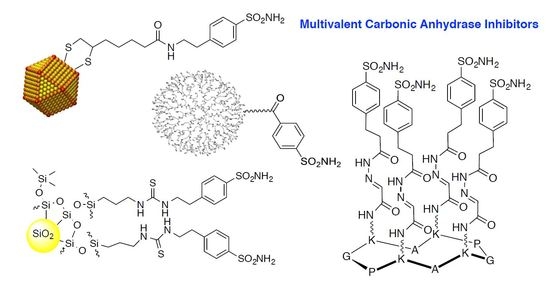
Multivalent Carbonic Anhydrases Inhibitors †
Abstract
:1. Introduction
2. Nanoparticles
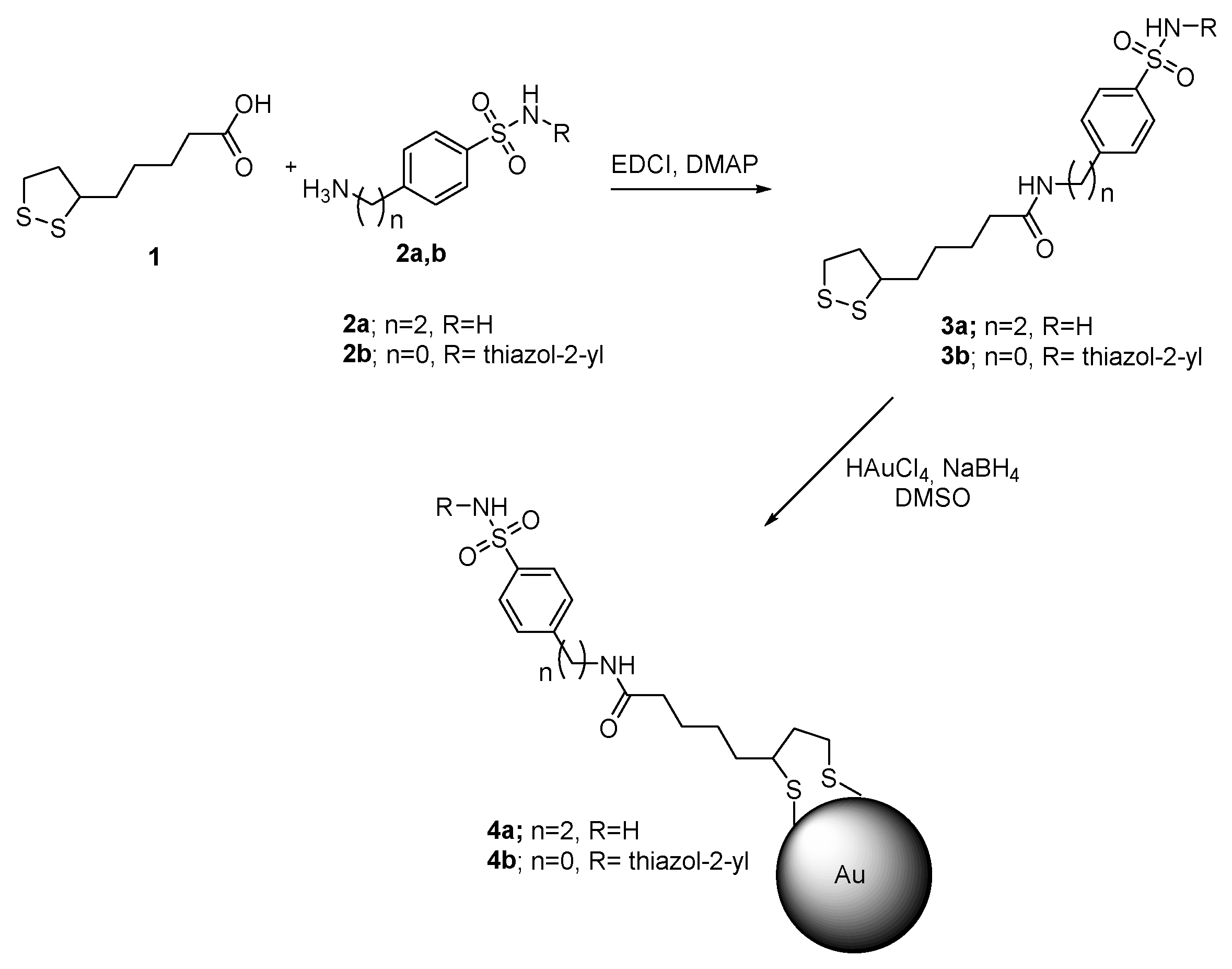
2.1. Gold Nanoparticles and Nanorods
2.2. Silica Nanoparticles
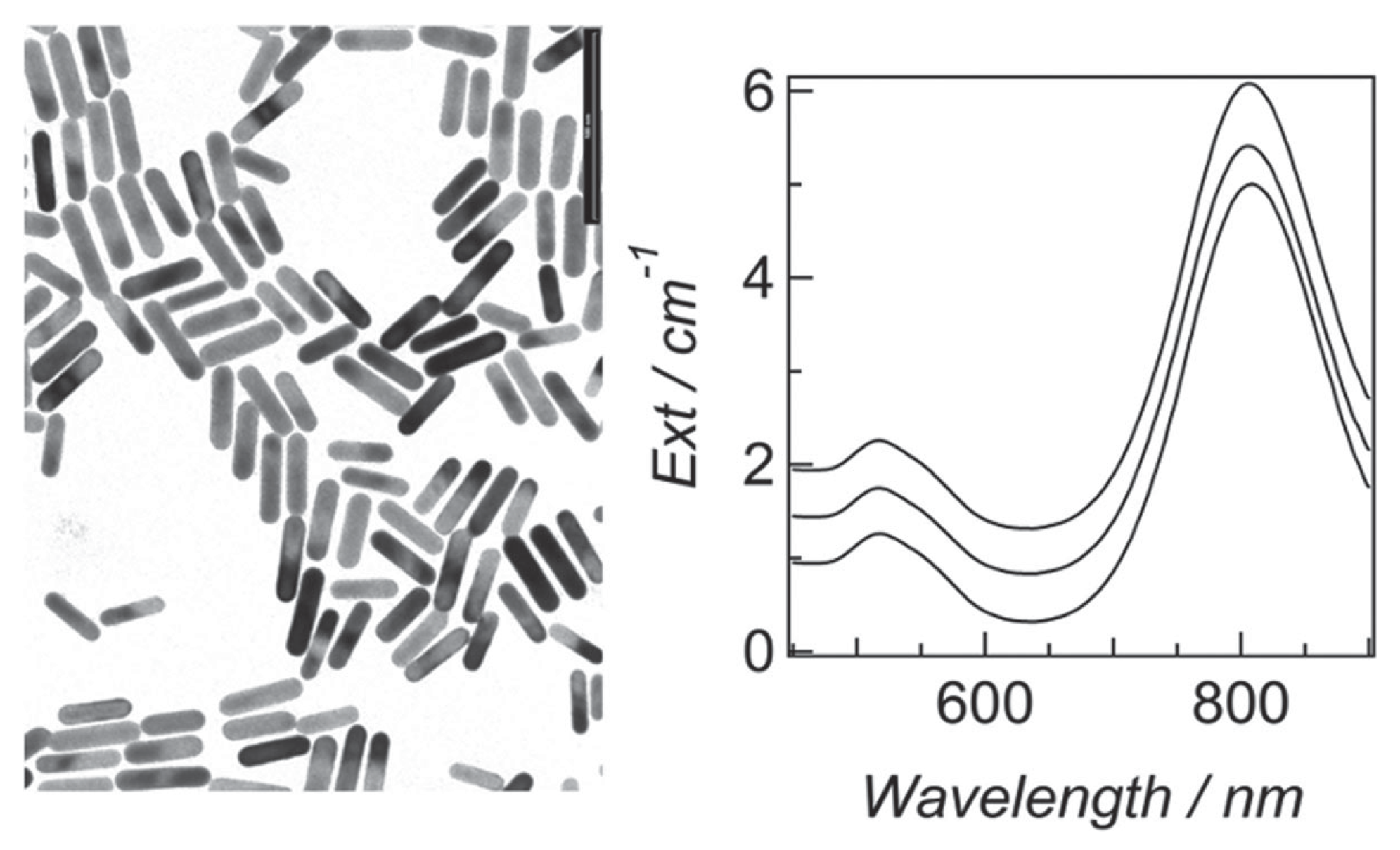
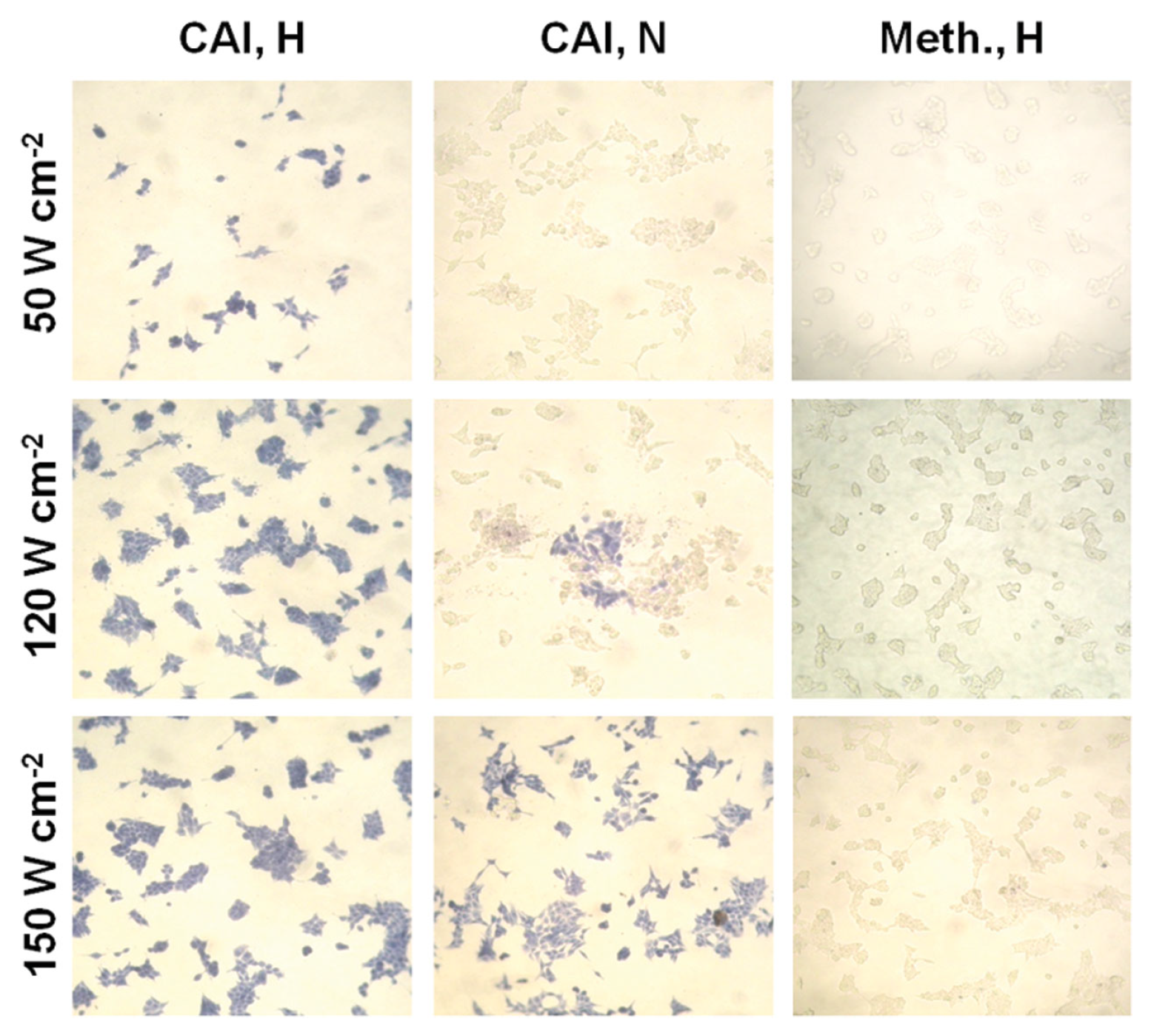
2.3. Gold Nanorods
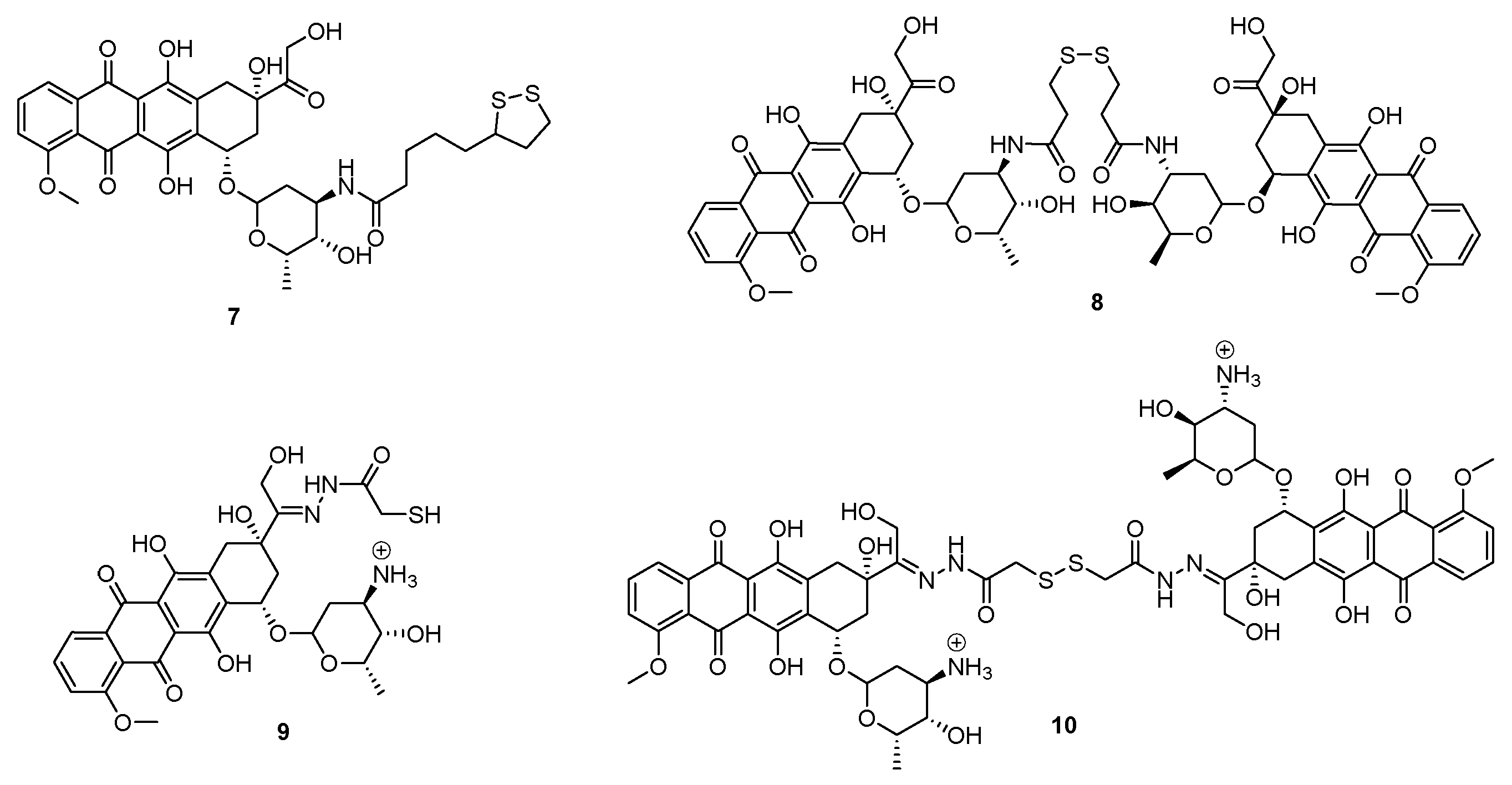
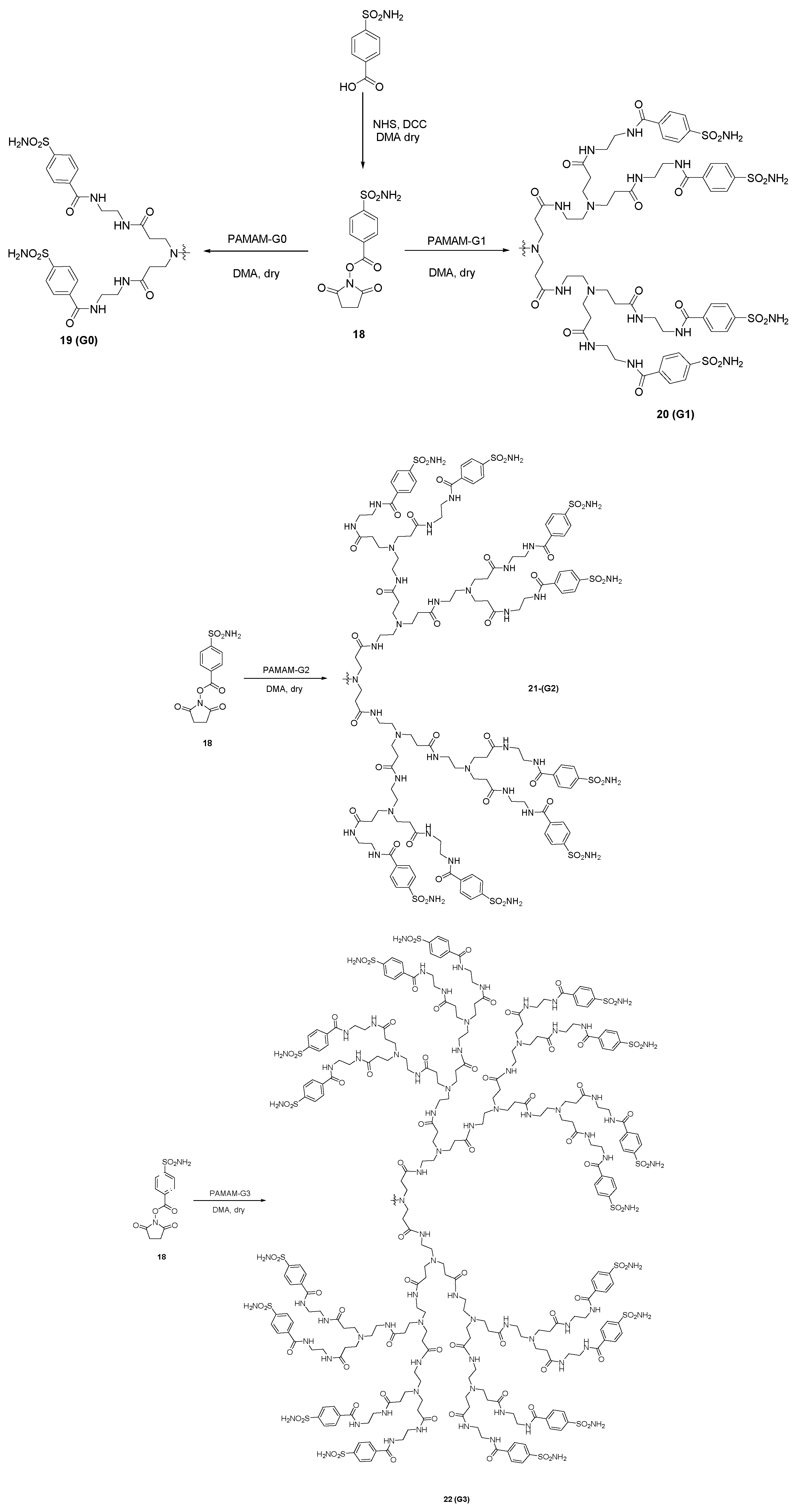
3. Poly(Amidoamine) (PAMAM) Dendrimers
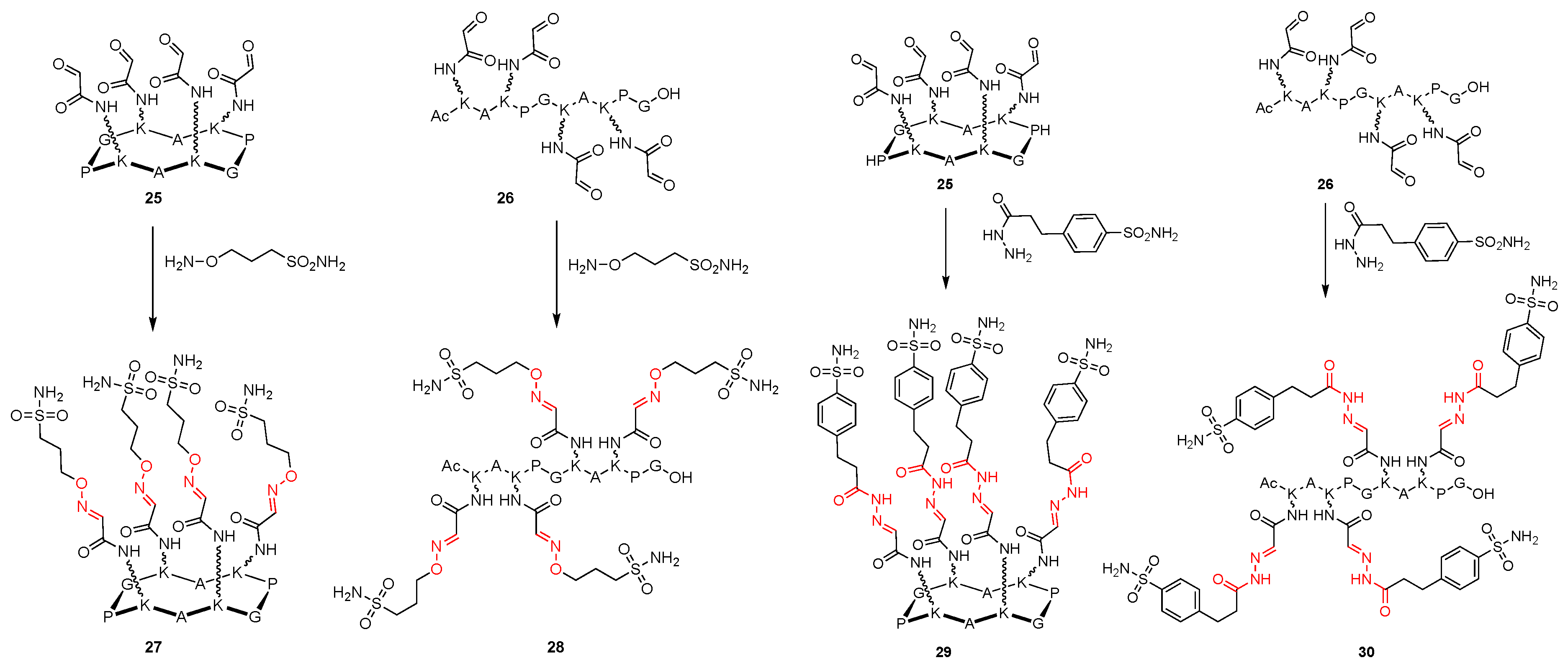
4. Other Platforms (Polyols, Fullerene, Cyclopeptide)
5. Conclusions and Prospects
Funding
Conflicts of Interest
Abbreviations
| hCA | Human carbonic anhydrase |
| CAI | Carbonic anhydrase inhibitor |
References
- Nocentini, A.; Supuran, C.T. Carbonic anhydrases: An overview. In Carbonic Anhydrases, Biochemistry and Pharmacology of en Evergreen Pharmaceutical Target; Elsevier—Academic Press: London, UK, 2019; pp. 3–16. [Google Scholar]
- Kanfar, N.; Bartolami, E.; Zelli, R.; Marra, A.; Winum, J.-Y.; Ulrich, S.; Dumy, P. Emerging trends in enzyme inhibition by multivalent nanoconstructs. Org. Biomol. Chem. 2015, 13, 9894–9906. [Google Scholar] [CrossRef] [PubMed]
- Compain, P. Multivalent Effect in Glycosidase Inhibition: The End of the Beginning. Chem. Rec. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Gouin, S.G. Multivalent inhibitors for carbohydrate-processing enzymes: Beyond the “lock-and-key” concept. Chem. Eur. J. 2014, 20, 11616–11628. [Google Scholar] [CrossRef] [PubMed]
- Matassini, C.; Parmeggiani, C.; Cardona, F.; Goti, A. Are enzymes sensitive to the multivalent effect? Emerging evidence with glycosidases. Tetrahedron Lett. 2016, 57, 5407–5415. [Google Scholar] [CrossRef]
- Raissi, A.J.; Scangarello, F.A.; Hulce, K.R.; Pontrello, J.K.; Paradis, S. Enhanced potency of the metalloprotease inhibitor TAPI-2 by multivalent display. Bioorg. Med. Chem. Lett. 2014, 24, 2002–2007. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Abbate, F.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: X-ray crystallographic structure of the adduct of human isozyme II with a bis-sulfonamide-two heads are better than one? Bioorg. Med. Chem. Lett. 2003, 13, 2759–2763. [Google Scholar] [CrossRef]
- Winum, J.-Y.; Pastorekova, S.; Jakubickova, L.; Montero, J.-L.; Scozzafava, A.; Pastorek, J.; Vullo, D.; Innocenti, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, and IX with bis-sulfamates. Bioorg. Med. Chem. Lett. 2005, 15, 579–584. [Google Scholar] [CrossRef]
- D’Ambrosio, K.; Smaine, F.Z.; Carta, F.; De Simone, G.; Winum, J.-Y.; Supuran, C.T. Development of potent carbonic anhydrase inhibitors incorporating both sulfonamide and sulfamide groups. J. Med. Chem. 2012, 55, 6776–6783. [Google Scholar] [CrossRef]
- Stiti, M.; Cecchi, A.; Rami, M.; Abdaoui, M.; Scozzafava, A.; Guari, Y.; Winum, J.-Y.; Supuran, C.T. Carbonic anhydrase inhibitor coated gold nanoparticles selectively inhibit the tumor-associated isoform IX over the cytosolic isozymes I and II. J. Am. Chem. Soc. 2008, 130, 16130–16131. [Google Scholar] [CrossRef]
- Bellissima, F.; Carta, F.; Innocenti, A.; Scozzafava, A.; Baglioni, P.; Supuran, C.T.; Berti, D. Structural modulation of the biological activity of gold nanoparticles functionalized with a carbonic anhydrase inhibitor. Eur. Phys. J. E Soft Matter 2013, 36, 48. [Google Scholar] [CrossRef]
- Shabana, A.M.; Mondal, U.K.; Alam, M.R.; Spoon, T.; Ross, C.A.; Madesh, M.; Supuran, C.T.; Ilies, M.A. pH-Sensitive Multiligand Gold Nanoplatform Targeting Carbonic Anhydrase IX Enhances the Delivery of Doxorubicin to Hypoxic Tumor Spheroids and Overcomes the Hypoxia-Induced Chemoresistance. ACS Appl. Mater. Interfaces 2018, 10, 17792–17808. [Google Scholar] [CrossRef] [PubMed]
- Touisni, N.; Kanfar, N.; Ulrich, S.; Dumy, P.; Supuran, C.T.; Mehdi, A.; Winum, J.Y. Fluorescent Silica Nanoparticles with Multivalent Inhibitory Effects towards Carbonic Anhydrases. Chem. Eur. J. 2015, 21, 10306–10309. [Google Scholar] [CrossRef] [PubMed]
- Kanfar, N.; Mehdi, A.; Dumy, P.; Ulrich, S.; Winum, J.Y. Polyhedral Oligomeric Silsesquioxane (POSS) Bearing Glyoxylic Aldehyde as Clickable Platform Towards Multivalent Conjugates. Chem. Eur. J. 2017, 23, 17867–17869. [Google Scholar] [CrossRef]
- Kim, C.; Cho, E.C.; Chen, J.Y.; Song, K.H.; Au, L.; Favazza, C.; Zhang, Q.A.; Cobley, C.M.; Gao, F.; Xia, Y.N. In Vivo Molecular Photoacoustic Tomography of Melanomas Targeted by Bioconjugated Gold Nanocages. ACS Nano 2010, 4, 4559–4564. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Thompson, L.B.; Boulos, S.P.; Sisco, P.N.; Murphy, C.J. Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv. Drug Deliv. Rev. 2012, 64, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.H.; Peng, X.H.; Wang, Y.Q.; Wang, Y.X.; Shin, D.M.; El-Sayed, M.A.; Nie, S.M. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano 2010, 4, 5887–5896. [Google Scholar] [CrossRef] [PubMed]
- Ratto, F.; Matteini, P.; Centi, S.; Rossi, F.; Pini, R. Gold nanorods as new nanochromophores for photothermal therapies. J. Biophotonics 2011, 4, 64–73. [Google Scholar] [CrossRef]
- Ratto, F.; Witort, E.; Tatini, F.; Centi, S.; Lazzeri, L.; Carta, F.; Lulli, M.; Vullo, D.; Fusi, F.; Supuran, C.T.; et al. Plasmonic Particles that Hit Hypoxic Cells. Adv. Funct. Mater. 2015, 25, 316–323. [Google Scholar] [CrossRef]
- Newkomem, G.R.; Moorefield, C.N.; Vögtle, F. Dendritic Molecules: Concepts, Syntheses, Perspectives; Wiley-VCH: New York, NY, USA, 1997. [Google Scholar]
- Ratto, F.; Matteini, P.; Rossi, F.; Pini, R. Size and shape control in the overgrowth of gold nanorods. J. Nanopart. Res. 2010, 12, 2029–2036. [Google Scholar] [CrossRef]
- Carta, F.; Osman, S.M.; Vullo, D.; Gullotto, A.; Winum, J.-Y.; AlOthman, Z.; Masini, E.; Supuran, C.T. Poly(amidoamine) Dendrimers with Carbonic Anhydrase Inhibitory Activity and Antiglaucoma Action. J. Med. Chem. 2015, 58, 4039–4045. [Google Scholar] [CrossRef]
- Carta, F.; Osman, S.M.; Vullo, D.; AlOthman, Z.; Supuran, C.T. Dendrimers incorporating benzenesulfonamide moieties strongly inhibit carbonic anhydrase isoforms I-XIV. Org. Biomol. Chem. 2015, 13, 6453–6457. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Osman, S.M.; Vullo, D.; AlOthman, Z.; Del Prete, S.; Capasso, C.; Supuran, C.T. Poly(amidoamine) dendrimers show carbonic anhydrase inhibitory activity against α-, β-, γ- and η-class enzymes. Bioorg. Med. Chem. 2015, 23, 6794–6798. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, T.F.; Brobeck, L. Poly(amidoamine) Dendrimers as Ophthalmic Vehicles for Ocular Delivery of Pilocarpine Nitrate and Tropicamide. J. Control. Release 2005, 102, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Barot, M.; Bagui, M.; Gokulgandhi, M.R.; Mitra, A.K. Prodrug strategies in ocular drug delivery. Med. Chem. 2012, 8, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Abellán-Flos, M.; Tanç, M.; Supuran, C.T.; Vincent, S.P. Multimeric xanthates as carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2016, 31, 946–952. [Google Scholar] [CrossRef]
- Abellán-Flos, M.; Tanç, M.; Supuran, C.T.; Vincent, S.P. Exploring carbonic anhydrase inhibition with multimeric coumarins displayed on a fullerene scaffold. Org. Biomol. Chem. 2015, 13, 7445–7451. [Google Scholar] [CrossRef]
- Supuran, C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin. Drug Discov. 2017, 12, 61–88. [Google Scholar] [CrossRef]
- Kanfar, N.; Tanc, M.; Dumy, P.; Supuran, C.T.; Ulrich, S.; Winum, J.-Y. Effective Access to Multivalent Inhibitors of Carbonic Anhydrases Promoted by Peptide Bioconjugation. Chem. Eur. J. 2017, 23, 6788–6794. [Google Scholar] [CrossRef]
- Nocentini, A.; Supuran, C.T. Advances in the structural annotation of human carbonic anhydrases and impact on future drug discovery. Expert Opin. Drug Discov. 2019, 14, 1175–1197. [Google Scholar] [CrossRef]
- Supuran, C.T. How many carbonic anhydrase inhibition mechanisms exist? J. Enzym. Inhib. Med. Chem. 2016, 31, 345–360. [Google Scholar] [CrossRef]



| Compound | KI (nM) * | ||
|---|---|---|---|
| hCA I | hCA II | hCA IX | |
| 3a | 214 ± 9 | 230 ± 10 | 41 ± 2 |
| 3b | >50000 | 50000 | >50000 |
| 4a | 581 ± 18 (128) | 451 ± 21 (116) | 32 ± 2 (34) |
| 4b | 28550 | 30400 | 31050 |
| Au | 32000 | 31600 | 29560 |
| AAZ | 250 (± 12) | 12 (± 1) | 25 (± 1) |
| Compound | KI (nM) * | |||
|---|---|---|---|---|
| hCA I | hCA II | hCA IX | hCA XII | |
| 18 | 7800 | 640 | 475 | 380 |
| 19 (G0) | 24.1 | 10.4 | 34.7 | 9.3 |
| rp | 323 | 61 | 13 | 40 |
| rp/n | 80.9 | 15.3 | 3.4 | 10.2 |
| 20 (G1) | 12.0 | 3.1 | 20.5 | 1.1 |
| rp | 650 | 206 | 23 | 345 |
| rp/n | 81.2 | 25.8 | 2.8 | 43.1 |
| 21 (G2) | 10.8 | 0.93 | 8.6 | 0.94 |
| rp | 722 | 688 | 55 | 404 |
| rp/n | 45.13 | 43 | 3.5 | 25.3 |
| 22 (G3) | 10.5 | 0.07 | 5.1 | 0.06 |
| rp | 742 | 9142 | 93 | 6333 |
| rp/n | 23.21 | 285.7 | 2.9 | 197.9 |
| AAZ | 250 | 12 | 25 | 5.7 |
| DRZ | 50000 | 9 | 52 | 3.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carta, F.; Dumy, P.; Supuran, C.T.; Winum, J.-Y. Multivalent Carbonic Anhydrases Inhibitors. Int. J. Mol. Sci. 2019, 20, 5352. https://doi.org/10.3390/ijms20215352
Carta F, Dumy P, Supuran CT, Winum J-Y. Multivalent Carbonic Anhydrases Inhibitors. International Journal of Molecular Sciences. 2019; 20(21):5352. https://doi.org/10.3390/ijms20215352
Chicago/Turabian StyleCarta, Fabrizio, Pascal Dumy, Claudiu T. Supuran, and Jean-Yves Winum. 2019. "Multivalent Carbonic Anhydrases Inhibitors" International Journal of Molecular Sciences 20, no. 21: 5352. https://doi.org/10.3390/ijms20215352