Staphylococcus aureus Nasal Carriage and Autoimmune Diseases: From Pathogenic Mechanisms to Disease Susceptibility and Phenotype
Abstract
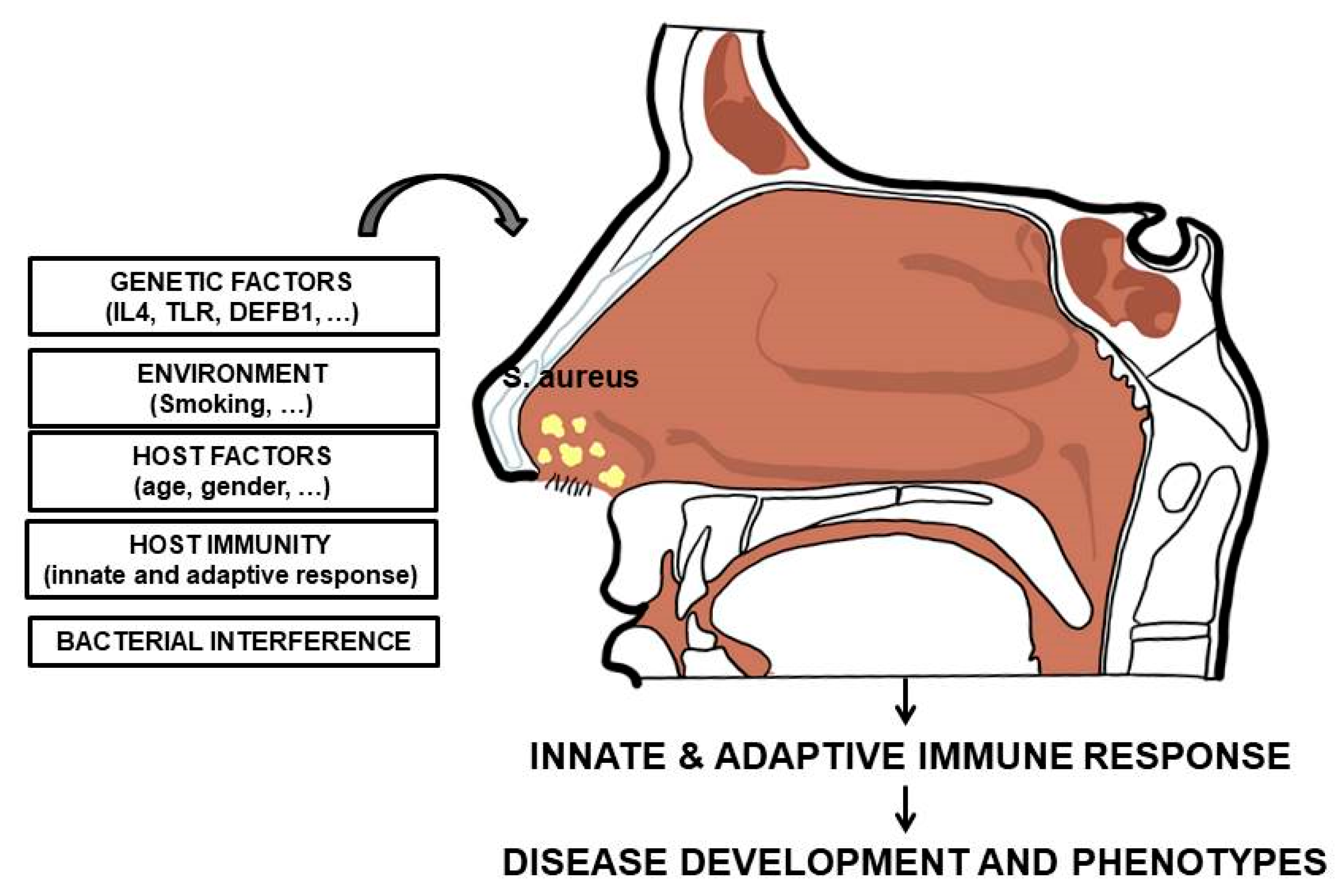
:1. Introduction
2. Staphylococcus aureus: General Aspects
3. Staphylococcus aureus Nasal Carriage: Interplay with the Immune System
4. Staphylococcus aureus and Granulomatosis with Polyangiitis
5. Staphylococcus aureus and Other Autoimmune Diseases
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| Autoimmune diseases | ADs |
| Rheumatoid arthritis | RA |
| Systemic lupus erythematosus | SLE |
| Staphylococcus aureus | S. aureus |
| Toll-like receptor 2 | TL2 |
| Stimulator of IFN Genes | STING |
| Granulomatosis with polyangiitis | GPA |
| Upper respiratory tract | URT |
References
- Ceccarelli, F.; Perricone, C.; Borgiani, P.; Ciccacci, C.; Rufini, S.; Cipriano, E.; Alessandri, C.; Spinelli, F.R.; Sili Scavalli, A.; Novelli, G.; et al. Genetic Factors in Systemic Lupus Erythematosus: Contribution to Disease Phenotype. J. Immunol. Res. 2015, 2015, 745647. [Google Scholar] [CrossRef] [PubMed]
- Perricone, C.; Ciccacci, C.; Ceccarelli, F.; Di Fusco, D.; Spinelli, F.R.; Cipriano, E.; Novelli, G.; Valesini, G.; Conti, F.; Borgiani, P. TRAF3IP2 gene and systemic lupus erythematosus: Association with disease susceptibility and pericarditis development. Immunogenetics 2013, 65, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.F. Infections and autoimmune diseases. J. Autoimmun. 2005, 25, 74–80. [Google Scholar] [CrossRef]
- Conti, F.; Ceccarelli, F.; Massaro, L.; Cipriano, E.; Di Franco, M.; Alessandri, C.; Spinelli, F.R.; Scrivo, R. Biological therapies in rheumatic diseases. Clin. Ther. 2013, 164, e413–e428. [Google Scholar]
- Mastrangelo, A.; Colasanti, T.; Barbati, C.; Pecani, A.; Sabatinelli, D.; Pendolino, M.; Truglia, S.; Massaro, L.; Mancini, R.; Miranda, F.; et al. The Role of Posttranslational Protein Modifications in Rheumatological Diseases: Focus on Rheumatoid Arthritis. J. Immunol. Res. 2015, 2015, 712490. [Google Scholar] [CrossRef] [PubMed]
- Perricone, C.; Ceccarelli, F.; Saccucci, M.; Di Carlo, G.; Bogdanos, D.P.; Lucchetti, R.; Pilloni, A.; Valesini, G.; Polimeni, A.; Conti, F. Porphyromonas gingivalis and rheumatoid arthritis. Curr. Opin. Rheumatol. 2019, 31, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Coit, P.; Sawalha, A.H. The human microbiome in rheumatic autoimmune diseases: A comprehensive review. Clin. Immunol. 2016, 170, 70–79. [Google Scholar] [CrossRef]
- Shin, C.; Kim, Y.K. Autoimmunity in microbiome-mediated diseases and novel therapeutic approaches. Curr. Opin. Pharmacol. 2019, 49, 34–42. [Google Scholar] [CrossRef]
- Brusca, S.B.; Abramson, S.B.; Scher, J.U. Microbiome and mucosal inflammation as extra-articular triggers for rheumatoid arthritis and autoimmunity. Curr. Opin. Rheumatol. 2014, 26, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Katz-Agranov, N.; Zandman-Goddard, G. The microbiome and systemic lupus erythematosus. Immunol. Res. 2017, 65, 432–437. [Google Scholar] [CrossRef]
- Yacoub, R.; Jacob, A.; Wlaschin, J.; McGregor, M.; Quigg, R.J.; Alexander, J.J. Lupus: The microbiome angle. Immunobiology 2018, 223, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.A.; Gallo, R.L. Functions of the skin microbiota in health and disease. Semin. Immunol. 2013, 25, 370–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiller, K.; Selkin, B.A.; Murakawa, G.J. Skin microflora and bacterial infections of the skin. J. Investig. Dermatol. Symp. Proc. 2001, 6, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Puhvel, S.M.; Reisner, R.M.; Amirian, D.A. Quantification of bacteria in isolated pilosebaceous follicles in normal skin. J. Invest. Dermatol. 1975, 65, 525–531. [Google Scholar] [CrossRef]
- Ulrich, R.G. Evolving superantigens of Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 2000, 27, 1–7. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Mehraj, J.; Witte, W.; Akmatov, M.K.; Layer, F.; Werner, G.; Krause, G. Epidemiology of Staphylococcus aureus Nasal Carriage Patterns in the Community. Curr. Top. Microbiol. Immunol. 2016, 398, 55–87. [Google Scholar]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Tam, K.; Torres, V.J. Staphylococcus aureus Secreted Toxins and Extracellular Enzymes. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Kusch, H.; Engelmann, S. Secrets of the secretome in Staphylococcus aureus. Int. J. Med. Microbiol. 2014, 304, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, A.R.; Salgado-Pabón, W.; Kohler, P.L.; Horswill, A.R.; Leung, D.Y.; Schlievert, P.M. Staphylococcal and streptococcal superantigen exotoxins. Clin. Microbiol. Rev. 2013, 26, 422–447. [Google Scholar] [CrossRef] [PubMed]
- Weigel, L.M.; Donlan, R.M.; Shin, D.H.; Jensen, B.; Clark, N.C.; McDougal, L.K.; Zhu, W.; Musser, K.A.; Thompson, J.; Kohlerschmidt, D.; et al. High-level vancomycin-resistant Staphylococcus aureus isolates associated with a polymicrobial biofilm. Antimicrob. Agents Chemother. 2007, 51, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.A.; Moreillon, P. Infective endocarditis. Nat. Rev. Cardiol. 2011, 8, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.L.; Perri, M.B.; Donabedian, S.M.; Manierski, C.; Singh, A.; Vager, D.; Haque, N.Z.; Speirs, K.; Muder, R.R.; Robinson-Dunn, B.; et al. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J. Clin. Microbiol. 2007, 45, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.G.; Perdreau-Remington, F.; Rieg, G.; Mehdi, S.; Perlroth, J.; Bayer, A.S.; Tang, A.W.; Phung, T.O.; Spellberg, B. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 2005, 352, 1445–1453. [Google Scholar] [CrossRef]
- Ruhe, J.J.; Smith, N.; Bradsher, R.W.; Menon, A. Community-onset methicillin-resistant Staphylococcus aureus skin and soft-tissue infections: Impact of antimicrobial therapy on outcome. Clin. Infect. Dis. 2007, 44, 777–784. [Google Scholar] [CrossRef]
- Guidance for Industry. Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment; Center for Drug Evaluation and Research, US Department of Health and Human Services, FDA: Washington, DC, USA, 2013. Available online: http://www.fda.gov/downloads/Drugs/ (accessed on 30 September 2019).
- Sheehy, S.H.; Atkins, B.A.; Bejon, P.; Byren, I.; Wyllie, D.; Athanasou, N.A.; Berendt, A.R.; McNally, M.A. The microbiology of chronic osteomyelitis: Prevalence of resistance to common empirical anti-microbial regimens. J. Infect. 2010, 60, 338–343. [Google Scholar] [CrossRef]
- Torda, A.J.; Gottlieb, T.; Bradbury, R. Pyogenic vertebral osteomyelitis: Analysis of 20 cases and review. Clin. Infect. Dis. 1995, 20, 320–328. [Google Scholar] [CrossRef]
- Mylona, E.; Samarkos, M.; Kakalou, E.; Fanourgiakis, P.; Skoutelis, A. Pyogenic vertebral osteomyelitis: A systematic review of clinical characteristics. Semin. Arthritis Rheum. 2009, 39, 10–17. [Google Scholar] [CrossRef]
- Kaandorp, C.J.; Van Schaardenburg, D.; Krijnen, P.; Habbema, J.D.; van de Laar, M.A. Risk factors for septic arthritis in patients with joint disease. A Prospective Study. Arthritis Rheum. 1995, 38, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- Lora-Tamayo, J.; Murillo, O.; Iribarren, J.A.; Soriano, A.; Sánchez-Somolinos, M.; Baraia-Etxaburu, J.M.; Rico, A.; Palomino, J.; Rodríguez-Pardo, D.; Horcajada, J.P.; et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin. Infect. Dis. 2013, 56, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Shorr, A.; Tabak, Y.P.; Gupta, V.; Liu, L.Z.; Johannes, R.S. Epidemiology and outcomes of health-care-associated pneumonia: Results from a large US database of culture-positive pneumonia. Chest 2005, 128, 3854–3862. [Google Scholar] [CrossRef]
- Lee, M.S.; Walker, V.; Chen, L.F.; Sexton, D.J.; Anderson, D.J. The epidemiology of ventilator-associated pneumonia in a network of community hospitals: A prospective multicenter study. Infect. Control. Hosp. Epidemiol. 2013, 34, 657–662. [Google Scholar] [CrossRef]
- DeRyke, C.A.; Lodise, T.P., Jr.; Rybak, M.J.; McKinnon, P.S. Epidemiology, treatment, and outcomes of nosocomial bacteremic Staphylococcus aureus pneumonia. Chest 2005, 128, 1414–1422. [Google Scholar] [CrossRef]
- Torres, A.; Ferrer, M.; Badia, J.R. Treatment guidelines and outcomes of hospital-acquired and ventilator-associated pneumonia. Clin. Infect. Dis. 2010, 51 (Suppl. 1), S48–S53. [Google Scholar] [CrossRef]
- Laupland, K.B.; Ross, T.; Gregson, D.B. Staphylococcus aureus bloodstream infections: Risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J. Infect. Dis. 2008, 198, 336–343. [Google Scholar] [CrossRef]
- Kaasch, A.J.; Barlow, G.; Edgeworth, J.D.; Fowler, V.G., Jr.; Hellmich, M.; Hopkins, S.; Kern, W.V.; Llewelyn, M.J.; Rieg, S.; Rodriguez-Baño, J.; et al. Staphylococcus aureus bloodstream infection: A pooled analysis of five prospective, observational studies. J. Infect. 2014, 68, 242–251. [Google Scholar] [CrossRef]
- Fowler, V.G., Jr.; Olsen, M.K.; Corey, G.R.; Woods, C.W.; Cabell, C.H.; Reller, L.B.; Cheng, A.C.; Dudley, T.; Oddone, E.Z. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch. Intern. Med. 2003, 163, 2066–2072. [Google Scholar] [CrossRef]
- Teh, B.W.; Slavin, M.A. Staphylococcus aureus meningitis: Barriers to treatment. Leuk. Lymphoma 2012, 53, 1443–1444. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.; Urday-Cornejo, V.; Donabedian, S.; Perri, M.; Tibbetts, R.; Zervos, M. Staphylococcus aureus meningitis: Case series and literature review. Medicine 2010, 89, 117–125. [Google Scholar] [CrossRef]
- Tunkel, A.R.; Hartman, B.J.; Kaplan, S.L.; Kaufman, B.A.; Roos, K.L.; Scheld, W.M.; Whitley, R.J. Practice guidelines for the management of bacterial meningitis. Clin. Infect. Dis. 2004, 39, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O. Spinal epidural abscess. N. Engl. J. Med. 2006, 355, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Reihsaus, E.; Waldbaur, H.; Seeling, W. Spinal epidural abscess: A meta-analysis of 915 patients. Neurosurg. Rev. 2000, 23, 175–204. [Google Scholar] [CrossRef] [PubMed]
- Herzer, C.M. Toxic shock syndrome: Broadening the differential diagnosis. J. Am. Board Fam. Pract. 2001, 14, 131–136. [Google Scholar] [PubMed]
- DeVries, A.S.; Lesher, L.; Schlievert, P.M.; Rogers, T.; Villaume, L.G.; Danila, R.; Lynfield, R. Staphylococcal toxic shock syndrome 2000-2006: Epidemiology, clinical features, and molecular characteristics. PLoS ONE 2011, 6, e22997. [Google Scholar] [CrossRef] [PubMed]
- Lappin, E.; Ferguson, A.J. Gram-positive toxic shock syndromes. Lancet Infect. Dis. 2009, 9, 281–290. [Google Scholar] [CrossRef]
- Fowler, V.G., Jr.; Miro, J.M.; Hoen, B.; Cabell, C.H.; Abrutyn, E.; Rubinstein, E.; Corey, G.R.; Spelman, D.; Bradley, S.F.; Barsic, B.; et al. Staphylococcus aureus endocarditis: A consequence of medical progress. JAMA 2005, 293, 3012–3021. [Google Scholar] [CrossRef]
- Tleyjeh, I.M.; Abdel-Latif, A.; Rahbi, H.; Scott, C.G.; Bailey, K.R.; Steckelberg, J.M.; Wilson, W.R.; Baddour, L.M. A systematic review of population-based studies of infective endocarditis. Chest 2007, 132, 1025–1035. [Google Scholar] [CrossRef]
- Wang, A.; Athan, E.; Pappas, P.A.; Fowler, V.G., Jr.; Olaison, L.; Paré, C.; Almirante, B.; Muñoz, P.; Rizzi, M.; Naber, C.; et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007, 297, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Alter, P.; Waldhans, S.; Plachta, E.; Moosdorf, R.; Grimm, W. Complications of implantable cardioverter defibrillator therapy in 440 consecutive patients. Pacing Clin. Electrophysiol. 2005, 28, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.J.; Afzal, A.; Evonich, R.; Haines, D.E. The prevalence of methicillin resistant organisms among pacemaker and defibrillator implant recipients. Am. J. Cardiovasc. Dis. 2012, 2, 116–122. [Google Scholar] [PubMed]
- Chitnis, A.S.; Edwards, J.R.; Ricks, P.M.; Sievert, D.M.; Fridkin, S.K.; Gould, C.V. Device-associated infection rates, device utilization, and antimicrobial resistance in long-term acute care hospitals reporting to the National Healthcare Safety Network, 2010. Infect. Control. Hosp. Epidemiol. 2012, 33, 993–1000. [Google Scholar] [CrossRef]
- Collignon, P.; Soni, N.; Pearson, I.; Sorrell, T.; Woods, P. Sepsis associated with central vein catheters in critically ill patients. Intensive Care Med. 1988, 14, 227–231. [Google Scholar] [CrossRef]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the prevention of intravascular catheter-related infections. Am. J. Infect. Control 2011, 39, S1–S34. [Google Scholar] [CrossRef] [Green Version]
- Muder, R.R.; Brennen, C.; Rihs, J.D.; Wagener, M.M.; Obman, A.; Stout, J.E.; Yu, V.L. Isolation of Staphylococcus aureus from the urinary tract: Association of isolation with symptomatic urinary tract infection and subsequent staphylococcal bacteremia. Clin. Infect. Dis. 2006, 42, 46–50. [Google Scholar] [CrossRef]
- Hooton, T.M.; Bradley, S.F.; Cardenas, D.D.; Colgan, R.; Geerlings, S.E.; Rice, J.C.; Saint, S.; Schaeffer, A.J.; Tambayh, P.A.; Tenke, P.; et al. Infectious Diseases Society of America. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 625–663. [Google Scholar] [CrossRef]
- Crowley, A.L.; Peterson, G.E.; Benjamin, D.K., Jr.; Rimmer, S.H.; Todd, C.; Cabell, C.H.; Reller, L.B.; Ryan, T.; Corey, G.R.; Fowler, V.G., Jr. Venous thrombosis in patients with short- and long-term central venous catheter-associated Staphylococcus aureus bacteremia. Crit. Care Med. 2008, 36, 385–390. [Google Scholar] [CrossRef]
- Mostafavifar, A.M.; Guilfoose, J.; Sarwari, A.R. Septic pelvic thrombophlebitis due to Staphylococcus aureus. W. Va. Med. J. 2009, 105, 20–22. [Google Scholar]
- Kuehnert, M.J.; Kruszon-Moran, D.; Hill, H.A. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001-2002. J. Infect. Dis. 2006, 193, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Brown, A.F.; Leech, J.M.; Rogers, T.R.; McLoughlin, R.M. Staphylococcus aureus Colonization: Modulation of Host Immune Response and Impact on Human Vaccine Design. Front. Immunol. 2014, 4, 507. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, M.E.; McLoughlin, R.M. Host-Bacterial Crosstalk Determines Staphylococcus aureus Nasal Colonization. Trends Microbiol. 2016, 24, 872–886. [Google Scholar] [CrossRef]
- Sakr, A.; Brégeon, F.; Mège, J.L.; Rolain, J.M.; Blin, O. Staphylococcus aureus Nasal Colonization: An Update on Mechanisms, Epidemiology, Risk Factors, and Subsequent Infections. Front. Microbiol. 2018, 9, 2419. [Google Scholar] [CrossRef]
- Emonts, M.; Uitterlinden, A.G.; Nouwen, J.L.; Kardys, I.; Maat, M.P.; Melles, D.C.; Witteman, J.; Jong, P.T.; Verbrugh, H.A.; Hofman, A.; et al. Host polymorphisms in interleukin 4, complement factor H, and C-reactive protein associated with nasal carriage of Staphylococcus aureus and occurrence of boils. J. Infect. Dis. 2008, 197, 1244–1253. [Google Scholar] [CrossRef]
- Nurjadi, D.; Herrmann, E.; Hinderberger, I.; Zanger, P. Impaired β-defensin expression in human skin links DEFB1 promoter polymorphisms with persistent Staphylococcus aureus nasal carriage. J. Infect. Dis. 2013, 207, 666–674. [Google Scholar] [CrossRef]
- Ruimy, R.; Angebault, C.; Djossou, F.; Dupont, C.; Epelboin, L.; Jarraud, S.; Lefevre, L.A.; Bes, M.; Lixandru, B.E.; Bertine, M.; et al. Are host genetics the predominant determinant of persistent nasal Staphylococcus aureus carriage in humans? J. Infect. Dis. 2010, 202, 924–934. [Google Scholar] [CrossRef]
- Zanger, P.; Nurjadi, D.; Vath, B.; Kremsner, P.G. Persistent nasal carriage of Staphylococcus aureus is associated with deficient induction of human beta-defensin 3 after sterile wounding of healthy skin in vivo. Infect. Immun. 2011, 79, 2658–2662. [Google Scholar] [CrossRef]
- Vuononvirta, J.; Toivonen, L.; Gröndahl-Yli-Hannuksela, K.; Barkoff, A.M.; Lindholm, L.; Mertsola, J.; Peltola, V.; He, Q. Nasopharyngeal bacterial colonization and gene polymorphisms of mannose-binding lectin and toll-like receptors 2 and 4 in infants. PLoS ONE 2011, 6, e26198. [Google Scholar] [CrossRef]
- Van den Akker, E.L.; Nouwen, J.L.; Melles, D.C.; van Rossum, E.F.; Koper, J.W.; Uitterlinden, A.G.; Hofman, A.; Verbrugh, H.A.; Pols, H.A.; Lamberts, S.W.; et al. Staphylococcus aureus nasal carriage is associated with glucocorticoid receptor gene polymorphisms. J. Infect. Dis. 2006, 194, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Messaritakis, I.; Samonis, G.; Dimopoulou, D.; Maraki, S.; Papadakis, J.A.; Daraki, V.; Fragaki, M.; Choulaki, C.; Andrianaki, A.M.; Kofteridis, D.P. Staphylococcus aureus nasal carriage might be associated with vitamin D receptor polymorphisms in type 2 diabetes. Clin. Microbiol. Infect. 2014, 20, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Kotpal, R.; Bhalla, P.; Dewan, R.; Kaur, R. Incidence and Risk Factors of Nasal Carriage of Staphylococcus aureus in HIV-Infected Individuals in Comparison to HIV-Uninfected Individuals: A Case-Control Study. J. Int Assoc. Provid. AIDS Care. 2016, 15, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, S.; Wasmer, S.; Hasler, P.; Vogt, T.; Nogarth, D.; Frei, R.; Widmer, A.F. Staphylococcus aureus in patients with rheumatoid arthritis under conventional and anti-tumor necrosis factor-alpha treatment. J. Rheumatol. 2005, 32, 2125–2129. [Google Scholar] [PubMed]
- Midorikawa, K.; Ouhara, K.; Komatsuzawa, H.; Kawai, T.; Yamada, S.; Fujiwara, T.; Yamazaki, K.; Sayama, K.; Taubman, M.A.; Kurihara, H.; et al. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infect. Immun. 2003, 71, 3730–3739. [Google Scholar] [CrossRef] [PubMed]
- Gröne, A. Keratinocytes and cytokines. Vet. Immunol. Immunopathol. 2002, 88, 1–12. [Google Scholar] [CrossRef]
- Simanski, M.; Rademacher, F.; Schröder, L.; Schumacher, H.M.; Gläser, R.; Harder, J. IL-17A and IFN-γ synergistically induce RNase 7 expression via STAT3 in primary keratinocytes. PLoS ONE. 2013, 8, e59531. [Google Scholar] [CrossRef]
- Kisich, K.O.; Howell, M.D.; Boguniewicz, M.; Heizer, H.R.; Watson, N.U.; Leung, D.Y. The constitutive capacity of human keratinocytes to kill Staphylococcus aureus is dependent on beta-defensin 3. J. Invest. Dermatol. 2007, 127, 2368–2380. [Google Scholar] [CrossRef]
- Zanger, P.; Holzer, J.; Schleucher, R.; Scherbaum, H.; Schittek, B.; Gabrysch, S. Severity of Staphylococcus aureus infection of the skin is associated with inducibility of human beta-defensin 3 but not human beta-defensin 2. Infect. Immun. 2010, 78, 3112–3117. [Google Scholar] [CrossRef]
- Yamasaki, K.; Gallo, R.L. Antimicrobial peptides in human skin disease. Eur. J. Dermatol. 2008, 18, 11–21. [Google Scholar]
- Noore, J.; Noore, A.; Li, B. Cationic antimicrobial peptide LL-37 is effective against both extra- and intracellular Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Spinelli, F.R.; Alessandri, C.; Valesini, G. Toll-like receptors and lupus nephritis. Clin. Rev. Allergy Immunol. 2011, 40, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Prince, A. Staphylococcus aureus induces type I IFN signaling in dendritic cells via TLR9. J. Immunol. 2012, 189, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Parcina, M.; Miranda-Garcia, M.A.; Durlanik, S.; Ziegler, S.; Over, B.; Georg, P.; Foermer, S.; Ammann, S.; Hilmi, D.; Weber, K.J.; et al. Pathogen-triggered activation of plasmacytoid dendritic cells induces IL-10-producing B cells in response to Staphylococcus aureus. J. Immunol. 2013, 190, 1591–1602. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Lu, Y.W.; Wu, S.; Wang, M.R.; Zhu, J.M. Possible Role of Staphylococcal Enterotoxin B in the Pathogenesis of Autoimmune Diseases. Viral Immunol. 2015, 28, 354–359. [Google Scholar] [CrossRef]
- Colque-Navarro, P.; Jacobsson, G.; Andersson, R.; Flock, J.I.; Möllby, R. Levels of antibody against 11 Staphylococcus aureus antigens in a healthy population. Clin. Vaccine Immunol. 2010, 17, 1117–1123. [Google Scholar] [CrossRef]
- Thwaites, G.E.; Gant, V. Are bloodstream leukocytes. Trojan Horses for the metastasis of Staphylococcus aureus? Nat. Rev. Microbiol 2011, 9, 215–222. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Heit, B.; Heinrichs, D.E. Antimicrobial mechanisms of macrophages and the immune evasion strategies of Staphylococcus aureus. Pathogens 2015, 4, 826–868. [Google Scholar] [CrossRef]
- Rollin, G.; Tan, X.; Tros, F.; Dupuis, M.; Nassif, X.; Charbit, A.; Coureuil, M. Intracellular survival of Staphylococcus aureus in endothelial cells: A matter of growth or persistence. Front. Microbiol. 2017, 8, 1354. [Google Scholar] [CrossRef]
- Zeng, Z.; Surewaard, B.G.; Wong, C.H.; Geoghegan, J.A.; Jenne, C.N.; Kubes, P. CRIg functions as a macrophage pattern recognition receptor to directly bind and capture blood-borne gram-positive bacteria. Cell Host Microbe 2016, 20, 99–106. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, S.R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014, 41, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Damania, B. The cGAS-STING Defense Pathway and Its Counteraction by Viruses. Cell Host Microbe 2016, 19, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, R.; Pratt, J.T.; Camilli, A. Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu. Rev. Microbiol. 2007, 61, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Scumpia, P.O.; Botten, G.A.; Norman, J.S.; Kelly-Scumpia, K.M.; Spreafico, R.; Ruccia, A.R.; Purbey, P.K.; Thomas, B.J.; Modlin, R.L.; Smale, S.T. Opposing roles of Toll-like receptor and cytosolic DNA-STING signaling pathways for Staphylococcus aureus cutaneous host defense. PLoS Pathog. 2017, 13, e1006496. [Google Scholar] [CrossRef] [PubMed]
- Swierstra, J.; Debets, S.; de Vogel, C.; Lemmens-den Toom, N.; Verkaik, N.; Ramdani-Bouguessa, N.; Jonkman, M.F.; van Dijl, J.M.; Fahal, A.; van Belkum, A.; et al. IgG4 subclass-specific responses to Staphylococcus aureus antigens shed new light on host-pathogen interaction. Infect. Immun. 2015, 83, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Verkaik, N.J.; de Vogel, C.P.; Boelens, H.A.; Grumann, D.; Hoogenboezem, T.; Vink, C.; Hooijkaas, H.; Foster, T.J.; Verbrugh, H.A.; van Belkum, A.; et al. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J. Infect. Dis. 2009, 199, 625–632. [Google Scholar] [CrossRef]
- Holtfreter, S.; Roschack, K.; Eichler, P.; Eske, K.; Holtfreter, B.; Kohler, C.; Engelmann, S.; Hecker, M.; Greinacher, A.; Broker, B.M. Staphylococcus aureus carriers neutralize superantigens by antibodies specific for their colonizing strain: A potential explanation for their improved prognosis in severe sepsis. J. Infect. Dis. 2006, 193, 1275–1278. [Google Scholar] [CrossRef]
- Laudien, M.; Gadola, S.D.; Podschun, R.; Hedderich, J.; Paulsen, J.; Reinhold-Keller, E.; Csernok, E.; Ambrosch, P.; Hellmich, B.; Moosig, F.; et al. Nasal carriage of Staphylococcus aureus and endonasal activity in Wegener s granulomatosis as compared to rheumatoid arthritis and chronic Rhinosinusitis with nasal polyps. Clin. Exp. Rheumatol. 2010, 28 (Suppl. 57), 51–55. [Google Scholar]
- Popa, E.R.; Stegeman, C.A.; Kallenberg, C.G.; Tervaert, J.W. Staphylococcus aureus and Wegener’s granulomatosis. Arthritis Res. 2002, 4, 77–79. [Google Scholar] [CrossRef]
- Popa, E.R.; Tervaert, J.W. The relation between Staphylococcus aureus and Wegener’s granulomatosis: Current knowledge and future directions. Intern. Med. 2003, 42, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Zycinska, K.; Wardyn, K.A.; Zielonka, T.M.; Demkow, U.; Traburzynski, M.S. Chronic crusting, nasal carriage of Staphylococcus aureus and relapse rate in pulmonary Wegener’s granulomatosis. J. Physiol. Pharmacol. 2008, 59 (Suppl. 6), 825–831. [Google Scholar] [PubMed]
- Styers, D.; Sheehan, D.J.; Hogan, P.; Sahm, D.F. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann. Clin. Microbiol. Antimicrob. 2006, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Stegeman, C.A.; Tervaert, J.W.; Sluiter, W.J.; Manson, W.L.; de Jong, P.E.; Kallenberg, C.G. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann. Intern. Med. 1994, 120, 12–17. [Google Scholar] [CrossRef]
- Salmela, A.; Rasmussen, N.; Tervaert, J.W.C.; Jayne, D.R.W.; Ekstrand, A.; European Vasculitis Study Group. Chronic nasal Staphylococcus aureus carriage identifies a subset of newly diagnosed granulomatosis with polyangiitis patients with high relapse rate. Rheumatology 2017, 56, 965–972. [Google Scholar] [CrossRef]
- Kallenberg, C.G.; Stegeman, C.A.; Tervaert, J.W. Staphylococcus aureus, trimethoprim-sulfamethoxazole, and Wegener’s granulomatosis. Sarcoidosis Vasc. Diffus. Lung Dis. 1996, 13, 253–255. [Google Scholar]
- Rhee, R.L.; Sreih, A.G.; Najem, C.E.; Grayson, P.C.; Zhao, C.; Bittinger, K.; Collman, R.G.; Merkel, P.A. Characterisation of the nasal microbiota in granulomatosis with polyangiitis. Ann. Rheum. Dis. 2018, 77, 1448–1453. [Google Scholar] [CrossRef]
- Lamprecht, P.; Fischer, N.; Huang, J.; Burkhardt, L.; Lütgehetmann, M.; Arndt, F.; Rolfs, I.; Kerstein, A.; Iking-Konert, C.; Laudien, M. Changes in the composition of the upper respiratory tract microbial community in granulomatosis with polyangiitis. J. Autoimmun. 2019, 97, 29–39. [Google Scholar] [CrossRef]
- Weppner, G.; Ohlei, O.; Hammers, C.M.; Holl-Ulrich, K.; Voswinkel, J.; Bischof, J.; Hasselbacher, K.; Riemekasten, G.; Lamprecht, P.; Ibrahim, S.; et al. In situ detection of PR3-ANCA+ B cells and alterations in the variable region of immunoglobulin genes support a role of inflamed tissue in the emergence of auto-reactivity in granulomatosis with polyangiitis. J. Autoimmun. 2018, 93, 89–103. [Google Scholar] [CrossRef]
- Pitzalis, C.; Jones, G.W.; Bombardieri, M.; Jones, S.A. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol. 2014, 14, 447–462. [Google Scholar] [CrossRef]
- Hui, Y.; Wohlers, J.; Podschun, R.; Hedderich, J.; Lamprecht, P.; Ambrosch, P.; Laudien, M. Antimicrobial peptides in nasal secretion and mucosa with respect to S. aureus colonisation in Wegener’s granulomatosis. Clin. Exp. Rheumatol. 2011, 29 (Suppl. 64), S49–S56. [Google Scholar] [PubMed]
- Cohen, M.A.; Huband, M.D. Activity of clinafloxacin, trovafloxacin, quinupristin/dalfopristin, and other antimicrobial agents versus Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Diagn. Microbiol. Infect. Dis. 1999, 33, 43–46. [Google Scholar] [CrossRef]
- Xu, H.; Tian, R.; Li, Y.; Chen, D.; Liu, Y.; Hu, Y.; Xiao, F. Ribosomal protein L3 mutations are associated with cfr-mediated linezolid resistance in clinical isolates of Staphylococcus cohnii. Curr. Microbiol. 2015, 70, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.R.; Stegeman, C.A.; Abdulahad, W.H.; van der Meer, B.; Arends, J.; Manson, W.M.; Bos, N.A.; Kallenberg, C.G.; Tervaert, J.W. Staphylococcal toxic-shock-syndrome-toxin-1 as a risk factor for disease relapse in Wegener’s granulomatosis. Rheumatology 2007, 46, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Fijołek, J.; Wiatr, E.; Petroniec, V.; Augustynowicz-Kopec, E.; Bednarek, M.; Gawryluk, D.; Martusewicz-Boros, M.M.; Modrzewska, K.; Radzikowska, E.; Roszkowski-Sliz, K. The presence of staphylococcal superantigens in nasal swabs and correlation with activity of granulomatosis with polyangiitis in own material. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 111), 40–45. [Google Scholar] [PubMed]
- Laudien, M.; Häsler, R.; Wohlers, J.; Böck, J.; Lipinski, S.; Bremer, L.; Podschun, R.; Ambrosch, P.; Lamprecht, P.; Rosenstiel, P.; et al. Molecular signatures of a disturbed nasal barrier function in the primary tissue of Wegener’s granulomatosis. Mucosal Immunol. 2011, 4, 564–573. [Google Scholar] [CrossRef]
- Wohlers, J.; Breucker, K.; Podschun, R.; Hedderich, J.; Lamprecht, P.; Ambrosch, P.; Laudien, M. Aberrant cytokine pattern of the nasal mucosa in granulomatosis with polyangiitis. Arthritis Res. Ther. 2012, 14, R203. [Google Scholar] [CrossRef]
- Glasner, C.; de Goffau, M.C.; van Timmeren, M.M.; Schulze, M.L.; Jansen, B.; Tavakol, M.; van Wamel, W.J.B.; Stegeman, C.A.; Kallenberg, C.G.M.; Arends, J.P.; et al. Genetic loci of Staphylococcus aureus associated with anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitides. Sci. Rep. 2017, 7, 12211. [Google Scholar] [CrossRef]
- Atzeni, F.; Masala, I.F.; di Franco, M.; Sarzi-Puttini, P. Infections in rheumatoid arthritis. Curr. Opin. Rheumatol. 2017, 29, 323–3304. [Google Scholar] [CrossRef]
- Dixon, W.G.; Watson, K.; Lunt, M.; Hyrich, K.L.; Silman, A.J.; Symmons, D.P.; British Society for Rheumatology Biologics Register. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: Results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006, 54, 2368–2376. [Google Scholar]
- Winthrop, K.L. Risk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factor. Nat. Clin. Pract. Rheumatol. 2006, 2, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Vaudaux, P.; Grau, G.E.; Huggler, E.; Schumacher-Perdreau, F.; Fiedler, F.; Waldvogel, F.A.; Lew, D.P. Contribution of tumor necrosis factor to host defense against staphylococci in a guinea pig model of foreign body infections. J. Infect. Dis. 1992, 166, 58. [Google Scholar] [CrossRef] [PubMed]
- Tabarya, D.; Hoffman, W.L. Staphylococcus aureus nasal carriage in rheumatoid arthritis: Antibody response to toxic shock syndrome toxin-1. Ann. Rheum. Dis. 1996, 55, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Varley, C.D.; Deodhar, A.A.; Ehst, B.D.; Bakke, A.; Blauvelt, A.; Vega, R.; Yamashita, S.; Winthrop, K.L. Persistence of Staphylococcus aureus colonization among individuals with immune-mediated inflammatory diseases treated with TNF-α inhibitor therapy. Rheumatology 2014, 53, 332–337. [Google Scholar] [CrossRef]
- Goodman, S.M.; Nocon, A.A.; Selemon, N.A.; Shopsin, B.; Fulmer, Y.; Decker, M.E.; Grond, S.E.; Donlin, L.T.; Figgie, M.P.; Sculco, T.P.; et al. Increased Staphylococcus aureus Nasal Carriage Rates in Rheumatoid Arthritis Patients on Biologic Therapy. J. Arthroplast. 2019, 34, 954–958. [Google Scholar] [CrossRef]
- Ataee, R.A.; Ataee, M.H.; Alishiri, G.H.; Esmaeili, D. Staphylococcal enterotoxin C in synovial fluid of patients with rheumatoid arthritis. Iran. Red. Crescent Med. J. 2014, 16, e16075. [Google Scholar] [CrossRef]
- Ataee, R.A.; Kashefi, R.; Alishiri, G.H.; Esmaieli, D. Assay of Blood and Synovial Fluid of Patients with Rheumatoid Arthritis for Staphylococcus aureus Enterotoxin D: Absence of Bacteria but Presence of Its Toxin. Jundishapur J. Microbiol. 2015, 8, e28395. [Google Scholar] [CrossRef]
- Lisnevskaia, L.; Murphy, G.; Isenberg, D. Systemic lupus erythematosus. Lancet 2014, 384, 1878–1888. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L. EBV and Autoimmunity. Curr. Top. Microbiol. Immunol. 2015, 390, 365–385. [Google Scholar]
- Rigante, D.; Mazzoni, M.B.; Esposito, S. The cryptic interplay between systemic lupus erythematosus and infections. Autoimmun. Rev. 2014, 13, 96–102. [Google Scholar] [CrossRef]
- Esposito, S.; Bosis, S.; Semino, M.; Rigante, D. Infections and systemic lupus erythematosus. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, V.R.; Tilahun, A.Y.; Clark, C.R.; Grande, J.P.; Rajagopalan, G. Chronic exposure to staphylococcal superantigen elicits a systemic inflammatory disease mimicking lupus. J. Immunol. 2012, 189, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Ceccarelli, F.; Iaiani, G.; Perricone, C.; Giordano, A.; Amori, L.; Miranda, F.; Massaro, L.; Pacucci, V.A.; Truglia, S.; et al. Association between Staphylococcus aureus nasal carriage and disease phenotype in patients affected by systemic lupus erythematosus. Arthritis Res. Ther. 2016, 18, 177. [Google Scholar] [CrossRef] [PubMed]
- Spaan, A.N.; Surewaard, B.G.; Nijland, R.; van Strijp, J.A. Neutrophils versus Staphylococcus aureus: A biological tug of war. Annu. Rev. Microbiol. 2013, 67, 629–650. [Google Scholar] [CrossRef]

| Resource | |
|---|---|
| Surface Structures [17] |
|
| Exotoxins [20,21] |
|
| Exoenzymes [20] |
|
| Infections | Details |
|---|---|
| Skin and Soft Tissues Infections [24,25,26,27,28] | From benign to life threating conditions (impetigo, cellulitis, surgical sites infections, cutaneous abscesses, purulent cellulitis) |
| Osteoarticular Infections [29,30,31,32,33,34] | Osteomyelitis, septic arthritis, prosthetic joint infections |
| Pleuropulmonary Infections [35,36,37,38] | Predominant role in hospital-acquired pneumonias in comparison to community-acquired ones. |
| Bacteremia [39,40,41] | Direct evolution of a local infection |
| Meningitis [42,43,44] | Complication of a primary non-central nervous system infection |
| Epidural Abscesses [45,46] | Rare intracranial or spinal condition, recognized in USA as second most common infection due to malpractice |
| Toxic shock Syndrome [47,48,49] | Sustained by super antigen-mediated process, linked to toxic shock syndrome toxin-1, able to activate T-cells, with massive cytokine release |
| Infective Endocarditis [50,51,52] | Observed in a proportion of S. aureus infected patients ranging from 16% to 34% |
| Cardiac Devices Infections [53,54] | Directly occurring during implantation or indirectly via haematogenous seeding from a distant source |
| Intravascular Catheter Infections [55,56,57] | Potentially leading to a bacterial spreading in the bloodstream, configuring the so-called central line-associated blood stream infection (mortality rate 7–21%) |
| Urinary Tract Infections [58,59] | Frequent in case of indwelling urinary catheter |
| Septic Thrombophlebitis [60,61] | Reported in up to 8% of all patients with bacteriema |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccarelli, F.; Perricone, C.; Olivieri, G.; Cipriano, E.; Spinelli, F.R.; Valesini, G.; Conti, F. Staphylococcus aureus Nasal Carriage and Autoimmune Diseases: From Pathogenic Mechanisms to Disease Susceptibility and Phenotype. Int. J. Mol. Sci. 2019, 20, 5624. https://doi.org/10.3390/ijms20225624
Ceccarelli F, Perricone C, Olivieri G, Cipriano E, Spinelli FR, Valesini G, Conti F. Staphylococcus aureus Nasal Carriage and Autoimmune Diseases: From Pathogenic Mechanisms to Disease Susceptibility and Phenotype. International Journal of Molecular Sciences. 2019; 20(22):5624. https://doi.org/10.3390/ijms20225624
Chicago/Turabian StyleCeccarelli, Fulvia, Carlo Perricone, Giulio Olivieri, Enrica Cipriano, Francesca Romana Spinelli, Guido Valesini, and Fabrizio Conti. 2019. "Staphylococcus aureus Nasal Carriage and Autoimmune Diseases: From Pathogenic Mechanisms to Disease Susceptibility and Phenotype" International Journal of Molecular Sciences 20, no. 22: 5624. https://doi.org/10.3390/ijms20225624
APA StyleCeccarelli, F., Perricone, C., Olivieri, G., Cipriano, E., Spinelli, F. R., Valesini, G., & Conti, F. (2019). Staphylococcus aureus Nasal Carriage and Autoimmune Diseases: From Pathogenic Mechanisms to Disease Susceptibility and Phenotype. International Journal of Molecular Sciences, 20(22), 5624. https://doi.org/10.3390/ijms20225624





