects of growth hormone on glucose, lipid and protein metabolism in human subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef] [PubMed]
- Roemmler, J.; Gockel, A.; Otto, B.; Bidlingmaier, M.; Schopohl, J. Effects on metabolic variables after 12-month treatment with a new once a week sustained-release recombinant growth hormone (GH:LB03002) in patients with GH deficiency. Clin. Endocrinol. 2012, 76, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Review: Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005, 11, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell. Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef]
- Frenette, P.S.; Pinho, S.; Lucas, D.; Scheiermann, C. Mesenchymal stem cell: Keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu. Rev. Immunol. 2013, 31, 285–316. [Google Scholar] [CrossRef] [PubMed]
- Krampera, M.; Pasini, A.; Pizzolo, G.; Cosmi, L.; Romagnani, S.; Annunziato, F. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr. Op. Pharm. 2006, 6, 435–441. [Google Scholar] [CrossRef]
- Siegel, G.; Schäfer, R.; Dazzi, F. The immunosuppressive properties of mesenchymal stem cells. Transplantation 2009, 87, S45–S49. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.j.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Calloni, R.; Cordero, E.A.A.; Henriques, J.A.P.; Bonatto, D. Reviewing and updating the major molecular markers for stem cells. Stem Cells Dev. 2013, 22, 1455–1476. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.-J.; Tuan, R.S.; Cheung, K.M.C.; Leung, V.Y.L. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Meirelles, L.; Fontes, A.M.; Caplan, A.I. Mechanisms involved in the herapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef]
- Kassis, I.; Zangi, L.; Rivkin, R.; Levdansky, L.; Samuel, S.; Marx, G.; Gorodetsky, R. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transpl. 2006, 37, 967–976. [Google Scholar] [CrossRef]
- Zou, Z.; Zhang, Y.; Hao, L.; Wang, F.; Liu, D.; Su, Y.; Sun, H. More insight into mesenchymal stem cells and their effects inside the body. Exp. Opin. Biol. Ther. 2010, 10, 215–230. [Google Scholar] [CrossRef]
- Lin, G.; Garcia, M.; Ning, H.; Banie, L.; Guo, Y.L.; Lue, T.F.; Lin, C.S. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008, 17, 1053–1063. [Google Scholar] [CrossRef]
- Corselli, M.; Chen, C.W.; Crisan, M.; Lazzari, L.; Péault, B. Perivascular ancestors of adultmultipotent stem cells. Arter. Thromb. Vasc. Biol. 2010, 30, 1104–1109. [Google Scholar] [CrossRef]
- Fong, C.Y.; Chak, L.L.; Biswas, A.; Tan, J.H.; Gauthaman, K.; Chan, W.K.; Bongso, A. Human Wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev. 2011, 7, 1–16. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Lin, P.; Correa, D.; Kean, T.J.; Awadallah, A.; Dennis, J.E.; Caplan, A.I. Serial transplantation and long-term engraftment of intra-arterially delivered clonally derived mesenchymal stem cells to injured bone marrow. Mol. Ther. 2014, 22, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Adult mesenchymal stem cells: When, where, and how. Stem Cells Int. 2015, 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Horn, P.; Castoldi, M.; Diehlmann, A.; Bork, S.; Saffrich, R.; Benes, V.; Blake, J.; Pfister, S.; Eckstein, V.; et al. Replicative Senescence of Mesenchymal Stem Cells: A Continuous and Organized Process. PLoS ONE 2008, 3, e2213. [Google Scholar] [CrossRef]
- Sethe, S.; Scutt, A.; Stolzing, A. Aging of mesenchymal stem cells. Ageing Res. Rev. 2006, 5, 91–116. [Google Scholar] [CrossRef]
- Zhou, S.; Greenberger, J.S.; Epperly, M.W.; Goff, J.P.; Adler, C.; Leboff, M.S.; Glowacki, J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008, 7, 335–343. [Google Scholar] [CrossRef]
- Olarescu, N.C.; Berryman, D.E.; Householder, L.A.; Lubbers, E.R.; List, E.O.; Benencia, F.; Kopchick, J.J.; Bollerslev, J. GH action influences adipogenesis of mouse adipose tissue-derived mesenchymal stem cells. J. Endocrinol. 2015, 226, 13–23. [Google Scholar] [CrossRef]
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; MacDougald, O.A. Inhibition of adipogenesis by Wnt signalling. Science 2000, 289, 950–953. [Google Scholar] [CrossRef]
- Prestwich, T.C.; McDougald, O.A. Wnt/β catenin signaling in adipogenesis and metabolism. Curr. Opin. Cell Biol. 2007, 19, 612–617. [Google Scholar] [CrossRef]
- Bolamperti, S.; Signo, M.; Spinello, A.; Moro, G.; Fraschini, G.; Guidobono, F.; Rubinacci, A.; Villa, I. GH prevents adipogenic differentiation of mesenchymal stromal stem cells derived from human trabecular bone via canonical Wnt signaling. Bone 2018, 112, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Zheng, W.; Jiang, H. Growth hormone facilitates 5′-azacytidine-induced myogenic but inhibits 5′-azacytidine-induced adipogenic commitment in C3H10T1/2 mesenchymal stem cells. Growth Horm. IGF Res. 2018, 40, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.M.; Jones, P.A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell 1979, 17, 771–779. [Google Scholar] [CrossRef]
- Huang, E.; Zhu, G.; Jiang, W.; Yang, K.; Gao, Y.; Luo, Q.; Gao, J.L.; Kim, S.H.; Liu, X.; Li, M.; et al. Growth hormone synergizes with BMP9 in osteogenic differentiation by activating the JAK/STAT/IGF1 pathway in murine multilineage cells. J. Bone Min. Res. 2012, 27, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.; Oreffo, R.O. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef]
- Guicheux, J.; Gauthier, O.; Aguado, E.; Pilet, P.; Couillaud, S.; Jegou, D.; Daculsi, G.; Heymann, D. Human growth hormone locally released in bone sites by calcium-phosphate biomaterial stimulates ceramic bone substitution without systemic effects: A rabbit study. J. Bone Min. Res. 1998, 13, 739–748. [Google Scholar] [CrossRef]
- Tresguerres, I.F.; Blanco, L.; Clemente, C.; Tresguerres, J.A. Effects of local administration of growth hormone in peri-implant bone: An experimental study with implants in rabbit tibiae. Int. J. Oral Maxillofac. Implant. 2003, 18, 807–811. [Google Scholar]
- Wang, J.R.; Ahmed, S.F.; Gadegaard, N.; Meek, R.M.; Dalby, M.J.; Yarwood, S.J. Nanotopology potentiates growth hormone signalling and osteogenesis of mesenchymal stem cells. Growth Horm. IGF Res. 2014, 24, 245–250. [Google Scholar] [CrossRef]
- Da Silveira Gerzson, A.; Machado, D.C.; Marinovic, D.R.; Pagnoncelli, R.M. Assessment of Adhesion and Proliferation of Bone Marrow Mesenchymal Stem Cells in Polymer Matrices with rhGH. Int. J. Oral Maxillofac. Implants. 2017, 32, e183–e189. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolamperti, S.; Guidobono, F.; Rubinacci, A.; Villa, I. The Role of Growth Hormone in Mesenchymal Stem Cell Commitment. Int. J. Mol. Sci. 2019, 20, 5264. https://doi.org/10.3390/ijms20215264
Bolamperti S, Guidobono F, Rubinacci A, Villa I. The Role of Growth Hormone in Mesenchymal Stem Cell Commitment. International Journal of Molecular Sciences. 2019; 20(21):5264. https://doi.org/10.3390/ijms20215264
Chicago/Turabian StyleBolamperti, Simona, Francesca Guidobono, Alessandro Rubinacci, and Isabella Villa. 2019. "The Role of Growth Hormone in Mesenchymal Stem Cell Commitment" International Journal of Molecular Sciences 20, no. 21: 5264. https://doi.org/10.3390/ijms20215264
APA StyleBolamperti, S., Guidobono, F., Rubinacci, A., & Villa, I. (2019). The Role of Growth Hormone in Mesenchymal Stem Cell Commitment. International Journal of Molecular Sciences, 20(21), 5264. https://doi.org/10.3390/ijms20215264