Variable Smear Layer and Adhesive Application: The Pursuit of Clinical Relevance in Bond Strength Testing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microtensile Bond Strengths (µTBS)
2.2. Fracture Modes
2.3. Transmission Electron Microscopy (TEM) Observation
2.4. Scanning Electron Microscopy (SEM) Observation
3. Materials and Methods
3.1. Teeth Selection, Preparation, and Bonding Procedures
3.2. µTBS Test
3.3. Fracture Mode Analysis
3.4. TEM of Resin-Dentin Interface
3.5. SEM of Dentin Surfaces Treated with Adhesives and Primer
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| µTBS | Microtensile bond strength |
| SiC | Silicon carbide paper |
| SB | Scotchbond Universal Adhesive |
| A | Adhesive failure |
| CD | Cohesive failure in dentin |
| CC | Cohesive failure in composite-resin |
| M | Mixed failure |
| NA | Non-adhesive failure |
| MB | Clearfil Megabond 2 |
| GP | G-Premio Bond |
| HAp | Hydroxyapatite |
| 10-MDP | 10-methacryloyloxydecyl dihydrogen phosphate |
| HEMA | 2-hydroxyethylmethacrylate |
| 4-META | 4-methacryloxyethyl trimellitic anhydride |
| MDTP | 10-methacryloxydecyl dihydrogen thiophosphate |
| CQ | Camphorquinone |
| Bis-GMA | Bisphenol-A-diglycidyl methacrylate |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| HL | Hybrid layer |
| MD | Mineralized dentin |
References
- Marshall, G.W., Jr.; Marshall, S.J.; Kinney, J.H.; Balooch, M. The dentin substrate: Structure and properties related to bonding. J. Dent. 1997, 25, 441–458. [Google Scholar] [CrossRef]
- Pashley, D.H.; Michelich, V.; Kehl, T. Dentin permeability: Effects of smear layer removal. J. Prosthet. Dent. 1981, 46, 531–537. [Google Scholar] [CrossRef]
- Van Landuyt, K.; De Munck, J.; Coutinho, E.; Peumans, M.; Lambrechts, P.; Van Meerbeek, B. Bonding to Dentin: Smear Layer and the Process of Hybridization. In Dental Hard Tissues and Bonding; Eliades, G., Watts, D.C., Eliades, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 89–122. [Google Scholar]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, F.R.; Pashley, D.H.; Suh, B.I.; Hiraishi, N.; Yiu, C.K.Y. Water treeing in simplified dentin adhesives—déjà vu? Oper. Dent. 2005, 30, 561–579. [Google Scholar] [PubMed]
- Zecin-Deren, A.; Sokolowski, J.; Szczesio-Wlodarczyk, A.; Piwonski, I.; Lukomska-Szymanska, M.; Lapinska, B. Multi-Layer Application of Self-Etch and Universal Adhesives and the Effect on Dentin Bond Strength. Molecules 2019, 24, 345. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, R.; de Leonardi, G.; Rosales-Leal, J.I.; Ceballos, L.; Cabrerizo-Vilchez, M.A. Influence of self-etching primer on the resin adhesion to enamel and dentin. Am. J. Dent. 2001, 14, 205–210. [Google Scholar]
- Perdigao, J.; Geraldeli, S.; Hodges, J.S. Total-etch versus self-etch adhesive: Effect on postoperative sensitivity. J. Am. Dent. Assoc. 2003, 134, 1621–1629. [Google Scholar] [CrossRef]
- Zander-Grande, C.; Amaral, R.C.; Loguercio, A.D.; Barroso, L.P.; Reis, A. Clinical Performance of One-step Self-etch Adhesives Applied Actively in Cervical Lesions: 24-month Clinical Trial. Oper. Dent. 2014, 39, 228–238. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Pucci, C.R.; Luo, T.; Breschi, L.; Pashley, D.H.; Niu, L.; Tay, F.R. No-waiting dentine self-etch concept-Merit or hype. J. Dent. 2017, 62, 54–63. [Google Scholar] [CrossRef]
- Alex, G. Universal adhesives: The next evolution in adhesive dentistry? Compend. Contin. Educ. Dent. 2015, 36, 15–26. [Google Scholar]
- Loguercio, A.D.; De Paula, E.A.; Hass, V.; Luque-Martinez, I.; Reis, A.; Perdigão, J. A new universal simplified adhesive: 36-month randomized double-blind clinical trial. J. Dent. 2015, 43, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.C.; Robles, A.; Fu, C.C.; Lin, C.P.; Sawlani, K.; Burgess, J.O. Two-year clinical trial of a universal adhesive in total-etch and self-etch mode in non-carious cervical lesions. J. Dent. 2015, 43, 1229–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.Y.; Tian, F.C.; Niu, L.N.; Ochala, K.; Chen, C.; Fu, B.P.; Wang, X.Y.; Pashley, D.H.; Tay, F.R. Defying ageing: An expectation for dentine bonding with universal adhesives? J. Dent. 2016, 45, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Tsubota, K.; Takamizawa, T.; Kurokawa, T.H.; Rikuta, A.; Ando, S. Factors affecting the in vitro performance of dentin-bonding systems. Jpn. Dent. Sci. Rev. 2012, 48, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Van Meerbeek, B.; De Munck, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; Van Landuyt, K.; Lambrechts, P.; Vanherle, G. Buonocore memorial lecture. Adhesion to enamel and dentin: Current status and future challenges. Oper. Dent. 2003, 28, 215–235. [Google Scholar]
- Perinka, L.; Sano, H.; Hosoda, H. Dentin thickness, hardness, and Ca-concentration vs bond strength of dentin adhesives. Dent. Mater. 1992, 8, 229–233. [Google Scholar] [CrossRef]
- Watanabe, I.; Nakabayashi, N.; Pashley, D.H. Bonding to ground dentin by a Phenyl-P self-etching primer. J. Dent. Res. 1994, 73, 1212–1220. [Google Scholar] [CrossRef]
- Sano, H.; Shono, T.; Sonoda, H.; Takatsu, T.; Ciucchi, B.; Carvalho, R.; Pashley, D.H. Relationship between surface area for adhesion and tensile bond strength—Evaluation of a micro-tensile bond test. Dent. Mater. 1994, 10, 236–240. [Google Scholar] [CrossRef]
- Perdigão, J.; Swift, E.J., Jr.; Denehy, G.E.; Wefel, J.S.; Donly, K.J. In vitro bond strengths and SEM evaluation of dentin bonding systems to different dentin substrates. J. Dent. Res. 1994, 73, 44–55. [Google Scholar] [CrossRef]
- Burrow, M.F.; Tagami, J.; Negishi, T.; Nikaido, T.; Hosoda, H. Early tensile bond strengths of several enamel and dentin bonding systems. J. Dent. Res. 1994, 73, 522–528. [Google Scholar] [CrossRef]
- Saikaew, P.; Chowdhury, A.F.; Fukuyama, M.; Kakuda, S.; Carvalho, R.M.; Sano, H. The effect of dentin surface preparation and reduced application time of adhesive on bonding strength. J. Dent. 2016, 47, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.S.; Pugach, M.K.; Hilton, J.F.; Watanabe, L.G.; Marshall, S.J.; Marshall, G.W., Jr. The influence of the dentin smear layer on adhesion: A self-etching primer vs. a total-etch system. Dent. Mater. 2003, 19, 758–767. [Google Scholar] [CrossRef]
- Suyama, Y.; Lührs, A.K.; De Munck, J.; Mine, A.; Poitevin, A.; Yamada, T.; Van Meerbeek, B.; Cardoso, M.V. Potential smear layer interference with bonding of self-etching adhesives to dentin. J. Adhes. Dent. 2013, 15, 317–324. [Google Scholar] [PubMed]
- Koibuchi, H.; Yasuda, N.; Nakabayashi, N. Bonding to dentin with a self-etching primer: The effect of smear layers. Dent. Mater. 2001, 17, 122–126. [Google Scholar] [CrossRef]
- Toledano, M.; Proença, J.P.; Erhardt, M.C.; Osorio, E.; Aguilera, F.S.; Osorio, R.; Tay, F.R. Increases in dentin-bond strength if doubling application time of an acetone-containing one-step adhesive. Oper. Dent. 2007, 32, 133–137. [Google Scholar] [CrossRef]
- Erhardt, M.C.; Osorio, R.; Pisani-Proenca, J.; Aguilera, F.S.; Osorio, E.; Breschi, L.; Toledano, M. Effect of double layering and prolonged application time on MTBS of water/ethanol-based self-etch adhesives to dentin. Oper. Dent. 2009, 34, 571–577. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Mine, A.; De Munck, J.; Jaecques, S.; Peumans, M.; Lambrechts, P.; Van Meerbeek, B. Are one-step adhesives easier to use and better performing? Multifactorial assessment of contemporary one-step self-etching adhesives. J. Adhes. Dent. 2009, 11, 175–190. [Google Scholar]
- Taschner, M.; Kümmerling, M.; Lohbauer, U.; Breschi, L.; Petschelt, A.; Frankenberger, R. Effect of Double-layer Application on Dentin Bond Durability of One-step Self-etch Adhesives. Oper. Dent. 2014, 39, 416–426. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, A.F.M.A.; Saikaew, P.; Alam, A.; Sun, J.; Carvalho, R.M.; Sano, H. Effects of Double Application of Contemporary Self-Etch Adhesives on Their Bonding Performance to Dentin with Clinically Relevant Smear Layers. J. Adhes. Dent. 2019, 21, 59–66. [Google Scholar]
- Sattabanasuk, V.; Vachiramon, V.; Qian, F.; Armstrong, S.R. Resin-dentin bond strength as related to different surface preparation methods. J. Dent. 2007, 35, 467–475. [Google Scholar] [CrossRef]
- Eick, J.D.; Wilko, R.A.; Anderson, C.H.; Sorensen, S.E. Scanning electron microscopy of cut tooth surfaces and identification of debris by use of the electron microprobe. J. Dent. Res. 1970, 49, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Takamizawa, T.; Barkmeier, W.W.; Sai, K.; Tsujimoto, A.; Imai, A.; Erickson, R.L.; Latta, M.A.; Miyazaki, M. Influence of different smear layers on bond durability of self-etch adhesives. Dent. Mater. 2018, 34, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Moszner, N.; Salz, U.; Zimmermann, J. Chemical aspects of self-etching enamel-dentin adhesives: A systematic review. Dent. Mater. 2005, 21, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Sano, H.; Yoshida, E.; Hori, H.; Kaga, M.; Oguchi, H.; Pashley, D.H. Effects of Multiple Adhesive Coatings on Dentin Bonding. Oper. Dent. 2004, 29, 416–423. [Google Scholar]
- Wei, S.; Shimada, Y.; Sadr, A.; Tagami, J. Effect of Double-application of Three Single-step Self-etch Adhesives on Dentin Bonding and Mechanical Properties of Resin-dentin Area. Oper. Dent. 2009, 34, 716–724. [Google Scholar] [CrossRef]
- Yoshihara, K.; Yoshida, Y.; Hayakawa, S.; Nagaoka, N.; Irie, M.; Ogawa, T.; Van Landuyt, K.L.; Osaka, A.; Suzuki, K.; Minagi, S.; et al. Nano-Layering of phosphoric-acid ester monomer on enamel and dentin. Acta. Biomater. 2011, 7, 3187–3195. [Google Scholar] [CrossRef]
- Yoshihara, K.; Yoshida, Y.; Nagaoka, N.; Fukegawa, D.; Hayakawa, S.; Mine, A.; Nakamura, M.; Minagi, S.; Osaka, A.; Suzuki, K.; et al. Nano-controlled molecular interaction at adhesive interfaces for hard tissue reconstruction. Acta. Biomater. 2010, 6, 3573–3582. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.I.A.; Kostiuk, L.W.; Kresta, S.M. The Effects of Mixing, Reaction Rates, and Stoichiometry on Yield for Mixing Sensitive Reactions—Part I: Model Development. Int. J. Chem. Eng. 2012, 2012, 750162. [Google Scholar] [CrossRef]
- Mitra, S.B.; Lee, C.Y.; Bui, H.T.; Tantbirojn, D.; Rusin, R.P. Long-term adhesion and mechanism of bonding of a paste-liquid resin-modified glass-ionomer. Dent. Mater. 2009, 25, 459–466. [Google Scholar] [CrossRef]
- Carvalho, R.M.; Mendonca, J.S.; Santiago, S.L.; Silveira, R.R.; Garcia, F.C.; Tay, F.R.; Pashley, D.H. Effects of HEMA/solvent combinations on bond strength to dentin. J. Dent. Res. 2003, 82, 597–601. [Google Scholar] [CrossRef]
- Yiu, C.K.; Pashley, E.L.; Hiraishi, N.; King, N.M.; Goracci, C.; Ferrari, M.; Carvalho, R.M.; Pashley, D.H.; Tay, F.R. Solvent and water retention in dental adhesive blends after evaporation. Biomaterials 2005, 26, 6863–6872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Hosaka, K.; Takahashi, M.; Ikeda, M.; Tian, F.; Komada, W.; Nakajima, M.; Foxton, R.; Nishitani, Y.; Pashley, D.H.; et al. Dentin Bonding Durability of Two-step Self-etch Adhesives with Improved of Degree of Conversion of Adhesive Resins. J. Adhes. Dent. 2017, 19, 31–37. [Google Scholar] [PubMed]
- Nassif, M.; Askary, F.E. Nanotechnology and nanoparticles in contemporary dental adhesives. In Nanobiomaterials in Clinical Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–198. [Google Scholar]
- Armstrong, S.; Breschi, L.; Özcan, M.; Pfefferkorn, F.; Ferrari, M.; Van Meerbeek, B. Academy of Dental Materials guidance on in vitro testing of dental composite bonding effectiveness to dentin/enamel using micro-tensile bond strength (µTBS) approach. Dent. Mater. 2017, 33, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.F.M.A.; Saikaew, P.; Matsumoto, M.; Sano, H.; Carvalho, R.M. Gradual dehydration affects the mechanical properties and bonding outcome of adhesives to dentin. Dent. Mater. J. 2019, 38, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Saikaew, P.; Matsumoto, M.; Chowdhury, A.; Carvalho, R.M.; Sano, H. Does Shortened Application Time Affect Long-Term Bond Strength of Universal Adhesives to Dentin? Oper. Dent. 2018, 43, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, S.; Mezzanzanica, D.; Spreafico, D.; Gagliani, M.; Re, D.; Tanaka, T.; Sidhu, S.K.; Sano, H. Effect of different bur grinding on the bond strength of self-etching adhesives. Oper. Dent. 2006, 31, 317–323. [Google Scholar] [CrossRef]
- Perdigao, J.; Lambrechts, P.; Van Meerbeek, B.; Vanherle, G.; Lopes, A.L. Field emission SEM comparison of four postfixation drying techniques for human dentin. J. Biomed. Mater. Res. 1995, 29, 1111–1120. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Spagnuolo, G.; Bramanti, E.; Laino, L.; Lauritano, F.; Cicciù, M. Interface Between MTA and Dental Bonding Agents: Scanning Electron Microscope Evaluation. J. Int. Soc. Prev. Community Dent. 2017, 7, 64–68. [Google Scholar]
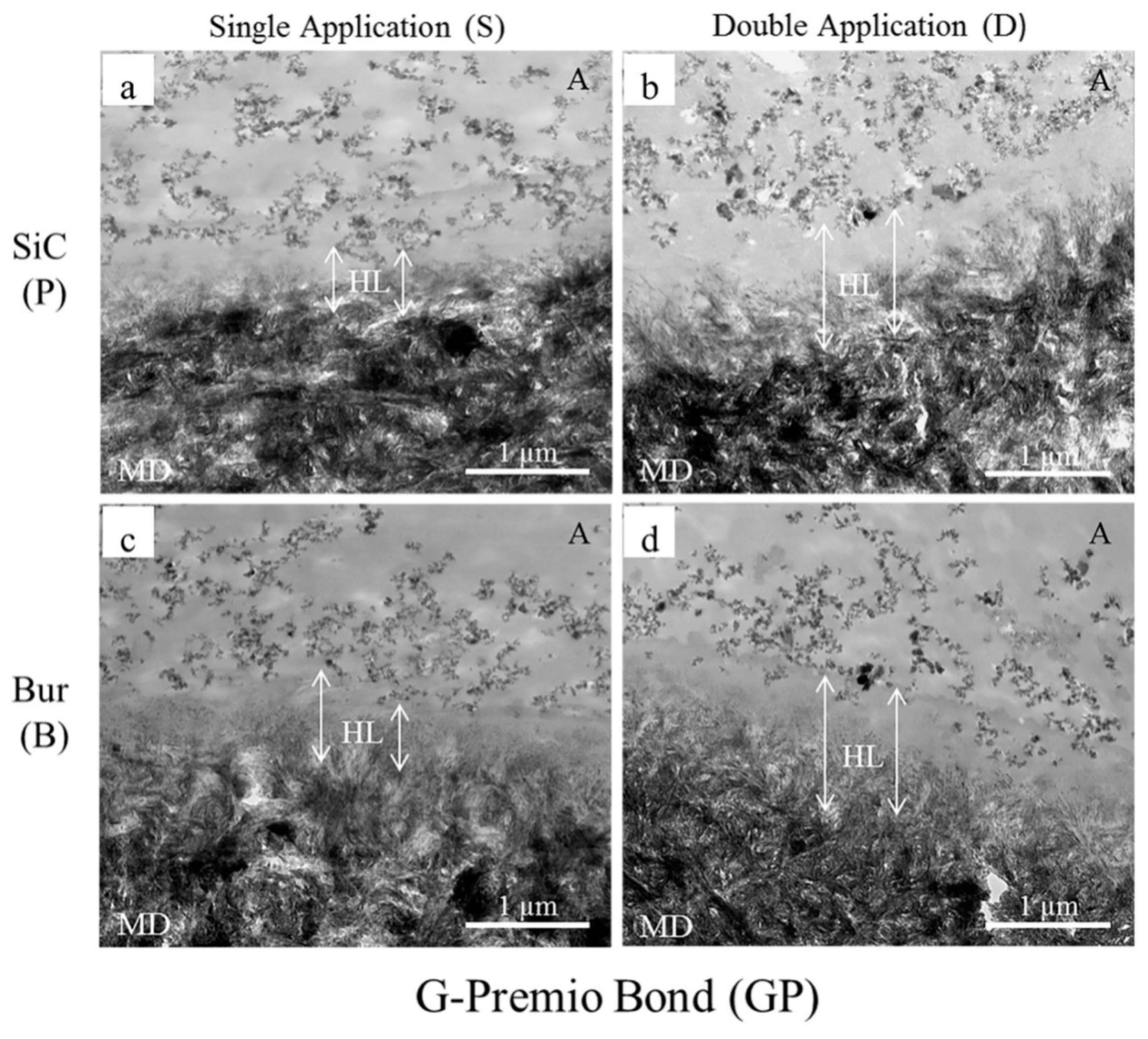

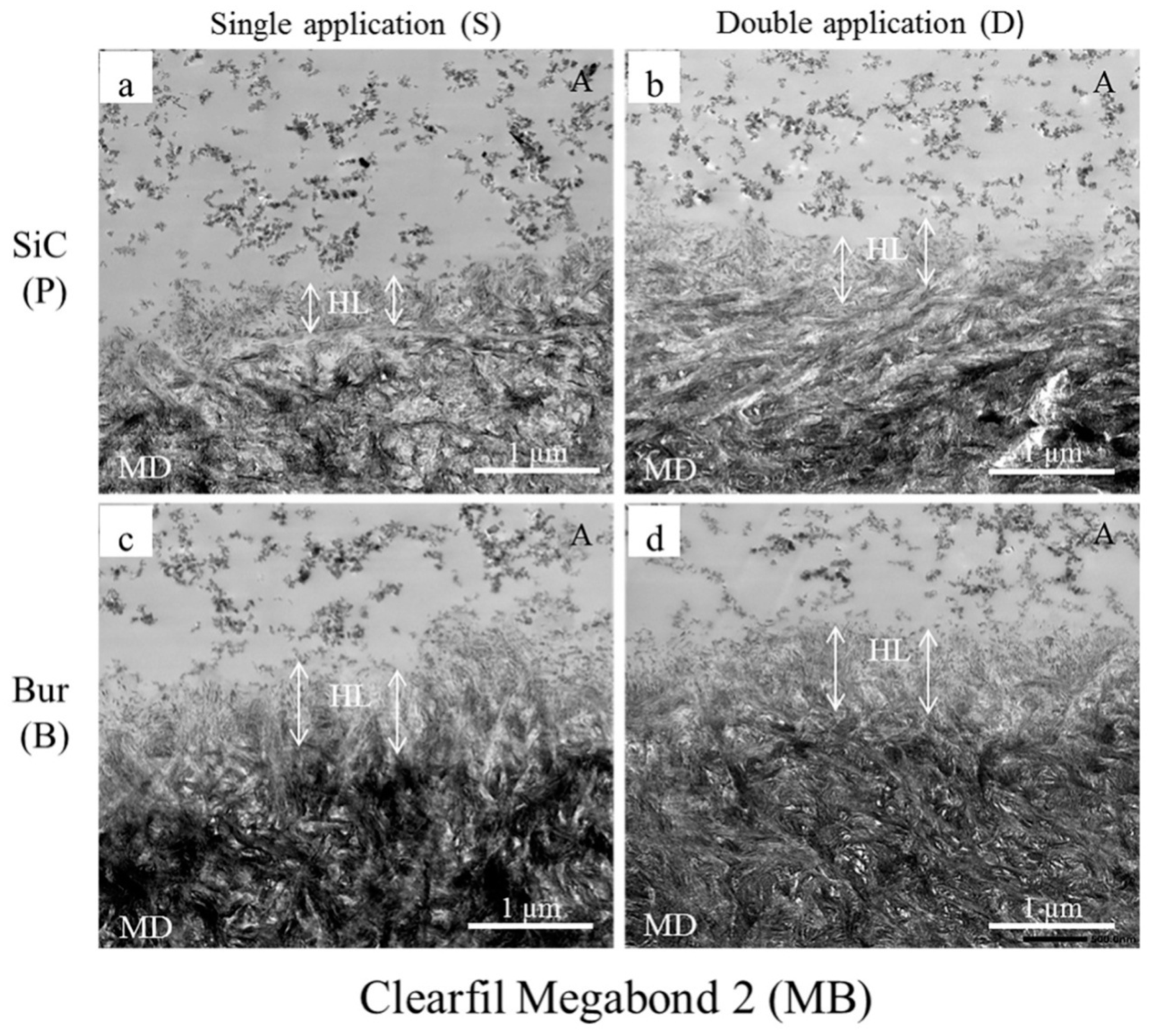

| Adhesives | Smear Layer Created with | Single Application as Per Manufacturers’ Instruction (S) | Double Application with Curing after Second Coat (D) | ||
|---|---|---|---|---|---|
| µTBS ± SD | A/CD/CC/M | µTBS ± SD | A/CD/CC/M | ||
| G-Premio Bond (GP) | 180-grit SiC (P) | 25.7 ± 3.7 a | 95/5/0/0 | 39.8 ± 5.5 b | 75/0/0/25 |
| Fine diamond bur (B) | 34.9 ± 4.4 a,b | 100/0/0/0 | 43.0 ± 2.9 b,c | 100/0/0/0 | |
| Scotchbond Universal Adhesive (SB) | 180-grit SiC (P) | 52.0 ± 4.3 c,d | 40/30/5/25 | 66.4 ± 4.5 e | 30/50/5/15 |
| Fine diamond bur (B) | 51.7 ± 4.5 c,d | 30/40/0/30 | 53.0 ± 4.4 d | 30/20/0/50 | |
| Clearfil Megabond 2 (MB) | 180-grit SiC (P) | 56.1 ± 4.7 d | 20/60/5/15 | 59.3 ± 2.7 d,e | 35/55/0/10 |
| Fine diamond bur (B) | 51.0 ± 4.4 c,d | 25/50/0/25 | 58.2 ± 6.3 d,e | 45/35/0/20 | |
| Adhesives (Batch Number) | pH * | Composition | Single Application as Per Manufacturers’ Instruction (S) | Double Application with Curing after Second Coat (D) |
|---|---|---|---|---|
| Scotchbond Universal Adhesive (649958) | 2.7 | 10-MDP, Vitrebond™ copolymer, HEMA, dimethacrylate resins, filler, silane, initiators, ethanol, water | 1. Apply the adhesive and rub for 20 s. 2. Dry gently for about 5 s until it no longer moves and the solvent evaporates. 3. Light cure for 10 s. | 1. Apply the adhesive and rub for 20 s. Repeat the step. 2. Dry gently for about 5 s until it no longer moves and the solvent evaporates. 3. Light cure for 10 s. |
| G-Premio Bond (1701111) | 1.5 | 10-MDP, 4-META, MDTP, methacrylate acid ester, distilled water, acetone, photo initiators, fine powdered silica | 1. Apply using a microbrush. 2. Leave undisturbed for 10 s. 3. Dry thoroughly with air under maximum air pressure. 4. Light cure for 10 s. | 1. Apply using a microbrush. 2. Leave undisturbed for 10 s. 3. Repeat step 1 and 2. 4. Dry thoroughly with air under maximum air pressure. 5. Light cure for 10 s. |
| Clearfil Megabond 2 (000033) | 2 | Primer: 10-MDP, HEMA, hydrophilic aliphatic dimethacrylate, dl-CQ, water Bond: 10-MDP, Bis-GMA, HEMA, dl-CQ, hydrophobic aliphatic dimethacrylate, initiators, accelerators, silanated colloidal silica | 1. Apply the primer and leave for 20 s. 2. Gentle air-blowing for > 5 s. 3. Apply the bond. 4. Gentle air-blowing to make the film uniform. 5. Light-cure for 10 s. | 1. Apply the primer and leave for 20 s. 2. Repeat step 1. 3. Gentle air-blowing for >5 s. 4. Apply the bond. 5. Gentle air-blowing to make the film uniform. 6. Light-cure for 10 s. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, A.F.M.A.; Islam, R.; Alam, A.; Matsumoto, M.; Yamauti, M.; Carvalho, R.M.; Sano, H. Variable Smear Layer and Adhesive Application: The Pursuit of Clinical Relevance in Bond Strength Testing. Int. J. Mol. Sci. 2019, 20, 5381. https://doi.org/10.3390/ijms20215381
Chowdhury AFMA, Islam R, Alam A, Matsumoto M, Yamauti M, Carvalho RM, Sano H. Variable Smear Layer and Adhesive Application: The Pursuit of Clinical Relevance in Bond Strength Testing. International Journal of Molecular Sciences. 2019; 20(21):5381. https://doi.org/10.3390/ijms20215381
Chicago/Turabian StyleChowdhury, Abu Faem Mohammad Almas, Rafiqul Islam, Arefin Alam, Mariko Matsumoto, Monica Yamauti, Ricardo Marins Carvalho, and Hidehiko Sano. 2019. "Variable Smear Layer and Adhesive Application: The Pursuit of Clinical Relevance in Bond Strength Testing" International Journal of Molecular Sciences 20, no. 21: 5381. https://doi.org/10.3390/ijms20215381
APA StyleChowdhury, A. F. M. A., Islam, R., Alam, A., Matsumoto, M., Yamauti, M., Carvalho, R. M., & Sano, H. (2019). Variable Smear Layer and Adhesive Application: The Pursuit of Clinical Relevance in Bond Strength Testing. International Journal of Molecular Sciences, 20(21), 5381. https://doi.org/10.3390/ijms20215381






