Immunomodulatory Effects of MSCs in Bone Healing
Abstract
:1. Introduction
2. Inflammation and Impact of Mesenchymal Stem Cells in Bone Healing
3. Mesenchymal Stem Cells Licensing as an Anti-Inflammatory Tool
4. Modulation of Macrophage Polarization and MSCs-Macrophage Crosstalk
5. Paracrine Signaling Molecules of MSCs and Macrophages in Bone Fracture Healing
6. Regulatory Effects of MSCs on T Lymphocytes in Bone Fracture Repair
7. MSCs Derived Exosomes in Bone Fracture Repair
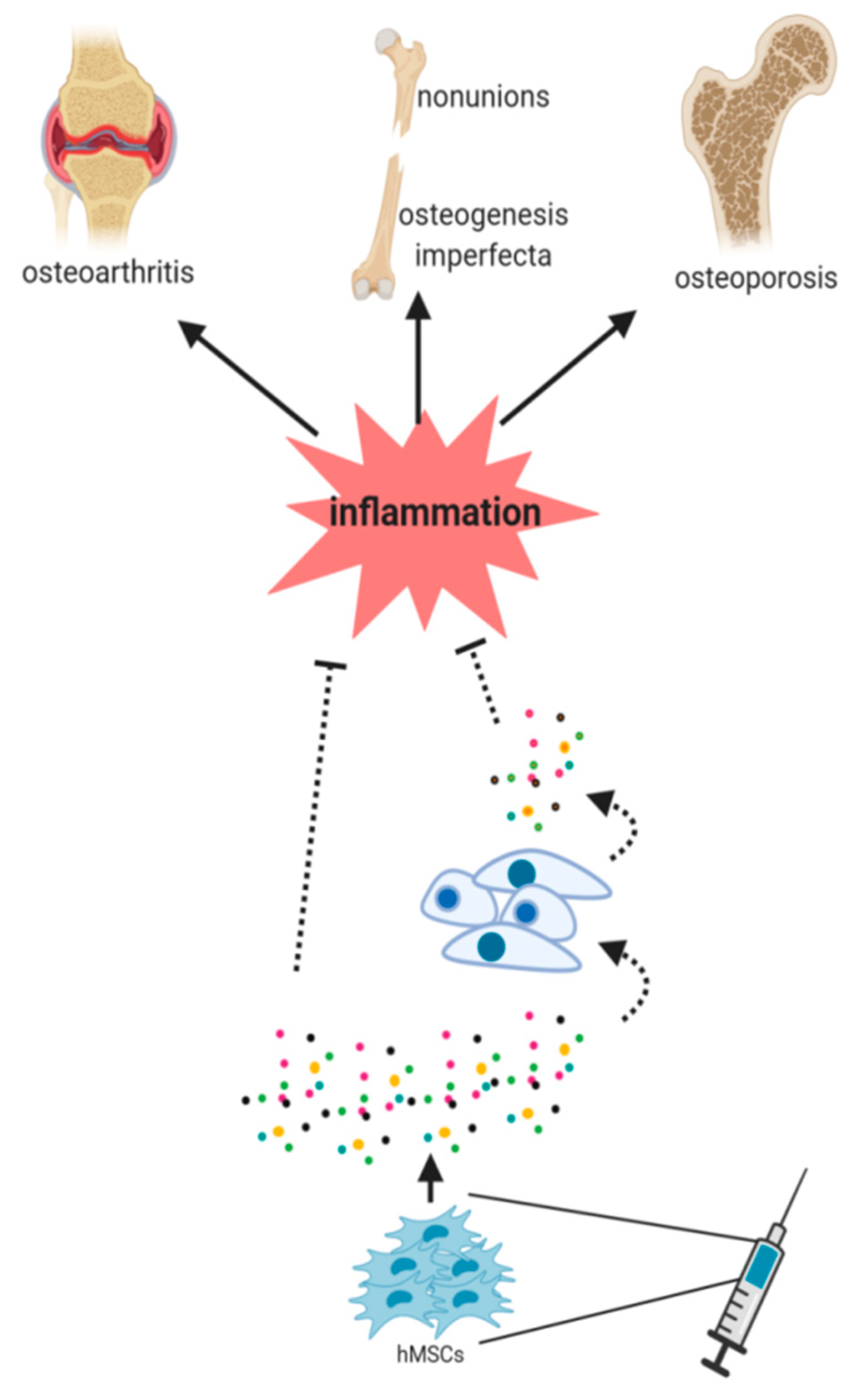
8. Immunomodulation as a Mechanism in MSC-Based Therapies for Bone Diseases
8.1. Nonunion Bone Fractures
8.2. Osteoporosis
8.3. Osteogenesis Imperfecta
8.4. Osteoarthritis
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MSC | Mesenchymal stem cells |
| ECM | Extracellular matrix |
| TNF-α | Tumor necrosis factor alpha |
| IL-1 | Interleukin-1 |
| PD-1 | Programmed death 1 |
| PD-L1 | Programmed cell death 1 ligand 1 |
| PD-L2 | Programmed cell death 1 ligand 2 |
| TGF-β | Transforming growth factor-β |
| Tregs | Regulatory T cells |
| TSG-6 | TNF-α-stimulated gene/protein 6 |
| CXCL8 | C-X-C motif chemokine ligand 8 |
| PMNs | Polymorphonuclear neutrophils |
| CCL2 | Chemokine ligand-2 |
| IL-6 | Interleukin-6 |
| CXCL12 | C-X-C motif chemokine ligand 12 |
| CXCR4 | C-X-C motif chemokine receptor 4 |
| BMP-2 | Bone morphogenetic protein 2 |
| ASCs | Adipose-derived stem cells |
| PSCs | Pericytes |
| IFNγ | Interferon gamma |
| iNOS | Inducible nitric oxide synthase |
| GVHD | Graft-versus-host disease |
| NO | Nitric oxide |
| IL-17A | Interleukin 17A |
| AUF1 | ARE-binding protein ARE/poly(U)-binding/degradation factor 1 |
| NFκB | Nuclear Factor Kappa B |
| TNFR1 | Tumor necrosis factor-alpha receptor 1 |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| COX-2 | Cyclooxygenase-2 |
| PGE2 | Prostaglandin E2 |
| IL-10 | Interleukin 10 |
| LPS | Lipopolysaccharide |
| IDO | Indoleamine 2,3-dioxygenase |
| BM-MSCs | Bone marrow-derived mesenchymal stem cells |
| VEGF | Vascular endothelial growth factor |
| HO-1 | Heme oxygenase-1 |
| EVs | Extracellular vesicles |
| MSC-Des | Mesenchymal stem cell-derived exosomes |
| CM | Conditioned Medium |
| LD | linear dichroism |
| miRNA | Micro RNA |
| OI | Osteogenesis imperfecta |
| C-RP | C-reactive protein |
| ESR | Erytrocyte sedimentation rate |
References
- Schell, H.; Duda, G.N.; Peters, A.; Tsitsilonis, S.; Johnson, K.A.; Schmidt-Bleek, K. The haematoma and its role in bone healing. J. Exp. Orthop. 2017, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Chen, J.; Gautam, S.C.; Zhang, Z.; Lu, M.; Chopp, M. Ischemic cerebral tissue and MCP-1 enhance rat bone marrow stromal cell migration in interface culture. Exp. Hematol. 2002, 30, 831–836. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Li, Z.; Crawford, R.; Xiao, Y.; Zhou, Y. Immunoregulatory role of exosomes derived from differentiating mesenchymal stromal cells on inflammation and osteogenesis. J. Tissue Eng. Regen. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lienau, J.; Schmidt-Bleek, K.; Peters, A.; Weber, H.; Bail, H.J.; Duda, G.N.; Perka, C.; Schell, H. Insight into the molecular pathophysiology of delayed bone healing in a sheep model. Tissue Eng. Part. A 2010, 16, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Mbalaviele, G.; Novack, D.V.; Schett, G.; Teitelbaum, S.L. Inflammatory osteolysis: A conspiracy against bone. J. Clin. Investig. 2017, 127, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Gibon, E.; Lu, L.Y.; Nathan, K.; Goodman, S.B. Inflammation, ageing, and bone regeneration. J. Orthop. Transl. 2017, 10, 28–35. [Google Scholar] [CrossRef]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Du, L. The role of secreted factors in stem cells-mediated immune regulation. Cell. Immunol. 2018, 326, 24–32. [Google Scholar] [CrossRef]
- Augello, A.; Tasso, R.; Negrini, S.M.; Amateis, A.; Indiveri, F.; Cancedda, R.; Pennesi, G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur. J. Immunol. 2005, 35, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, E.; Krulova, M.; Zajicova, A.; Pokorna, K.; Prochazkova, J.; Trosan, P.; Holan, V. The role of mouse mesenchymal stem cells in differentiation of naive T-cells into anti-inflammatory regulatory T-cell or proinflammatory helper T-cell 17 population. Stem Cells Dev. 2012, 21, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Dyer, D.P.; Thomson, J.M.; Hermant, A.; Jowitt, T.A.; Handel, T.M.; Proudfoot, A.E.; Day, A.J.; Milner, C.M. TSG-6 inhibits neutrophil migration via direct interaction with the chemokine CXCL8. J. Immunol. 2014, 192, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef]
- Yellowley, C. CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair. Bonekey Rep. 2013, 2, 300. [Google Scholar] [CrossRef] [Green Version]
- Kidd, L.J.; Stephens, A.S.; Kuliwaba, J.S.; Fazzalari, N.L.; Wu, A.C.; Forwood, M.R. Temporal pattern of gene expression and histology of stress fracture healing. Bone 2010, 46, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Kitaori, T.; Ito, H.; Schwarz, E.M.; Tsutsumi, R.; Yoshitomi, H.; Oishi, S.; Nakano, M.; Fujii, N.; Nagasawa, T.; Nakamura, T. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheumatol. 2009, 60, 813–823. [Google Scholar] [CrossRef] [Green Version]
- Wynn, R.F.; Hart, C.A.; Corradi-Perini, C.; O’Neill, L.; Evans, C.A.; Wraith, J.E.; Fairbairn, L.J.; Bellantuono, I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 2004, 104, 2643–2645. [Google Scholar] [CrossRef]
- Li, X.; Gao, Z.; Wang, J. Single percutaneous injection of stromal cell-derived factor-1 induces bone repair in mouse closed tibial fracture model. Orthopedics 2011, 34, 450. [Google Scholar]
- Granero-Moltó, F.; Weis, J.A.; Miga, M.I.; Landis, B.; Myers, T.J.; O’Rear, L.; Longobardi, L.; Jansen, E.D.; Mortlock, D.P.; Spagnoli, A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar] [CrossRef]
- Marcacci, M.; Kon, E.; Moukhachev, V.; Lavroukov, A.; Kutepov, S.; Quarto, R.; Mastrogiacomo, M.; Cancedda, R. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007, 13, 947–955. [Google Scholar] [CrossRef]
- Zwingenberger, S.; Yao, Z.; Jacobi, A.; Vater, C.; Valladares, R.D.; Li, C.; Nich, C.; Rao, A.J.; Christman, J.E.; Antonios, J.K.; et al. Enhancement of BMP-2 induced bone regeneration by SDF-1α mediated stem cell recruitment. Tissue Eng. Part A 2014, 20, 810–818. [Google Scholar] [CrossRef]
- Gimble, J.M.; Grayson, W.; Guilak, F.; Lopez, M.J.; Vunjak-Novakovic, G. Adipose tissue as a stem cell source for musculoskeletal regeneration. Front. Biosci. 2011, 3, 69–81. [Google Scholar] [CrossRef]
- Sunay, O.; Can, G.; Cakir, Z.; Denek, Z.; Kozanoglu, I.; Erbil, G.; Yilmaz, M.; Baran, Y. Autologous rabbit adipose tissue-derived mesenchymal stromal cells for the treatment of bone injuries with distraction osteogenesis. Cytotherapy 2013, 15, 690–702. [Google Scholar] [CrossRef] [Green Version]
- Hsu, W.K.; Wang, J.C.; Liu, N.Q.; Krenek, L.; Zuk, P.A.; Hedrick, M.H.; Benhaim, P.; Lieberman, J.R. Stem cells from human fat as cellular delivery vehicles in an athymic rat posterolateral spine fusion model. J. Bone Joint Surg. Am. 2008, 90, 1043–1052. [Google Scholar] [CrossRef]
- Traktuev, D.O.; Merfeld-Clauss, S.; Li, J.; Kolonin, M.; Arap, W.; Pasqualini, R.; Johnstone, B.H.; March, K.L. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ. Res. 2008, 102, 77–85. [Google Scholar] [CrossRef]
- James, A.W.; Zara, J.N.; Corselli, M.; Askarinam, A.; Zhou, A.M.; Hourfar, A.; Nguyen, A.; Megerdichian, S.; Asatrian, G.; Pang, S.; et al. An abundant perivascular source of stem cells for bone tissue engineering. Stem Cells Transl. Med. 2012, 1, 673–684. [Google Scholar] [CrossRef]
- Caplan, A.I. All MSCs are pericytes? Cell Stem Cell 2008, 3, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Feng, J.; Seidel, K.; Shi, S.; Klein, O.; Sharpe, P.; Chai, Y. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell 2014, 14, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.G.; James, A.W.; Asatrian, G.; Chang, L.; Nguyen, A.; Le, K.; Bayani, G.; Lee, R.; Stoker, D.; Zhang, X.; et al. Human perivascular stem cell-based bone graft substitute induces rat spinal fusion. Stem Cells Transl. Med. 2014, 3, 1231–1241. [Google Scholar] [CrossRef]
- Supakul, S.; Yao, K.; Ochi, H.; Shimada, T.; Hashimoto, K.; Sunamura, S.; Mabuchi, Y.; Tanaka, M.; Akazawa, C.; Nakamura, T.; et al. Pericytes as a Source of Osteogenic Cells in Bone Fracture Healing. Int. J. Mol. Sci. 2019, 20, 1079. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Jeong, B.C.; Hur, S.W.; Kim, J.W.; Lee, K.B.; Koh, J.T. The Angiopoietin-1 Variant COMP-Ang1 Enhances BMP2-Induced Bone Regeneration with Recruiting Pericytes in Critical Sized Calvarial Defects. PLoS ONE 2015, 10, e0140502. [Google Scholar] [CrossRef]
- Dorronsoro, A.; Ferrin, I.; Salcedo, J.M.; Jakobsson, E.; Fernández-Rueda, J.; Lang, V.; Sepulveda, P.; Fechter, K.; Pennington, D.; Trigueros, C. Human mesenchymal stromal cells modulate T-cell responses through TNF-α-mediated activation of NF-κB. Eur. J. Immunol. 2014, 44, 480–488. [Google Scholar] [CrossRef]
- Krampera, M. Mesenchymal stromal cells: More than inhibitory cells. Leukemia 2011, 25, 565–566. [Google Scholar] [CrossRef]
- Shi, Y.; Su, J.; Roberts, A.I.; Shou, P.; Rabson, A.B.; Ren, G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012, 33, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef]
- Han, X.; Yang, Q.; Lin, L.; Xu, C.; Zheng, C.; Chen, X.; Han, Y.; Li, M.; Cao, W.; Cao, K.; et al. Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death Differ. 2014, 21, 1758–1768. [Google Scholar] [CrossRef]
- Anderson, P. Post-transcriptional regulons coordinate the initiation and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Kronsteiner, B.; Wolbank, S.; Peterbauer, A.; Hackl, C.; Redl, H.; van Griensven, M.; Gabriel, C. Human mesenchymal stem cells from adipose tissue and amnion influence T-cells depending on stimulation method and presence of other immune cells. Stem Cells Dev. 2011, 20, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, Y.; Liu, Y.; Akiyama, K.; Chen, C.; Qu, C.; Jin, Y.; Shi, S. IFN-γ and TNF-α synergistically induce mesenchymal stem cell impairment and tumorigenesis via NFκB signaling. Stem Cells 2013, 31, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Polchert, D.; Sobinsky, J.; Douglas, G.; Kidd, M.; Moadsiri, A.; Reina, E.; Genrich, K.; Mehrotra, S.; Setty, S.; Smith, B.; et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur. J. Immunol. 2008, 38, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Vi, L.; Baht, G.S.; Whetstone, H.; Ng, A.; Wei, Q.; Poon, R.; Mylvaganam, S.; Grynpas, M.; Alman, B.A. Macrophages promote osteoblastic differentiation in-vivo: Implications in fracture repair and bone homeostasis. J. Bone Miner. Res. 2015, 30, 1090–1102. [Google Scholar] [CrossRef] [PubMed]
- Maggini, J.; Mirkin, G.; Bognanni, I.; Holmberg, J.; Piazzón, I.M.; Nepomnaschy, I.; Costa, H.; Cañones, C.; Raiden, S.; Vermeulen, M.; et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS ONE 2010, 5, e9252. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, D.; Zhang, Y.; Li, M. Inflammation, mesenchymal stem cells and bone regeneration. Histochem. Cell Biol. 2018, 149, 393–404. [Google Scholar] [CrossRef]
- Loi, F.; Córdova, L.A.; Zhang, R.; Pajarinen, J.; Lin, T.H.; Goodman, S.B.; Yao, Z. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res. Ther. 2016, 7, 15. [Google Scholar] [CrossRef]
- Huang, S.C.; Everts, B.; Ivanova, Y.; O’Sullivan, D.; Nascimento, M.; Smith, A.M.; Beatty, W.; Love-Gregory, L.; Lam, W.Y.; O’Neill, C.M.; et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 2014, 15, 846–855. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Xue, M.; Chen, H.; Jiao, J.; Herschman, H.R.; O’Keefe, R.J.; Zhang, X. The spatiotemporal role of COX-2 in osteogenic and chondrogenic differentiation of periosteum-derived mesenchymal progenitors in fracture repair. PLoS ONE 2014, 9, e100079. [Google Scholar] [CrossRef]
- Lu, L.Y.; Loi, F.; Nathan, K.; Lin, T.H.; Pajarinen, J.; Gibon, E.; Nabeshima, A.; Cordova, L.; Jämsen, E.; Yao, Z.; et al. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J. Orthop. Res. 2017, 35, 2378–2385. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, E.; van der Poll, T.; DelaRosa, O.; Dalemans, W. Mesenchymal stem cells as a therapeutic tool to treat sepsis. World J. Stem Cells 2015, 7, 368–379. [Google Scholar] [CrossRef]
- Cho, D.I.; Kim, M.R.; Jeong, H.Y.; Jeong, H.C.; Jeong, M.H.; Yoon, S.H.; Kim, Y.S.; Ahn, Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp. Mol. Med. 2014, 46, e70. [Google Scholar] [CrossRef]
- Kim, J.; Hematti, P. Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Exp. Hematol. 2009, 37, 1445–1453. [Google Scholar] [CrossRef]
- François, M.; Romieu-Mourez, R.; Li, M.; Galipeau, J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 2012, 20, 187–195. [Google Scholar] [CrossRef]
- Seebach, E.; Freischmidt, H.; Holschbach, J.; Fellenberg, J.; Richter, W. Mesenchymal stroma cells trigger early attraction of M1 macrophages and endothelial cells into fibrin hydrogels, stimulating long bone healing without long-term engraftment. Acta Biomater. 2014, 10, 4730–4741. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef]
- Alexander, K.A.; Chang, M.K.; Maylin, E.R.; Kohler, T.; Müller, R.; Wu, A.C.; Van Rooijen, N.; Sweet, M.J.; Hume, D.A.; Raggatt, L.J.; et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J. Bone Miner. Res. 2011, 26, 1517–1532. [Google Scholar] [CrossRef]
- Champagne, C.M.; Takebe, J.; Offenbacher, S.; Cooper, L.F. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone 2002, 30, 26–31. [Google Scholar] [CrossRef]
- Guihard, P.; Danger, Y.; Brounais, B.; David, E.; Brion, R.; Delecrin, J.; Richards, C.D.; Chevalier, S.; Rédini, F.; Heymann, D.; et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells 2012, 30, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.K.; Raggatt, L.J.; Alexander, K.A.; Kuliwaba, J.S.; Fazzalari, N.L.; Schroder, K.; Maylin, E.R.; Ripoll, V.M.; Hume, D.A.; Pettit, A.R. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J. Immunol. 2008, 181, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, R.; Fan, W.; Prasadam, I.; Crawford, R.; Xiao, Y. Mesenchymal stromal cells regulate the cell mobility and the immune response during osteogenesis through secretion of vascular endothelial growth factor A. J. Tissue Eng. Regen. Med. 2018, 12, e566–e578. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.; Geissler, S.; Taylor, W.R.; Schmidt-Bleek, K.; Juelke, K.; Schwachmeyer, V.; Dahne, M.; Hartwig, T.; Akyüz, L.; Meisel, C.; et al. Terminally differentiated CD8⁺ T cells negatively affect bone regeneration in humans. Sci. Transl. Med. 2013, 5, 177ra36. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, R.; Shi, S. Systemic infusion of mesenchymal stem cells improves cell-based bone regeneration via upregulation of regulatory T cells. Tissue Eng. Part A 2015, 21, 498–509. [Google Scholar] [CrossRef]
- Linero, I.; Chaparro, O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS ONE 2014, 9, e107001. [Google Scholar] [CrossRef]
- Klyushnenkova, E.; Mosca, J.D.; Zernetkina, V.; Majumdar, M.K.; Beggs, K.J.; Simonetti, D.W.; Deans, R.J.; McIntosh, K.R. T cell responses to allogeneic human mesenchymal stem cells: Immunogenicity, tolerance, and suppression. J. Biomed. Sci. 2005, 12, 47–57. [Google Scholar] [CrossRef]
- Duffy, M.M.; Ritter, T.; Ceredig, R.; Griffin, M.D. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res. Ther. 2011, 2, 34. [Google Scholar] [CrossRef]
- Glennie, S.; Soeiro, I.; Dyson, P.J.; Lam, E.W.; Dazzi, F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005, 105, 2821–2827. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Chen, C.; Wang, D.; Xu, X.; Qu, C.; Yamaza, T.; Cai, T.; Chen, W.; Sun, L.; Shi, S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 2012, 10, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Heldring, N.; Kadri, N.; le Blanc, K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells 2017, 35, 766–776. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Wood, K.J. Mesenchymal stromal cells in transplantation rejection and tolerance. Cold Spring Harb. Perspect. Med. 2013, 3, a015560. [Google Scholar] [CrossRef] [PubMed]
- Melief, S.M.; Schrama, E.; Brugman, M.H.; Tiemessen, M.M.; Hoogduijn, M.J.; Fibbe, W.E.; Roelofs, H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells 2013, 31, 1980–1991. [Google Scholar] [CrossRef]
- Yan, Z.; Zhuansun, Y.; Chen, R.; Li, J.; Ran, P. Immunomodulation of mesenchymal stromal cells on regulatory T cells and its possible mechanism. Exp. Cell Res. 2014, 324, 65–74. [Google Scholar] [CrossRef]
- De Toro, J.; Herschlik, L.; Waldner, C.; Mongini, C. Emerging roles of exosomes in normal and pathological conditions: New insights for diagnosis and therapeutic applications. Front. Immunol. 2015, 6, 203. [Google Scholar] [CrossRef]
- Basu, J.; Ludlow, J.W. Exosomes for repair, regeneration and rejuvenation. Expert Opin. Biol. Ther. 2016, 16, 489–506. [Google Scholar] [CrossRef]
- Fierabracci, A.; Del Fattore, A.; Luciano, R.; Muraca, M.; Teti, A. Recent advances in mesenchymal stem cell immunomodulation: The role of microvesicles. Cell Transplant. 2015, 24, 133–149. [Google Scholar] [CrossRef]
- Hannafon, B.N.; Ding, W.Q. Intercellular communication by exosome-derived microRNAs in cancer. Int. J. Mol. Sci. 2013, 14, 14240–14269. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Miyaki, S.; Ishitobi, H.; Ogura, T.; Kato, Y.; Kamei, N.; Miyado, K.; Higashi, Y.; Ochi, M. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl. Med. 2016, 5, 1620–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.Y.; Yang, H.B.; Hsu, H.S.; Chen, L.L.; Tsai, C.C.; Tsai, K.S.; Yew, T.L.; Kao, Y.H.; Hung, S.C. Mesenchymal stem cell-conditioned medium facilitates angiogenesis and fracture healing in diabetic rats. J. Tissue Eng. Regen. Med. 2012, 6, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yang, F.; Wang, Z.; Fu, Q.; Liang, A. MicroRNA-21 promotes osteogenic differentiation by targeting small mothers against decapentaplegic 7. Mol. Med. Rep. 2015, 12, 1561–1567. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, L.; Huang, S.; Hou, Y.; Liu, Y.; Chan, K.M.; Pan, X.H.; Li, G. mir-21 overexpressing mesenchymal stem cells accelerate fracture healing in a rat closed femur fracture model. BioMed Res. Int. 2015, 2015, 412327. [Google Scholar] [CrossRef]
- Martin, I.; Galipeau, J.; Kessler, C.; Le Blanc, K.; Dazzi, F. Challenges for mesenchymal stromal cell therapies. Sci. Transl. Med. 2019, 11, eaat2189. [Google Scholar] [CrossRef]
- Frölke, J.P.; Patka, P. Definition and classification of fracture non-unions. Injury 2007, 38, S19–S22. [Google Scholar] [CrossRef]
- Mathieu, M.; Rigutto, S.; Ingels, A.; Spruyt, D.; Stricwant, N.; Kharroubi, I.; Albarani, V.; Jayankura, M.; Rasschaert, J.; Bastianelli, E.; et al. Decreased pool of mesenchymal stem cells is associated with altered chemokines serum levels in atrophic nonunion fractures. Bone 2013, 53, 391–398. [Google Scholar] [CrossRef]
- Iwakura, T.; Miwa, M.; Sakai, Y.; Niikura, T.; Lee, S.Y.; Oe, K.; Hasegawa, T.; Kuroda, R.; Fujioka, H.; Doita, M.; et al. Human hypertrophic nonunion tissue contains mesenchymal progenitor cells with multilineage capacity in vitro. J. Orthop. Res. 2009, 27, 208–215. [Google Scholar] [CrossRef]
- Connolly, J.F.; Guse, R.; Tiedeman, J.; Dehne, R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin. Orthop. Relat. Res. 1991, 266, 259–270. [Google Scholar] [CrossRef]
- Garg, N.K.; Gaur, S.; Sharma, S. Percutaneous autogenous bone marrow grafting in 20 cases of ununited fracture. Acta Orthop. Scand. 1993, 64, 671–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, A.; Sangwan, S.S.; Siwach, R.C.; Ali, A.M. Percutaneous bone marrow grafting for the treatment of tibial non-union. Injury 2005, 36, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.D.; Phedy, P.; Kholinne, E.; Djaja, Y.P.; Kusnadi, Y.; Merlina, M.; Yulisa, N.D. Mesenchymal stem cell implantation in atrophic nonunion of the long bones: A translational study. Bone Joint Res. 2016, 5, 287–293. [Google Scholar] [CrossRef]
- O’Keefe, R.J.; Mao, J. Bone tissue engineering and regeneration: From discovery to the clinic--an overview. Tissue Eng. Part B Rev. 2011, 17, 389–392. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Kuiper, J.H.; Roberts, S.; Harrison, P.E.; Cassar-Pullicino, V.N.; Tins, B.; Bajada, S.; Richardson, J.B. Predictors of fracture healing in patients with recalcitrant nonunions treated with autologous culture expanded bone marrow-derived mesenchymal stromal cells. J. Orthop. Res. 2019, 37, 1303–1309. [Google Scholar] [CrossRef]
- Briot, K.; Geusens, P.; Em Bultink, I.; Lems, W.F.; Roux, C. Inflammatory diseases and bone fragility. Osteoporos. Int. 2017, 28, 3301–3314. [Google Scholar] [CrossRef]
- Li, F.; Zhou, C.; Xu, L.; Tao, S.; Zhao, J.; Gu, Q. Effect of Stem Cell Therapy on Bone Mineral Density: A Meta-Analysis of Preclinical Studies in Animal Models of Osteoporosis. PLoS ONE 2016, 11, e0149400. [Google Scholar] [CrossRef]
- Rauch, F.; Glorieux, F.H. Osteogenesis imperfecta. Lancet 2004, 363, 1377–1385. [Google Scholar] [CrossRef]
- Marini, J.C.; Forlino, A.; Bächinger, H.P.; Bishop, N.J.; Byers, P.H.; Paepe, A.; Fassier, F.; Fratzl-Zelman, N.; Kozloff, K.M.; Krakow, D.; et al. Osteogenesis imperfecta. Nat. Rev. Dis. Primers 2017, 3, 17052. [Google Scholar] [CrossRef]
- Kang, H.; Aryal, A.C.S.; Marini, J.C. Osteogenesis imperfecta: New genes reveal novel mechanisms in bone dysplasia. Transl. Res. 2017, 181, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Gordon, P.L.; Koo, W.K.; Marx, J.C.; Neel, M.D.; McNall, R.Y.; Muul, L.; Hofmann, T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc. Natl. Acad. Sci. USA 2002, 99, 8932–8937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuru, S.; Desbourdes, L.; Guess, A.J.; Hofmann, T.J.; Relation, T.; Kaito, T.; Dominici, M.; Iwamoto, M.; Horwitz, E.M. Extracellular vesicles released from mesenchymal stromal cells stimulate bone growth in osteogenesis imperfecta. Cytotherapy 2018, 20, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Götherström, C.; Ringdén, O.; Hassan, M.; McMahon, R.; Horwitz, E.; Anneren, G.; Axelsson, O.; Nunn, J.; Ewald, U.; et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation 2005, 79, 1607–1614. [Google Scholar] [CrossRef]
- Salter, L.; Offiah, A.C.; Bishop, N. Elevated platelet counts in a cohort of children with moderate-severe osteogenesis imperfecta suggest that inflammation is present. Arch. Dis. Child. 2018, 103, 767–771. [Google Scholar] [CrossRef]
- Cao, Y.J.; Wei, Z.; Zhang, H.; Zhang, Z.L. Expanding the Clinical Spectrum of Osteogenesis Imperfecta Type V: 13 Additional Patients and Review. Front. Endocrinol. 2019, 10, 375. [Google Scholar] [CrossRef]
- Rauch, F.; Geng, Y.; Lamplugh, L.; Hekmatnejad, B.; Gaumond, M.H.; Penney, J.; Yamanaka, Y.; Moffatt, P. Crispr-Cas9 engineered osteogenesis imperfecta type V leads to severe skeletal deformities and perinatal lethality in mice. Bone 2018, 107, 131–142. [Google Scholar] [CrossRef]
- O’Brien, K.; Tailor, P.; Leonard, C.; DiFrancesco, L.M.; Hart, D.A.; Matyas, J.R.; Frank, C.B.; Krawetz, R.J. Enumeration and Localization of Mesenchymal Progenitor Cells and Macrophages in Synovium from Normal Individuals and Patients with Pre-Osteoarthritis or Clinically Diagnosed Osteoarthritis. Int. J. Mol. Sci. 2017, 18, 774. [Google Scholar] [CrossRef]
- Vega, A.; Martín-Ferrero, M.A.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef]
- Chahal, J.; Gómez-Aristizábal, A.; Shestopaloff, K.; Bhatt, S.; Chaboureau, A.; Fazio, A.; Chisholm, J.; Weston, A.; Chiovitti, J.; Keating, A.; et al. Bone Marrow Mesenchymal Stromal Cell Treatment in Patients with Osteoarthritis Results in Overall Improvement in Pain and Symptoms and Reduces Synovial Inflammation. Stem Cells Transl. Med. 2019, 8, 746–757. [Google Scholar] [Green Version]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Chullikana, A.; Rengasamy, M.; Shetty, N.; Pandey, V.; Agarwal, V.; Wagh, S.Y.; Vellotare, P.K.; Damodaran, D.; Viswanathan, P.; et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res. Ther. 2016, 18, 301. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medhat, D.; Rodríguez, C.I.; Infante, A. Immunomodulatory Effects of MSCs in Bone Healing. Int. J. Mol. Sci. 2019, 20, 5467. https://doi.org/10.3390/ijms20215467
Medhat D, Rodríguez CI, Infante A. Immunomodulatory Effects of MSCs in Bone Healing. International Journal of Molecular Sciences. 2019; 20(21):5467. https://doi.org/10.3390/ijms20215467
Chicago/Turabian StyleMedhat, Dalia, Clara I. Rodríguez, and Arantza Infante. 2019. "Immunomodulatory Effects of MSCs in Bone Healing" International Journal of Molecular Sciences 20, no. 21: 5467. https://doi.org/10.3390/ijms20215467
APA StyleMedhat, D., Rodríguez, C. I., & Infante, A. (2019). Immunomodulatory Effects of MSCs in Bone Healing. International Journal of Molecular Sciences, 20(21), 5467. https://doi.org/10.3390/ijms20215467




